Characterization of Adult Functional Traits of Local Populations and Cultivars of Sandberg Bluegrass and Bottlebrush Squirreltail Perennial Bunchgrasses
Abstract
:1. Introduction
2. Results
2.1. Sandberg Bluegrass Aboveground Traits
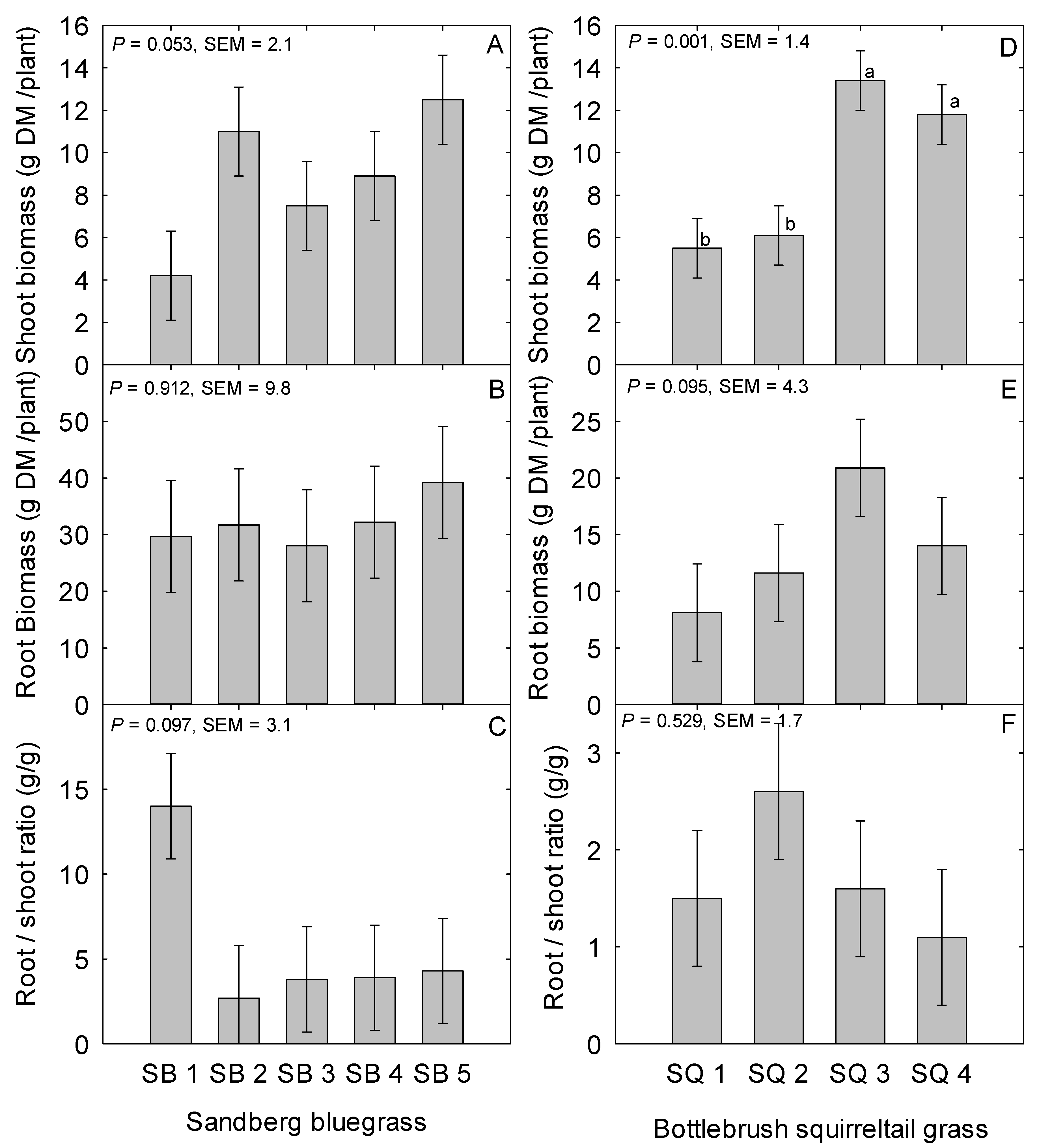
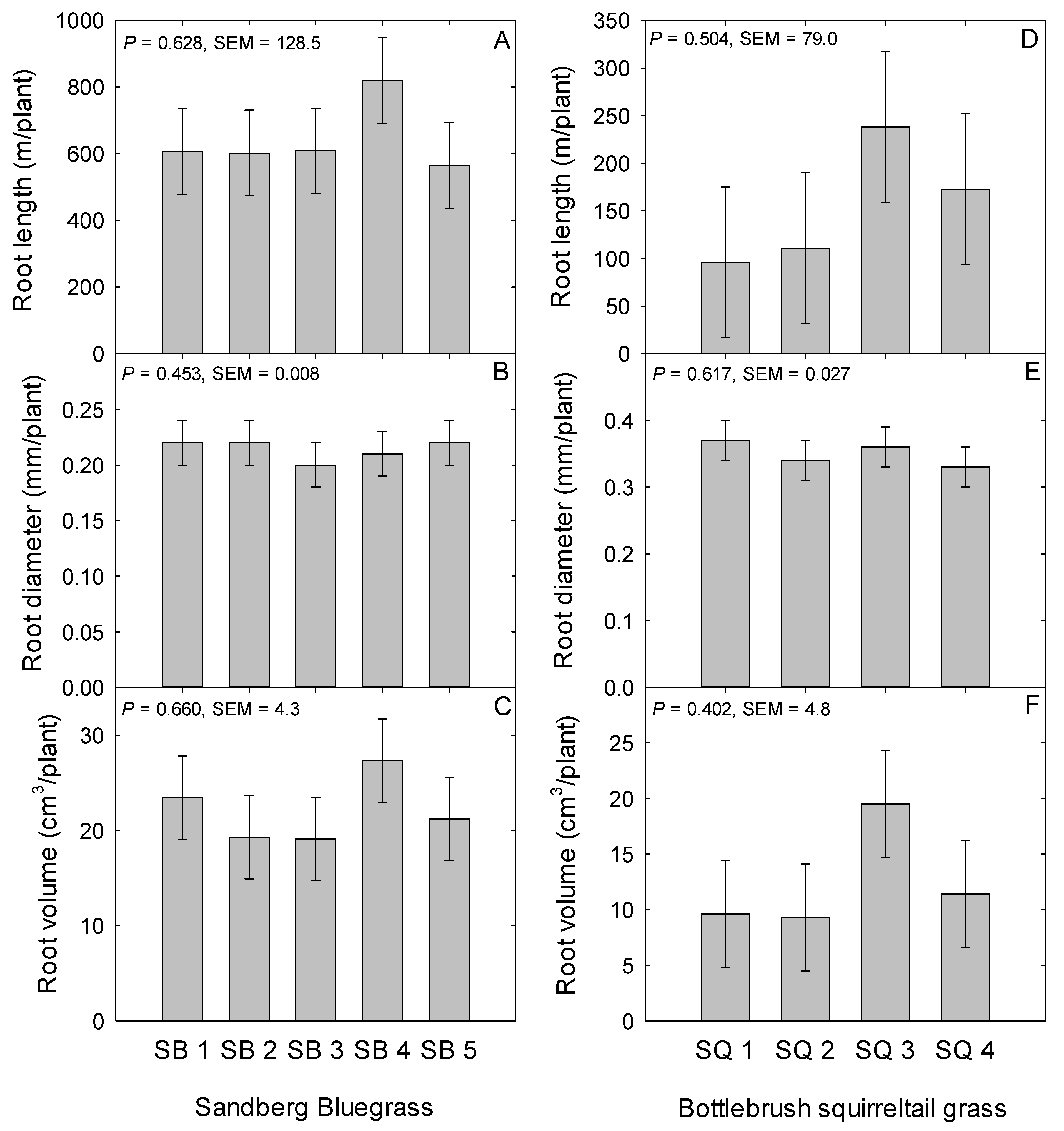
2.2. Sandberg Bluegrass Belowground Traits
2.3. Sandberg Bluegrass Forage Nutritive Value
2.4. Bottlebrush Squirreltail Aboveground Traits
2.5. Bottlebrush Squirreltail Belowground Traits
2.6. Bottlebrush Squirreltail Forage Nutritive Value
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Study Location
4.2. Seedling Establishment and Transplant, Experimental Design, and Management
4.3. Data Collected
4.4. Root Image Scanning and Analysis
4.5. Data Analysis
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Booth, T.B. Seed longevity and seeding strategies affect sagebrush revegetation. J. Range Manag. 2002, 55, 188–193. [Google Scholar] [CrossRef]
- Pellant, M.; Abbey, B.; Karl, S. Restoring the Great Basin Desert, U.S.A.: Integrating science, management, and people. Environ. Monit. Assess. 2004, 99, 169–179. [Google Scholar] [CrossRef]
- Davies, G.M.; Bakker, J.D.; Dettweiler-Robinson, E.; Dunwiddie, P.W.; Hall, S.A.; Downs, J.; Evans, J. Trajectories of change in sagebrush steppe vegetation communities in relation to multiple wildfires. Ecol. Appl. 2012, 22, 1562–1577. [Google Scholar] [CrossRef] [PubMed]
- DiTomaso, J.M. Invasive weeds in rangelands: Species, impacts, and management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Chambers, J.C.; Roundy, B.A.; Blank, R.R.; Meyer, S.E.; Whittaker, A. What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol. Monogr. 2007, 77, 117–145. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in Mediterranean-climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.E.; Saab, V.A.; Rich, T.D.; Dobkin, D.S. Effects of livestock grazing on neotropical migratory landbirds in western North America. In Status and Management of Neotropical Migratory Birds; Finch, D.M., Stangel, P.W., Eds.; United States Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station, General Technical Report RM-229: Fort Collins, CO, USA, 1993; pp. 296–309. [Google Scholar]
- Knick, S.T.; Dobkin, D.S.; Rotenberry, J.T.; Schroeder, M.A.; Vander Haegen, W.M.; Van Riper III, C. Teetering on the edge or too late? Conservation and research issues for avifauna of sagebrush habitats. Condor 2003, 105, 611–634. [Google Scholar] [CrossRef]
- Alder, P.B.; Milchunas, D.G.; Sala, O.E.; Burke, I.C.; Lauenroth, W.K. Plant traits and ecosystem grazing effects: Comparison of U.S. sagebrush steppe and Patagonian steppe. Ecol. Appl. 2005, 15, 774–792. [Google Scholar]
- McKay, J.K.; Christian, C.E.; Harrison, S.; Rice, K.J. “How local is local?” – A review of practical conceptual issues in the genetics of restoration. Restor. Ecol. 2005, 13, 432–440. [Google Scholar] [CrossRef]
- Bureau of Land Management. The Great Basin Restoration Initiative: A Hand to Nature: Progress to Date; National Interagency Fire Center, US Department of Interior: Boise, ID, USA, 2001; pp. 1–33.
- Jones, T.A. Ecologically appropriate plant materials for restoration applications. BioScience 2013, 63, 211–219. [Google Scholar] [CrossRef]
- Schröder, R.; Prasse, R. Cultivation and hybridization alter the germination behavior of native plants used in revegetation and restoration. Restor. Ecol. 2013, 21, 793–800. [Google Scholar] [CrossRef]
- Kimball, S.; Lulow, M.E.; Mooney, K.A.; Sorenson, Q.M. Establishment and management of native functional groups in restoration. Restor. Ecol. 2014, 22, 81–88. [Google Scholar] [CrossRef]
- Buisson, E.; Alvarado, S.T.; Stradic, S.L.; Morellato, L.P.C. Plant phenological research enhances ecological restoration. Restor. Ecol. 2017, 25, 164–171. [Google Scholar] [CrossRef]
- James, J.J.; Drenovsky, R.E.; Monaco, T.A.; Rinella, M.J. Managing soil nitrogen to restore annual grass-infested plant communities: Effective strategy or incomplete framework? Ecol. Appl. 2011, 21, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Mukherjee, J.R.; Jones, T.A.; Alder, P.B.; Monaco, T.A. Contrasting mechanism of recovery from defoliation in two Intermountain-Native Bunchgrasses. Rangel. Ecol. Manag. 2015, 68, 485–493. [Google Scholar] [CrossRef]
- Sandel, B.; Corbin, J.D.; Krupa, M. Using plant functional traits to guide restoration: A case study in California coastal grassland. Ecosphere 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Ferrero-Serrano, A.; Hild, A.L.; Mealor, B.A. Can invasive species enhance competitive ability and restoration potential in native grass. Restor. Ecol. 2011, 19, 545–551. [Google Scholar] [CrossRef]
- Espeland, E.K.; Emery, N.C.; Mercer, K.L.; Woolbright, S.A.; Kettenring, K.M.; Gepts, P.; Etterson, J.R. Evolution of plant materials for ecological restoration: Insights from the applied and basic literature. J. Appl. Ecol. 2017, 54, 102–115. [Google Scholar] [CrossRef]
- Leger, E.A.; Baughman, O.W. What seeds to plant in the Great Basin? Comparing traits prioritized in native plant cultivars and releases with those that promote survival in the field. Nat. Areas J. 2015, 35, 54–68. [Google Scholar] [CrossRef]
- Casper, B.D.; Jackson, R.B. Plant competition underground. Annu. Rev. Ecol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Leger, E.A.; Li, J.; Nowak, R.S. Natural selection favors root investment in native grasses during restoration of invaded fields. J. Arid Environ. 2015, 116, 11–17. [Google Scholar] [CrossRef]
- Judd, L.A.; Jackson, B.E.; Fonteno, W.C. Advancements in root growth measurement technologies and observation capabilities for container-grown plants. Plants 2015, 4, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.C.; Jones, T.A.; Monaco, T.A. Genetic variation for adaptive traits in bottlebrush squirreltail in the Northern Intermountain West, United States. Restor. Ecol. 2011, 19, 460–469. [Google Scholar] [CrossRef]
- Smith, B.M.; Diaz, A.; Daniels, R.; Winder, L.; Holland, J.M. Regional and ecotype traits in Lotus corniculatus L., with reference to restoration ecology. Restor. Ecol. 2009, 17, 12–23. [Google Scholar] [CrossRef]
- Lambert, A.M.; Baer, S.G.; Gibson, D.J. Intraspecific variation in ecophysiology of three dominant prairie grasses used in restoration: Cultivar versus non-cultivar population sources. Restor. Ecol. 2011, 19, 43–52. [Google Scholar] [CrossRef]
- Kulpa, S.M.; Leger, E.A. Strong natural selection during plant restoration favors an unexpected suite of plant traits. Evol. Appl. 2013, 6, 510–523. [Google Scholar] [CrossRef]
- Atwater, D.Z.; James, J.J.; Leger, E.A. Seedling root traits strongly influence field survival and performance of a common bunchgrass. Basic Appl. Ecol. 2015, 1439–1791. [Google Scholar] [CrossRef]
- Pokorny, M.L.; Sheley, R.L.; Zabinski, C.A.; Engel, R.E.; Svejcar, T.J.; Borkowski, J.J. Plant functional group diversity as a mechanism for invasion resistance. Restor. Ecol. 2005, 13, 448–459. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Skinner, R.H.; Barker, D.J.; Edwards, G.R.; Tracy, B.F.; Wedin, D.A. Plant species diversity and management of temperate forage and grazing land ecosystems. Crop Sci. 2004, 44, 1132–1144. [Google Scholar] [CrossRef]
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.A.; Nielson, D.C.; Arredondo, J.T.; Redinbaugh, M.G. Characterization of diversity among 3 squirreltail taxa. J. Range Manag. 2003, 56, 474–482. [Google Scholar] [CrossRef]
- Johnson, R.; Stritch, L.; Olwell, P.; Lambert, S.; Horning, M.E.; Cronn, R. What are the best sources for ecosystem restoration on BLM and USFS lands? Nativ. Plants 2010, 11, 117–131. [Google Scholar] [CrossRef]
- Collins, R.P.; Fothergill, M.; Macduff, J.H.; Puzio, S. Morphological Compatibility of white clover and perennial ryegrass cultivars grown under two nitrate levels in flowing solution culture. Ann. Bot. 2003, 92, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- Johnson, R.C.; Horning, M.E.; Espeland, E.K.; Vance-Borland, K. Relating adaptive genetic traits to climate for Sandberg bluegrass from the Intermountain Western United States. Evol. Appl. 2015, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.E.; Backer, D.M.; Belnap, J.; Bradford, J.B.; Butterfield, B.J.; Copeland, S.M.; Duniway, M.C.; Faist, A.M.; Fick, S.E.; Jensen, S.L.; et al. Beyond traditional ecological restoration on the Colorado Plateau. Restor. Ecol. 2018, 26, 1055–1106. [Google Scholar] [CrossRef]
- Winkler, D.E.; Belnap, J.; Hoover, D.; Reed, S.C.; Duniway, M.C. Shrub persistence and increased grass mortality in response to drought in dryland systems. Glob. Chang. Biol. 2019, 00, 1–15. [Google Scholar] [CrossRef]
- Harper, K.T.; Belnap, J. The influence of biological soil crust on mineral uptake by associated vascular plants. J. Arid Environ. 2001, 47, 347–357. [Google Scholar] [CrossRef]
- Su, Y.G.; Li, X.R.; Cheng, Y.W.; Tan, H.J.; Jia, R.L. Effects of biological soil crusts on emergence of desert vascular plants in North China. Plant Ecol. 2007, 191, 11–19. [Google Scholar] [CrossRef]
- Bowker, M.A.; Belnap, J.; Davidson, D.W. Microclimate and propagule availability are equally important for rehabilitation of dryland N-fixing lichens. Restor. Ecol. 2010, 18, 30–33. [Google Scholar] [CrossRef]
- Raventos, J.; Silva, J.F. Architecture, seasonal growth and interference in three grass species with different flowering phenologies in a tropical savanna. Vegetatio 1988, 75, 115–123. [Google Scholar] [CrossRef]
- Mangla, S.; Sheley, R.L.; James, J.J.; Radosevich, S.R. Role of competition in restoring resource poor arid systems dominated by invasive grasses. J. Arid Environ. 2011, 75, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.A.; Conradi, T.; Meimberg, H.; Kollmann, J. Seed selection for grassland restoration: Competitive effect of a dominant grass is mediated by seed source and nutrient availability. Restor. Ecol. 2015, 23, 261–267. [Google Scholar] [CrossRef]
- McGlone, C.M.; Sieg, C.H.; Kolb, T.E.; Nietupsky, T. Established native perennial grasses out-compete an invasive annual grass regardless of soil water and nutrient availability. Plant Ecol. 2012, 213, 445–457. [Google Scholar] [CrossRef]
- Tiwari, T.P.; Brook, R.M.; Wagstaff, P.; Sinclair, F.L. Effects of light environment on maize in hillside agroforestry systems of Nepal. Food Secur. 2012, 4, 103–114. [Google Scholar] [CrossRef]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis: Do leaf structure and orientation interact to regulate internal light and carbon dioxide? BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Zolotareva, N.V.; Ronzhina, D.A.; Podgaevskaya, E.N.; Migalina, S.V.; Ivanov, L.A. Leaf functional traits of abundant species predict productivity in three temperate herbaceous communities along an environmental gradient. Flora 2018, 239, 11–19. [Google Scholar] [CrossRef]
- Huber-Sannwald, E.; Pyke, D.A.; Caldwell, M.M. Morphological plasticity following species-specific recognition and competition in two perennial grasses. Am. J. Bot. 1996, 83, 919–931. [Google Scholar] [CrossRef]
- Espeland, E.; Johnson, R.C.; Horning, M.E. Plasticity in native perennial grass populations: Implications for restoration. Evol. Appl. 2018, 11, 340–349. [Google Scholar] [CrossRef]
- Shaw, A.N.; Mummey, D.L. Poa secunda local collections and commercial releases: A genotypic evaluation. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Herget, M.E.; Hufford, K.M.; Mummey, D.L.; Shreading, L.N. Consequences of seed origin and biological invasion for early establishment in restoration of a north American grass species. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Arredondo, J.T.; Jones, T.A.; Johnson, D.A. Seedling growth of Intermountain perennial and weedy annual grasses. J. Range Manag. 1998, 51, 584–589. [Google Scholar] [CrossRef]
- Valencia, E.; Quero, J.L.; Maestre, F.T. Functional leaf and size traits determine the photosynthetic response of 10 dryland species to warming. J. Plant Ecol. 2016, 9, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Klopf, R.P.; Baer, S.G. Root dynamics of cultivar and non-cultivar population sources of two dominant grasses during initial establishment of Tallgrass Prairie. Restor. Ecol. 2011, 19, 112–117. [Google Scholar] [CrossRef]
- Hufford, K.M.; Mazer, S.J.; Camara, M.D. Local adaptation and effects of grazing among seedlings of two native California bunchgrass species: Implications for restoration. Restor. Ecol. 2008, 16, 59–69. [Google Scholar] [CrossRef]
- Poelman, M.E.; Pilmanis, A.M.; Hufford, K.M. Testing the cultivar vigor hypothesis: Comparisons of the competitive ability of wild and cultivated populations of Pascopyrum smithii along a restoration chronosequence. Restor. Ecol. 2019, 27, 92–101. [Google Scholar] [CrossRef]
- Sartie, A.M.; Easton, H.S.; Matthew, C. Plant morphology differences in two perennial ryegrass cultivars. N. Z. J. Agric. Res. 2009, 52, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.C.; Jones, T.A.; Larson, S.R.; Mott, I.W.; Monaco, T.A. Ecotypic variation in Elymus elymoides subsp. brevifolius in the northern Intermountain West. Rangel. Ecol. Manag. 2011, 64, 649–658. [Google Scholar]
- Burns, J.C. Nutritive Value. In Tall Fescue for the Twenty-first Century, Agronomy Monograph; Fribourg, H.A., Hannaway, D.B., West, C.P., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2009; Volume 53, pp. 159–201. [Google Scholar]
- Caddel, J.; Allen, E. Forage Quality Interpretations; Oklahoma Cooperative Extension Service. Extension Facts F2117; Oklahoma State University: Stillwater, OK, USA, 1994. [Google Scholar]
- Cruz, R.; Ganskopp, D. Seasonal preferences of steers for prominent norther Great Basin grasses. J. Range Manag. 1998, 51, 557–565. [Google Scholar] [CrossRef]
- Ganskopp, D.; Bohnert, D. Nutritional dynamics of 7 Northern Great Basin grasses. J. Range Manag. 2001, 54, 640–647. [Google Scholar] [CrossRef]
- Demarchi, R.A. Chemical composition of Bighorn winter forages. J. Range Manag. 1968, 21, 385–388. [Google Scholar] [CrossRef]
- Jefferies, N.W.; Rice, R.W. Nutritive value of clipped and grazed range forage samples. J. Range Manag. 1969, 22, 192–195. [Google Scholar] [CrossRef]
- Jensen, K.B.; Johnson, D.A.; Asay, K.H.; Olson, K.C. Seasonal-accumulated growth and forage quality of range grasses for fall and winter grazing. Can. J. Plant Sci. 2002, 82, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Copeland, S.M.; Bradford, J.B.; Duniway, M.C.; Schuster, R.M. Potential impacts of overlapping land-use and climate in a sensitive dryland: A case study of the Colorado Plateau, USA. Ecosphere 2017, 8, e01823. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W.; Ghimire, R.P. Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crop. Res. 2017, 210, 183–191. [Google Scholar] [Green Version]
- Hereford, R.; Webb, R.H. Historic variation of warm-season rainfall, southern Colorado Plateau, Southern USA. Clim. Chang. 1992, 22, 239–256. [Google Scholar] [CrossRef]
- Buckley, R.C. Seed size and seedling establishment in tropical and dunecrest plants. Biotropica 1982, 14, 314–315. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Maskova, T.; Herben, T. Root:shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.L.; Leger, E.A. Competitive seedlings and inherited traits: A test of rapid evolution of Elymus multisetus (big squirreltail) in response to cheatgrass invasion. Evol. Appl. 2011, 4, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, F.A.; Schnyder, H.; Thornton, B. Defoliation effects on carbon and nitrogen substrate import and tissue-bound efflux in leaf growth zones of grasses. Plant Cell Environ. 2004, 27, 347–356. [Google Scholar] [CrossRef]
- Skinner, R.H.; Morgan, J.A.; Hanson, J.D. Carbon and nitrogen reserve remobilization following defoliation: Nitrogen and elevated CO2 effects. Crop Sci. 1999, 39, 1749–1756. [Google Scholar] [CrossRef]
- Hamerlynck, E.P.; Smith, B.S.; Sheley, R.L.; Svejcar, T.J. Compensatory photosynthesis, water-use efficiency, and biomass allocation of defoliated exotic and native bunchgrass seedlings. Rangel. Ecol. Manag. 2016, 69, 206–214. [Google Scholar] [CrossRef]
- Marshall, A.H.; Collins, R.P.; Humphreys, M.W.; Scullion, J. A new emphasis on root traits for perennial grass and legume varieties with environmental and ecological benefits. Food Energy Secur. 2016, 5, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Li, M.-H.; Sardans, J.; Lu, X.-T.; Wang, C.; Penuelas, J.; Wang, Z.; Han, X.-G.; Jiang, Y. Carbon and nitrogen allocation shifts in plants and soils along aridity and fertility gradients in grasslands of China. Ecol. Evol. 2017, 7, 6927–6934. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.L.; Larson, S.R.; Jensen, K.B.; Harrison, R.D.; Palazzo, A.J.; Cary, T.J. Registration of Reliable Sandberg bluegrass germplasm. Crop Sci. 2006, 46, 487–488. [Google Scholar] [CrossRef]
- Kellogg, E.A. Apomixis in the Poa secunda complex. Am. J. Bot. 1987, 74, 1431–1437. [Google Scholar] [CrossRef]
- Majerus, M.; Holzworth, L.; Tilley, D.; Stannard, M. Plant Guide for Sandberg Bluegrass (Poa Secunda J. Presl); USDA-Natural Resources Conservation Service, Idaho Plant Materials Center: Aberdeen, ID, USA, 2009.
- Welsh, S.L. A Utah Flora. The Great Basin Naturalist Memoir No. 9; Atwood, N.D., Higgins, L.C., Goodrich, S., Eds.; Brigham Young University: Provo, UT, USA, 1987; p. 894. [Google Scholar]
- Van Vuren, D. Summer diets of bison and cattle in southern Utah. J. Range Manag. 1984, 37, 260–261. [Google Scholar] [CrossRef]
- Jones, T.A.; Nielson, D.C.; Larson, S.R.; Johnson, D.A.; Monaco, T.A.; Caicco, S.L.; Ogle, D.G.; Young, S.A.; Carlson, J.R. Registration of Toe Jam Creek bottlebrush squirreltail germplasm. Crop Sci. 2004, 44, 1880–1881. [Google Scholar] [CrossRef]
- Jones, T.A. A nomenclatural guide and simplified key to the squirreltail taxa. Nativ. Plants J. 2014, 15, 51–55. [Google Scholar] [CrossRef]
- Fisher, D.S.; Burns, J.C.; Moore, J.E. The nutritive evaluation of forage. In Forages. Volume 1: An Introduction to Grassland Agriculture; Barnes, R.F., Miller, D.A., Nelson, C.J., Eds.; Iowa State Univ. Press: Ames, IA, USA, 1995. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agriculture. Handbook No. 379; USDA-ARS: Washington, DC, USA, 1970.
- Tilley, J.M.A.; Terry, R.A. A two stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.4 Users’ Guide; SAS Institute: Cary, NC, USA, 2017. [Google Scholar]


| Plant Species | Entry | Plant Height (cm) | Leaf Length (cm) | Tiller (plant−1) | Inflorescence Length (cm) | Root Carbon Content (g/plant) | Root Nitrogen Content (mg/plant) |
|---|---|---|---|---|---|---|---|
| Sandberg bluegrass | Button Point (LP)¶ | 20.5 | 13.2c | 268 | - | 341.3 | 13.4 |
| Sandberg bluegrass | Panther Valley (LP) | 23.4 | 16.3ab | 252 | - | 328.5 | 14.2 |
| Sandberg bluegrass | Winnemucca Mountain (LP) | 23.1 | 15.5abc | 224 | - | 287.5 | 12.8 |
| Sandberg bluegrass | Hanford (CV) | 23.6 | 18.4a | 312 | - | 383.3 | 14.6 |
| Sandberg bluegrass | Mountain Home (CV) | 22.9 | 15.0bc¶ | 258 | - | 385.7 | 17.7 |
| SEM† | 1.7 | 1.1 | 33 | - | 97.4 | 4.5 | |
| P value‡ | 0.714 | 0.032 | 0.451 | - | 0.899 | 0.920 | |
| Squirreltail | George St. Sonoma (LP) | 71.0a¶ | 17.3 | 150 | 8.9c | 89.3b | 3.7b |
| Squirreltail | Grass Valley (LP) | 71.6a | 15.9 | 135 | 11.4bc | 120.7ab | 5.2ab |
| Squirreltail | Toe Jam Creek (CV) | 43.0b | 20.4 | 135 | 13.2ab | 237.9a | 9.4a |
| Squirreltail | Vale (CV) | 37.4b | 17.1 | 152 | 16.3a | 160.5ab | 6.4ab |
| SEM† | 3.9 | 1.1 | 15 | 1.2 | 48.8 | 1.9 | |
| P Value‡ | <0.001 | 0.064 | 0.482 | 0.005 | 0.076 | 0.094 |
| Plant Species | Classification | Seed Source | Site Elevation (m) | Average Annual Precipitation (mm) | Seed Collection Reference Number | Latitude | Longitude | Number of Plants Sampled and Area in Parentheses | Soil Type |
|---|---|---|---|---|---|---|---|---|---|
| Poa secunda | Wildland | Button Point | 1340 | 203.2 | NV020-02 | 41° 1′ 22.92” N | 117° 35′ 40.07′ W | 10,000 (20 acres) | Silt |
| Poa secunda | Wildland | Panther Valley | 1495 | 188.0 | NV020-03 | 40° 32′ 51.15” N | 117° 35′ 48.16” W | Not available | Droughty loam |
| Poa secunda | Wildland | Winnemucca Mountain | 1372 | 208.3 | NV020-04 | 41° 02′ 26.10” N | 117° 43′ 28.57” W | 10,000 (50 acres) | Sandy loam |
| Poa secunda | Cultivar | Hanford† | |||||||
| Poa secunda | Cultivar | Mountain Home‡ | |||||||
| Elymus elymoides | Wildland | George St. Sonoma | 1368 | 208.3 | NV020-01 | 40° 44′ 53.61” N | 117° 43′ 19.33” W | 100 (20 acres) | Droughty loam |
| Elymus elymoides | Wildland | Grass Valley | 1485 | 208.3 | NV020-07 | 40° 29′ 29.04” N | 117° 36′ 12.83” | 1000 (3 acres) | Droughty loam |
| Elymus elymoides | Cultivar | Toe Jam Creek | |||||||
| Elymus elymoides | Cultivar | Vale§ |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, J.K.Q. Characterization of Adult Functional Traits of Local Populations and Cultivars of Sandberg Bluegrass and Bottlebrush Squirreltail Perennial Bunchgrasses. Plants 2019, 8, 166. https://doi.org/10.3390/plants8060166
Solomon JKQ. Characterization of Adult Functional Traits of Local Populations and Cultivars of Sandberg Bluegrass and Bottlebrush Squirreltail Perennial Bunchgrasses. Plants. 2019; 8(6):166. https://doi.org/10.3390/plants8060166
Chicago/Turabian StyleSolomon, Juan K. Q. 2019. "Characterization of Adult Functional Traits of Local Populations and Cultivars of Sandberg Bluegrass and Bottlebrush Squirreltail Perennial Bunchgrasses" Plants 8, no. 6: 166. https://doi.org/10.3390/plants8060166
APA StyleSolomon, J. K. Q. (2019). Characterization of Adult Functional Traits of Local Populations and Cultivars of Sandberg Bluegrass and Bottlebrush Squirreltail Perennial Bunchgrasses. Plants, 8(6), 166. https://doi.org/10.3390/plants8060166





