Environmental Factors Influence Plant Vascular System and Water Regulation
Abstract
:1. Understanding the Effects of Climate Change on Plant Water Status
2. Plant Transpiration and Its Regulating Factors
2.1. Plant Vascular Structure and Function
2.2. Vascular Cambium and Plant Growth
2.3. Plant Hydraulic Conductance
3. Environmental Factors and Plant Water Status
3.1. Thermal-Related Responses of Plant Growth and Vascular System
3.2. CO2-Dependent Responses of Plant Growth and Vascular System
3.3. Drought-Related Responses of Plant Growth and Vascular System
4. Plant Responses to Multiple Environmental Factors
4.1. Plant Responses to Temperature and Carbon Dioxide
4.2. Plant Responses to Temperature and Drought Stress
4.3. Plant Responses to Carbon Dioxide and Drought
4.4. Interactive Effects of Temperature, Carbon Dioxide, and Drought on the Form and Function of Plant Vascular System
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S.; et al. Technical summary. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 33–115. [Google Scholar]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.-Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M.; Coopman, R.E.; Gago, J.; Galmés, J.; Martorell, S.; Morales, F.; Diaz-Espejo, A. Stomatal and mesophyll conductances to CO2 in different plant groups: Underrated factors for predicting leaf photosynthesis responses to climate change? Plant Sci. 2014, 226, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, J.J.; Goicoechea, N.; Antolín, M.C.; Pascual, I.; Sánchez-Díaz, M.; Aguirreolea, J.; Morales, F. Growth, photosynthetic acclimation and yield quality in legumes under climate change simulations: An updated survey. Plant Sci. 2014, 226, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flexas, J.; Niinemets, Ü.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water- use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Centritto, M.; Brilli, F.; Fodale, R.; Loreto, F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.F. Researches on transpiration and assimilation. Bot. Gaz. 1896, 21, 26–33. [Google Scholar] [CrossRef]
- Pittermann, J. The evolution of water transport in plants: An integrated approach. Geobiology 2010, 8, 112–139. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 2013, 171, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Milhinhos, A.; Miguel, C.M. Hormone interactions in xylem development: A matter of signals. Plant Cell Rep. 2013, 32, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant Cell Environ. 2015, 38, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Dhirendra Singh, N.; Venugopal, N. Cambial activity and annual rhythm of xylem production of Pinus kesiya Royle ex. Gordon (Pinaceae) in relation to phenology and climatic factors growing in sub-tropical wet forest of North East India. Flora 2011, 206, 198–204. [Google Scholar] [CrossRef]
- Medeiros, J.S.; Ward, J.K. Increasing atmospheric [CO2] from glacial to future concentrations affects drought tolerance via impacts on leaves, xylem and their integrated function. New Phytol. 2013, 199, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Secchi, F. Threats to xylem hydraulic function of trees under ‘new climate normal’ conditions. Plant Cell Environ. 2015, 38, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ye, L.; Nii, N. Effects of soil water availability on development of suberin lamellae in the endodermis and exodermis and on cortical cell wall thickening in red bayberry (Myrica rubra Sieb. et Zucc.) tree roots. Sci. Hort. 2011, 129, 554–560. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Yamane, K.; Islam, M.A.; Oribe, Y.; Ko, J.-H.; Jin, H.-O.; Funada, R. A rapid decrease in temperature induces latewood formation in artificially reactivated cambium of conifer stems. Ann. Bot. 2012, 110, 875–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.; David, J.S.; Cochard, H.; Caldeira, M.C.; Henriques, M.O.; Quilhó, T.; Paço, T.A.; Pereira, J.S.; David, T.S. Drought-induced embolism in current-year shoots of two Mediterranean evergreen oaks. For. Ecol. Manag. 2012, 285, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef] [PubMed]
- Swidrak, I.; Gruber, A.; Oberhuber, W. Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees 2014, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabeshima, E.; Kubo, T.; Yasue, K.; Hiura, T.; Funada, R. Changes in radial growth of earlywood in Quercus crispula between 1970 and 2004 reflect climate change. Trees 2015, 29, 1273–1281. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiol. Plant. 2006, 126, 458–468. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Response of nodulated alfalfa to water supply, temperature and elevated CO2: Productivity and water relations. Environ. Exp. Bot. 2006, 55, 130–141. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Combined effects of temperature and carbon dioxide on plant growth and subsequent seed germinability of Silene noctiflora. Int. J. Plant Sci. 2008, 169, 1200–1209. [Google Scholar] [CrossRef]
- Čufar, K.; Cherubini, M.; Gričar, J.; Prislan, P.; Spina, S.; Romagnoli, M. Xylem and phloem formation in chestnut (Castanea sativa Mill.) during the 2008 growing season. Dendrochronologia 2011, 29, 127–134. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Lynch, A.L.; Godin, V.J.; Reid, D.M. Single and interactive effects of temperature, carbon dioxide, and watering regime on the invasive weed black knapweed (Centaurea nigra). Écoscience 2013, 20, 328–338. [Google Scholar] [CrossRef]
- Beerling, D.J.; Franks, P.J. The hidden cost of transpiration. Nature 2010, 464, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J. Capillarität und saftsteigen. Ber. Deutsch. Bot. Ges. 1893, 11, 203–212. [Google Scholar]
- Dixon, H.H.; Joly, J. On the ascent of sap. Philos. Trans. R. Soc. Lond. B 1894, 186, 563–576. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, J.; Hwang, I. Investigating water transport through the xylem network in vascular plants. J. Exp. Bot. 2014, 65, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.S. Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. J. Exp. Bot. 2015, 66, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakimova, E.T.; Woltering, E.J. Xylogenesis in zinnia (Zinnia elegans) cell cultures: Unravelling the regulatory steps in a complex developmental programmed cell death event. Planta 2017, 245, 681–705. [Google Scholar] [CrossRef] [PubMed]
- Payvandi, S.; Daly, K.R.; Jones, D.L.; Talboys, P.; Zygalakis, K.C.; Roose, T. A mathematical model of water and nutrient transport in xylem vessels of a wheat plant. Bull. Math. Biol. 2014, 76, 566–596. [Google Scholar] [CrossRef] [PubMed]
- Levanič, T.; Čater, M.; McDowell, N.G. Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol. 2011, 31, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, B.; Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Hall, S.A.; Duniway, M.C.; Hochstrasser, T.; Jia, G.; Munson, S.M.; Pyke, D.A.; et al. Climate change-induced vegetation shifts lead to more ecological droughts despite projected rainfall increases in many global temperate drylands. Glob. Chang. Biol. 2017, 23, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Dié, A.; Kitin, P.; Kouamé, F.N.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann. Bot. 2012, 110, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Pramod, S.; Rao, K.S. Cambial activity, annual rhythm of xylem production in relation to phenology and climatic factors and lignification pattern during xylogenesis in drum-stick tree (Moringa oleifera). Flora 2014, 209, 556–566. [Google Scholar] [CrossRef]
- Pramod, S.; Patel, P.B.; Rao, K.S. Influence of exogenous ethylene on cambial activity, xylogenesis and ray initiation in young shoots of Leucaena leucocephala (Lam.) de Wit. Flora 2013, 208, 549–555. [Google Scholar] [CrossRef]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tähtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K.; et al. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Pivovaroff, A.L.; Sack, L.; Santiago, L.S. Coordination of stem and leaf hydraulic conductance in southern California shrubs: A test of the hydraulic segmentaion hypothesis. New Phytol. 2014, 203, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; John, G.P.; Scoffoni, C.; Sack, L. How does leaf anatomy influence water transport outside the xylem? Plant Physiol. 2015, 168, 1616–1635. [Google Scholar] [CrossRef] [PubMed]
- Pieruschka, R.; Huber, G.; Berry, J.A. Control of transpiration by radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 13372–13377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peak, D.; Mott, K.A. A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ. 2011, 34, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Voelker, S.L.; Lachenbruch, B.; Meinzer, F.C.; Kitin, P.; Strauss, S.H. Transgenic poplars with reduced lignin show impaired xylem conductivity, growth efficiency and survival. Plant Cell Environ. 2011, 34, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.J.; Peterson, C.A. Casparian bands occur in the periderm of Pelargonium hortorum stem and root. Ann. Bot. 2011, 107, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jingmin, L.; Chong, L.; Zheng, X.; Kaiping, Z.; Xue, K.; Liding, W. A microfluidic pump/valve inspired by xylem embolism and transpiration in plants. PLoS ONE 2012, 7, e50320. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Tyree, M.T. Mechanism of water stress-induced xylem embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem network transport capacity: A review of embolism repair in vascular plants. Front. Plant Sci. 2013, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Matthews, M.A.; Shackel, K.A. The dynamics of embolism repair in xylem: In vivo visualizations using high-resolution computed tomography. Plant Physiol. 2010, 154, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Ribas-Carbo, M.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachez, C.; Besserer, A.; Chevalier, A.S.; Chaumont, F. Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci. 2013, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Laur, J.; Hacke, U.G. Transpirational demand affects aquaporin expression in poplar roots. J. Exp. Bot. 2013, 64, 2283–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaldenhoff, R.; Kai, L.; Uehlein, N. Aquaporins and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta 2014, 1840, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.D.C.; Carvajal, M. New challenges in plant aquaporin biotechnology. Plant Sci. 2014, 217–218, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.B.; MacKinnon, E.D.; Venturas, M.D.; Crous, C.J.; Jacobsen, A.L. Root resistance to cavitation is accurately measured using a centrifuge technique. Tree Physiol. 2015, 35, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, T.; Zeppel, M.J.B.; Anderegg, W.R.L.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; Ruehr, N.K.; Powell, T.L.; von Arx, G.; Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: Processes and trade-offs. Ecol. Res. 2018, 33, 839–855. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostiainen, K.; Kaakinen, S.; Saranpää, P.; Sigurdsson, B.D.; Linder, S.; Vapaavuori, E. Effect of elevated [CO2] on stem wood properties of mature Norway spruce grown at different soil nutrient availability. Glob. Chang. Biol. 2004, 10, 1526–1538. [Google Scholar] [CrossRef]
- Lovisola, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Paul, S.; Das, M.K.; Baishya, P.; Ramteke, A.; Farooq, M.; Baroowa, B.; Sunkar, R.; Gogoi, N. Effect of high temperautre on yield associated parameters and vascular bundle development in five potato cultivars. Sci. Hort. 2017, 225, 134–140. [Google Scholar] [CrossRef]
- Twumasi, P.; van Ieperen, W.; Woltering, E.J.; Emons, A.M.C.; Schel, J.H.N.; Snel, J.F.H.; van Meeteren, U.; van Marwijk, D. Effects of water stress during growth on xylem anatomy, xylem functioning and vase life in three Zinnia elegans cultivars. Acta Hort. 2005, 669, 303–311. [Google Scholar] [CrossRef]
- Dos Santos, A.B.; Bottcher, A.; Kiyota, E.; Mayer, J.L.S.; Vicentini, R.; dos Santos Brito, M.; Creste, S.; Landell, M.G.A.; Mazzafera, P. Water sterss alters lignin content and related gene expression in two sugarcane genotypes. J. Agric. Food Chem. 2015, 63, 4708–4720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and post-drought recovery between the small-leafed and large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant. 2013, 35, 213–222. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Allakhverdiev, S.I.; Hurry, V.; Hüner, N.P.A. Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 2015, 126, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; O’Hara, K.H.; Campion, C.M.; Walker, A.V.; Edwards, N.T. Thermal plasticity of photosynthesis: The role of acclimation in forest responses to a warming climate. Glob. Chang. Biol. 2010, 16, 2272–2286. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Prislan, P.; Gričar, J.; de Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- De Schepper, V.; De Swaef, T.; Bauweraerts, I.; Steppe, K. Phloem transport: a review of mechanisms and controls. J. Exp. Bot. 2013, 64, 4839–4850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, M.B.; Johnson, E.A. Temperature-dependent rate models of vascular cambium cell mortality. Can. J. For. Res. 2004, 34, 546–559. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A.; Tyree, M.T. Moving byond the cambium necrosis hypothesis of post-fire tree mortality: Cavitation and deformation of xylem in forest fires. New Phytol. 2012, 194, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.G.; Shafroth, P.B.; Blumenthal, D.M.; Morgan, J.A.; LeCain, D.R. Elevated CO2 does not offset greater water stress predicted under climate change for native and exotic riparian plants. New Phytol. 2013, 197, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Abrams, M.D. Adaptations of forest ecosystems to air pollution and climate change. Tree Physiol. 2011, 31, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 2012, 111, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.U.; Brüggemann, W. Photosynthetic responses of a C3 and three C4 species of the genus Panicum (s.l.) with different metabolic subtypes to drought stress. Photosynth. Res. 2012, 112, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.; Pittermann, J.; Polley, H.W.; Aspinwall, M.J.; Fay, P.A. The effect of subambient to elevated atmospheric CO2 concentration on vascular function in Helianthus annuus: Implications for plant response to climate change. New Phytol. 2013, 199, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Smith, D.D.; McCulloh, K.A. A synthesis of the effects of atmospheric carbon dioxide enrichment on plant hydraulics: Implications for whole-plant water use efficiency and resistance to drought. Plant Cell Environ. 2017, 40, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Atwell, B.J.; Henery, M.L.; Whitehead, D. Sapwood development in Pinus radiata trees grown for three years at ambient and elevated carbon dioxide partial pressures. Tree Physiol. 2003, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Salomón, R.L.; Limousin, J.M.; Ourcival, J.M.; Rodríguez-Calcerrada, J.; Steppe, K. Stem hydraulic capacitance decreases with drought stress: Implications for modelling tree hydraulics in the Mediterranean oak Quercus ilex. Plant Cell Environ. 2017, 40, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Love, D.M. What plant hydraulics can tell us about responses to climate- change droughts. New Phytol. 2015, 207, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Masias, F.H.; Knipfer, T.; McElrone, A.J. Differential responses of grapevine rootstocks to water stress are associated with adjustments in fine root hydraulic physiology and suberization. J. Exp. Bot. 2015, 66, 6069–6078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eapen, D.; Barroso, M.L.; Ponce, G.; Campos, M.E.; Cassab, G.I. Hydrotropism: Root growth responses to water. Trends Plant Sci. 2005, 10, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Sai Prasad, S.V.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.A.; Esteban, L.G.; de Palacios, P.; García Fernández, F. Variation in wood anatomical traits of Pinus sylvestris L. between Spanish regions of provenance. Trees 2010, 24, 1017–1028. [Google Scholar] [CrossRef]
- Lens, F.; Picon-Cochard, C.; Delmas, C.E.L.; Signarbieux, C.; Buttler, A.; Cochard, H.; Jansen, S.; Chauvin, T.; Chacon-Doria, L.; del Arco, M.; et al. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiol. 2016, 172, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Volaire, F.; Lens, F.; Cochard, H.; Xu, H.; Chacon-Doria, L.; Bristiel, P.; Balachowski, J.; Rowe, N.; Violle, C.; Picon-Cochard, C. Embolism and mechanical resistances play a key role in dehydration tolerance of a perennial grass Dactylis glomerata L. Ann. Bot. 2018, 122, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S. Drought impacts on phloem transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Stangl, Z.R.; Ivanov, A.G.; Bui, V.; Mema, M.; Hüner, N.P.A.; Öquist, G.; Way, D.; Hurry, V. Contrasting acclimation abilities of two dominant boreal conifers to elevated CO2 and temperature. Plant Cell Environ. 2018, 41, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Fauset, S.; Oliveira, L.; Buckeridge, M.S.; Foyer, C.H.; Galbraith, D.; Tiwari, R.; Gloor, M. Contrasting responses of stomatal conductance and photosynthetic capacity to warming and elevated CO2 in the tropical tree species Alchornea glandulosa under heatwave conditions. Environ. Exp. Bot. 2019, 158, 28–39. [Google Scholar] [CrossRef]
- Paudel, I.; Halpern, M.; Wagner, Y.; Raveh, E.; Yermiyahu, U.; Hoch, G.; Klein, T. Elevated CO2 compensates for drought effects in lemon saplings via stomatal downregulation, increased soil moisture, and increased wood carbon storage. Environ. Exp. Bot. 2018, 148, 117–127. [Google Scholar] [CrossRef]
- Wertin, T.M.; McGuire, M.A.; Teskey, R.O. Effects of predicted future and current atmospheric temperature and [CO2] and high and low soil moisture on gas exchange and growth of Pinus taeda seedlings at cool and warm sites in the species range. Tree Physiol. 2012, 32, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Huang, G.; Zhou, S.; Tissue, D.T. Dry mass productions, allocation patterns and water use efficientcy of two conifers with different water use strategies under elevated [CO2], warming and drought conditions. Eur. J. For. Res. 2018, 137, 605–618. [Google Scholar] [CrossRef]
- Lewis, J.D.; Smith, R.A.; Ghannoum, O.; Logan, B.A.; Phillips, N.G.; Tissue, D.T. Industrial-age changes in atmosphere [CO2] and temperature differentially alter responses of faster- and slower-growing Eucalyptus seedlings to short-term drought. Tree Physiol. 2013, 33, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, W.; Chang, S.X.; Anyia, A.O. Water-deficit and high temperature affected water use efficiency and arabinoxylan concentration in spring wheat. J. Cereal Sci. 2010, 52, 263–269. [Google Scholar] [CrossRef]
- Wang, A.; Lam, S.K.; Hao, X.; Li, F.X.; Zong, Y.; Wang, H.; Li, P. Elevated CO2 reduces the adverse effects of drought stress on a high-yielding soybean (Glycine max (L.) Merr.) cultivar by increasing water use efficiency. Plant Physiol. Biochem. 2018, 132, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Pérez, P.; Hernández, L.; Irigoyen, J.J.; Zita, G.; Martínez-Carrasco, R.; Sánchez-Díaz, M. The response of nodulated alfalfa to water supply, temperature and elevated CO2: Photosynthetic downregulation. Physiol. Plant. 2005, 23, 348–358. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; de Vos, D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schütz, M.; Fangmeier, A. Growth and yield responses of spring wheat (Triticum aestivum L. cv. Minaret) to elevated CO2 and water limitation. Environ. Pollut. 2001, 114, 187–194. [Google Scholar] [CrossRef]
- Smith, N.G.; Dukes, J.S. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob. Chang. Biol. 2013, 19, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Swann, A.L.S.; Hoffman, F.M.; Koven, C.D.; Randerson, J.T. Plant responses to increasing CO2 reduce estimates of climate impacts on drought severity. Proc. Natl. Acad. Sci. USA 2016, 113, 10019–10024. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.M.; Linares, J.C.; García-Cervigón, A.I.; Arzac, A.; Delgado, A.; Rozas, V. Drought-induced increase in water-use efficiency reduces secondary tree growth and tracheid wall thickness in a Mediterranean conifer. Oecologia 2014, 176, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on dry matter partitioning and photosynthesis before and after cutting of nodulated alfalfa. Plant Sci. 2006, 170, 1059–1067. [Google Scholar] [CrossRef]
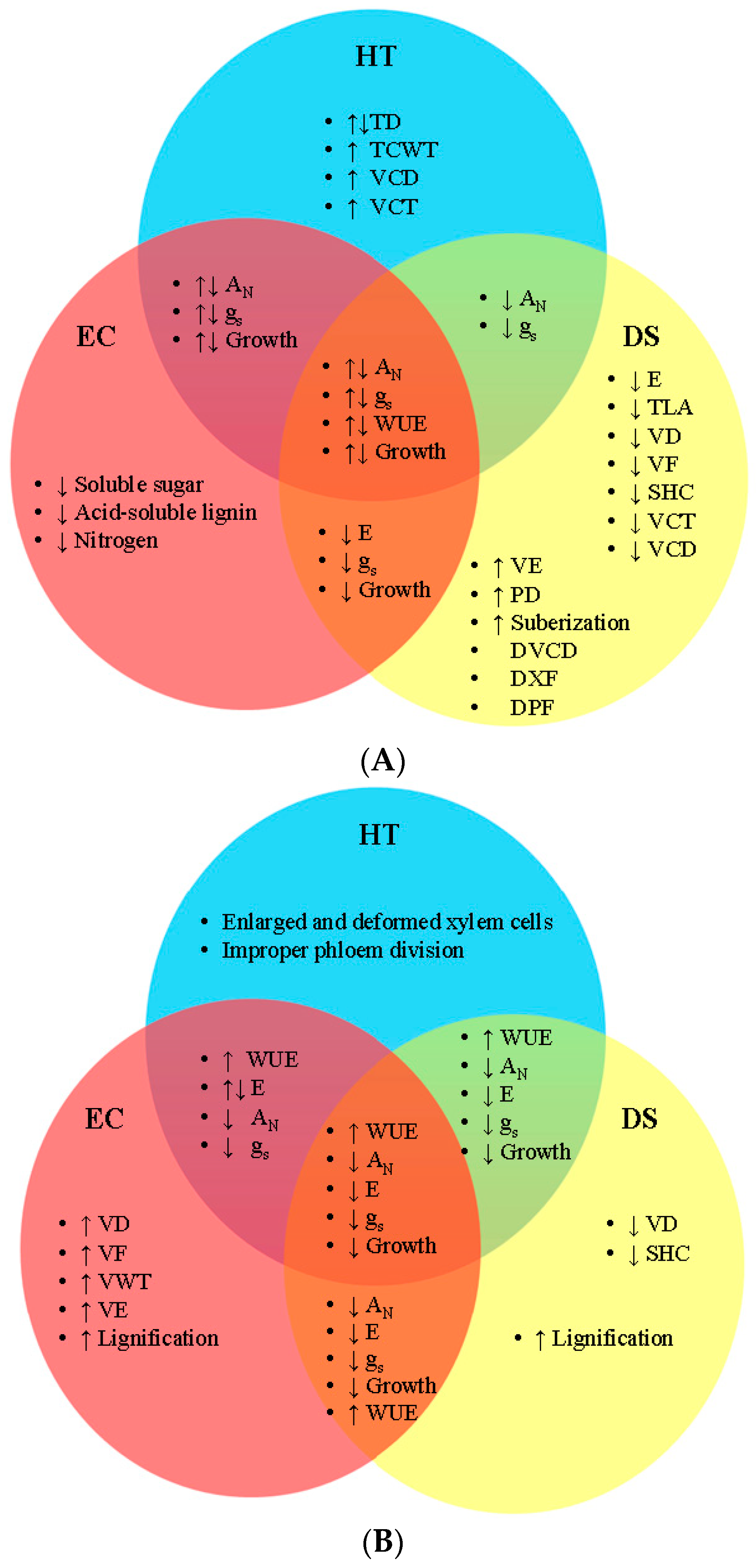
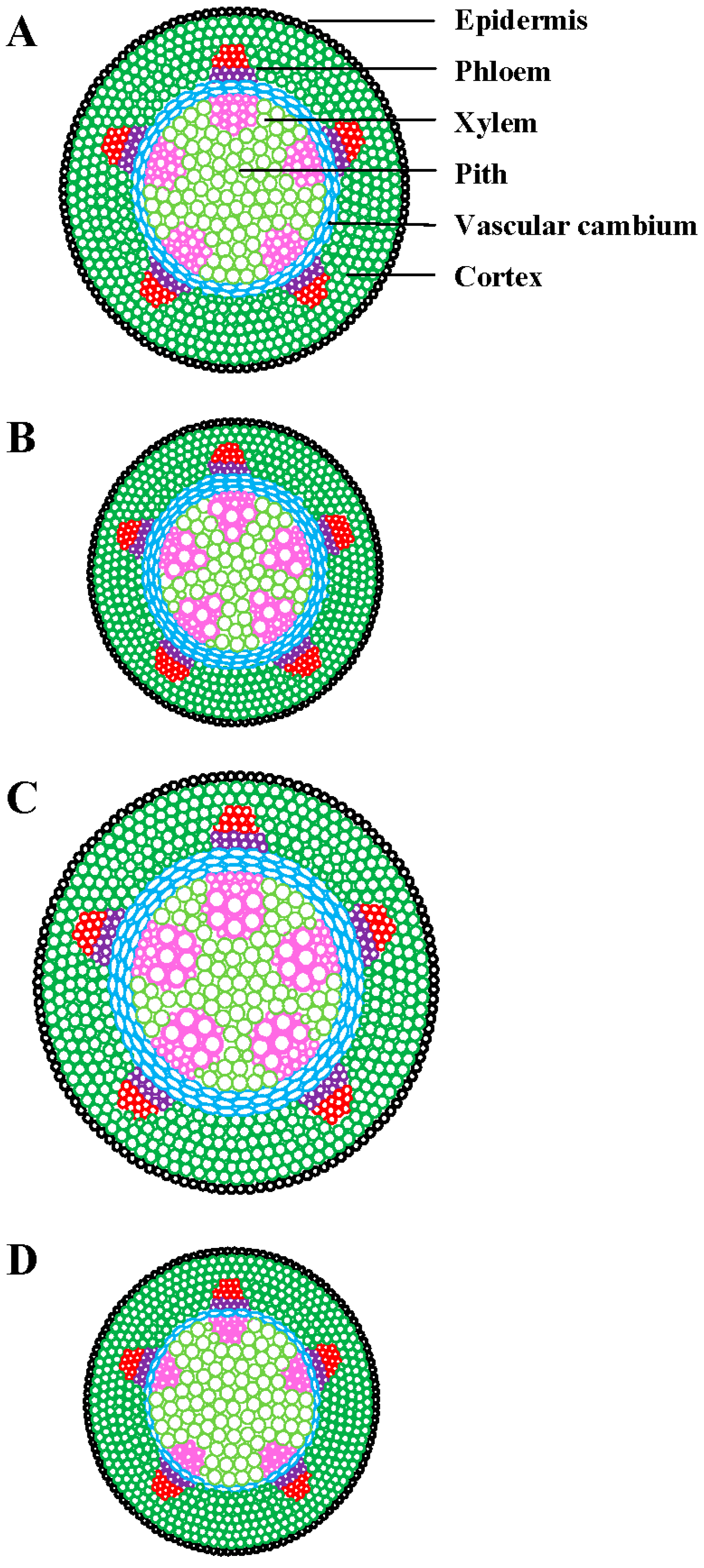

| Environmental Factor | Common Name | SCIENTIFIC NAME | Xylem | Phloem | Vascular Cambium | Structural Polymers | Plant Organ | Experimental Condition | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Woody plants | |||||||||
| HT | Khasi pine | Pinus kesiya Royle ex. Gordon | ↑ tracheid diameter | NM | ↑ thickness | NM | Stem | Field | [16] |
| Momi fir | Abies firma Siebold & Zucc. | ↓ tracheid diameter ↑ tracheid cell wall thickness | NM | Induced division | NM | Stem | Field nursery | [20] | |
| EC | Norway spruce | Picea abies (L.) Karst. | NM | NM | NM | ↓ concentrations of soluble sugar, acid-soluble lignin and nitrogen | Stem | Whole-tree chamber | [70] |
| DS | Common grape | Vitis vinifera L. | ↓ vessel diameter ↑ embolism | NM | NM | NM | Stem | Greenhouse | [71] |
| Cork oak | Quercus suber L. | ↓ vessel diameter - vessel density | NM | NM | ↑ suberization | Stem | Field | [22] | |
| European larch | Larix decidua Mill. | Delayed formation ↓ tracheid diameter | Delayed formation ↑ diameter | ↓ thickness ↓ division | NM | Stem | Field | [24] | |
| Teak | Tectona grandis (L.f.) Kuntze | Delayed formation ↓ vessel thickness | Delayed formation | Delayed division ↓ thickness | - lignification | Stem | Field | [43] | |
| Herbaceous plants | |||||||||
| HT | Potato | Solanum tuberosum L. | Enlarged and deformed cells | Improper division | NM | NM | Stem | Field | [72] |
| EC | Common bean | Phaseolus vulgaris L. | ↑ vessel diameter ↓ vessel density ↑ embolism | NM | NM | NM | Stem | Growth chamber | [17] |
| DS | Common zinnia | Zinnia elegans Sessé & Moc. | ↓ vessel diameter | NM | NM | NM | Stem | Greenhouse | [73] |
| Sugarcane | Saccharum spp. | NM | NM | NM | ↑ lignification | Stem | Greenhouse | [74] | |
| White clover | Trifolium repens L. | NM | NM | NM | ↑ lignification | Leaf and root | Greenhouse | [75] | |
| Environmental factor | Common Name | Scientific name | Growth/Biomass | AN | gs | E | WUE | Reference |
|---|---|---|---|---|---|---|---|---|
| Woody plants | ||||||||
| HT × EC | Norway spruce | Picea abies (L.) H. Karst | ↓ | ↓ | ↓ | NM | NM | [105] |
| Scots pine | Pinus sylvestris L. | ↑ | ↑ | ↑ | NM | NM | [105] | |
| Tamanqueiro, local name | Alchornea glandulosa Poepp. & Endle. | NM | ↑ | ↓ | NM | NM | [106] | |
| HT × DS | Black poplar | Populus nigra L. | NM | ↓ * | ↓ * | NM | NM | [7] |
| EC × DS | Lemon tree | Citrus limon (L.) Burm. F. var. ‘Villafranca’ | - | - | ↓ * | ↓ * | NM | [107] |
| HT × EC × DS | Loblolly pine | Pinus taeda L. | ↑ * (warm site) | ↑ * (June) | ↓ | NM | NM | [108] |
| Monterey pine | Pinus radiata D. Don | ↓ * | - | - | - | ↑ * | [109] | |
| Oyster Bay pine | Callitris rhomboidea R. Br. Ex Rich. & A. Rich. | ↓ * | - | - | - | ↑ * | [109] | |
| Red ironbark | Eucalyptus sideroxylon A. Cunn ex. Woolls | ↑ | ↓ | ↓ | NM | NM | [110] | |
| Sydney blue gum | Eucalyptus saligna Sm. | ↑ | ↓ | ↓ | NM | NM | [110] | |
| Herbaceous plants | ||||||||
| HT × EC | Common bean | Phaseolus vulgaris L. | - | ↓ * | ↓ * | ↓ * | ↑ * | [17] |
| Night-flowering catchfly | Silene noctiflora L. | - | - | NM | ↑ * | ↑ | [28] | |
| HT × DS | Spring wheat | Triticum aestivum L. | ↓ * | ↓ | ↓ | ↓ | ↑ * | [111] |
| EC × DS | Soybean | Glycine max (L.) Merr. | ↓ | ↓ | ↓ | ↓ | ↑ ** | [112] |
| HT × EC × DS | Alfalfa | Medicago sativa L. | ↓ * | ↓ * | ↓ * | NM | ↑ * | [26,27,113] |
| Bird’s-foot trefoil | Lotus corniculatus L. | ↓ * FM | ↓ * | ↓ * | NM | NM | [114] | |
| Black medick | Medicago lupulina L. | ↓ * FM | ↓ * | ↓ * | NM | NM | [114] | |
| Canola | Brassica napus L. | - | - | ↓ * | ↓ * | ↑ * | [1] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants 2019, 8, 65. https://doi.org/10.3390/plants8030065
Qaderi MM, Martel AB, Dixon SL. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants. 2019; 8(3):65. https://doi.org/10.3390/plants8030065
Chicago/Turabian StyleQaderi, Mirwais M., Ashley B. Martel, and Sage L. Dixon. 2019. "Environmental Factors Influence Plant Vascular System and Water Regulation" Plants 8, no. 3: 65. https://doi.org/10.3390/plants8030065
APA StyleQaderi, M. M., Martel, A. B., & Dixon, S. L. (2019). Environmental Factors Influence Plant Vascular System and Water Regulation. Plants, 8(3), 65. https://doi.org/10.3390/plants8030065






