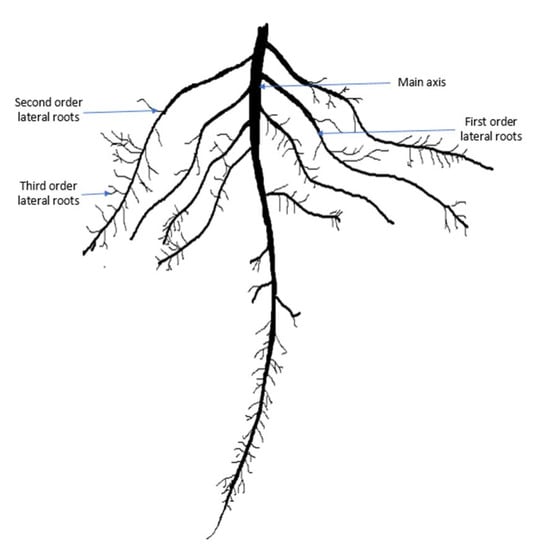
Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Management
2.2. Salinity Treatment, Injury Scoring, and Data Collection
2.3. Estimation of Root Surface Area
2.4. Statistical Analysis
3. Results
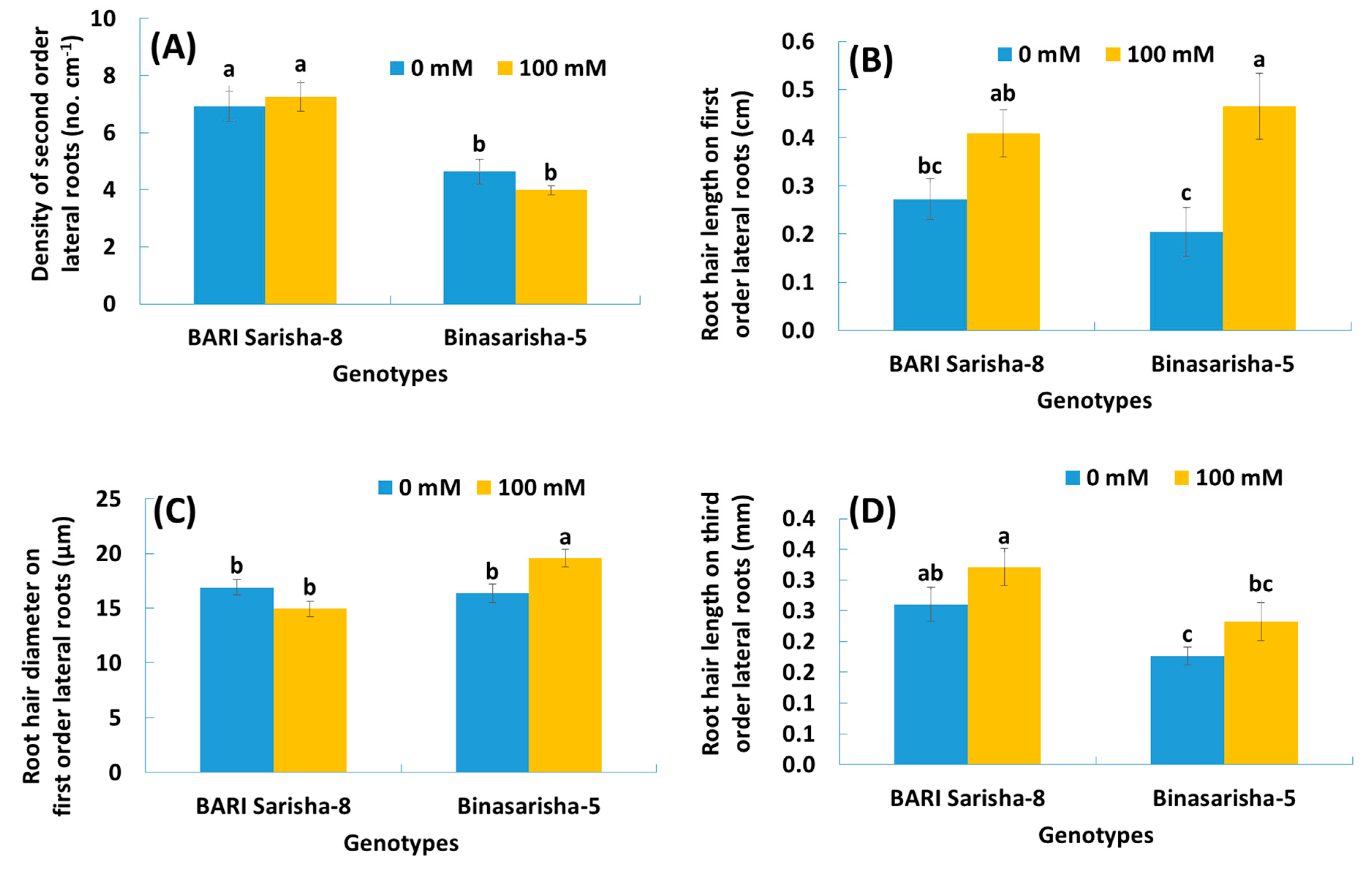
3.1. Effects of Salt Treatment
3.2. Varietal Differences
3.3. Treatment × Variety Differences
3.4. Trait Associations
4. Discussion
4.1. Effects of Salinity Stress on Shoot and Root Morphologies
4.2. Varietal Variations
4.3. Trait Associations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- USDA: United States Department of Agriculture, World Agricultural Production. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 29 November 2018).
- Anonymous. Yearbook of Agricultural Statistics 2017. Statistics and Informatics Division (SID), Ministry of Planning, Government of the People’s Republic of Bangladesh. 2017. Available online: http://bbs.portal.gov.bd/sites/default/files/files/bbs.portal.gov.bd/page/1b1eb817_9325_4354_a756_3d18412203e2/Yearbook-2017-Final-05-05-2018.pdf (accessed on 29 November 2018).
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: Cambridge, MA, USA, 2000; p. 533. [Google Scholar]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching out in roots: Uncovering form, function, and regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Ann. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Park, W.J.; Sauer, M.; Woll, K. From weeds to crops: Genetic analysis of root development in cereals. Trends Plant Sci. 2004, 9, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zobel, R.W.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- Osmont, K.S.; Sibout, R.; Hardtke, C.S. Hidden branches: Developments in root system architecture. Ann. Rev. Plant Biol. 2007, 58, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Li, K.; Sun, F.; Han, C.; Wang, Y.; Li, X. Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J. Plant Res. 2008, 121, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Salt Stress in Plants; Springer: Heidelberg, Germany, 2013. [Google Scholar]
- Kauser, R.; Athar, H.U.R.; Ashraf, M. Chlorophyll fluorescence: A potential indicator for rapid assessment of water stress tolerance in canola (Brassica napus L.). Pak. J. Bot. 2006, 38, 1501–1509. [Google Scholar]
- Shah, S.H. Effects of salt stress on mustard as affected by gibberellic acid application. Gen. Appl. Plant Physiol. 2007, 33, 97–106. [Google Scholar]
- García Morales, S.; Trejo-Téllez, L.I.; Gómez Merino, F.C.; Caldana, C.; Espinosa-Victoria, D.; Herrera Cabrera, B.E. Growth, photosynthetic activity, and potassium and sodium concentration in rice plants under salt stress. Agronomy 2012, 34, 317–324. [Google Scholar]
- Qiu, N.; Lu, Q.; Lu, C. Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica. New Phytol. 2003, 159, 479–486. [Google Scholar] [CrossRef]
- Villalta, I.; Reina-Sánchez, A.; Bolarín, M.C.; Cuartero, J.; Belver, A.; Venema, K.; Asins, M.J. Genetic analysis of Na+ and K+ concentrations in leaf and stem as physiological components of salt tolerance in tomato. Theor. Appl. Gen. 2008, 116, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Blumwald, E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 2001, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzedah, H.; Bemani, M. Influence of salinity at early stage of flowering on the development of male gametophyte in canola (Brassica napus L.) cv. Symbol. Res. J. Environ. Sci. 2008, 2, 415–423. [Google Scholar] [CrossRef]
- Ashraf, M.; McNeilly, T. Salinity tolerance in Brassica oilseeds. Crit. Rev. Plant Sci. 2004, 23, 157–174. [Google Scholar] [CrossRef]
- Kumar, D. Salt tolerance in oilseed brassicas-present status and future prospects. Plant Breed. Abs. 1995, 65, 1439–1447. [Google Scholar]
- Maggio, A.; De Pascale, S.; Ruggiero, C.; Barbieri, G. Physiological response of field-grown cabbage to salinity and drought stress. Eur. J. Agron. 2004, 23, 57–67. [Google Scholar] [CrossRef]
- Badruddin, M.; Rhaman, M.M.; Nehar, N.A.; Hossain, M.M.; Hasan, M.B. Physiological characterization of mustard (Brassica sp.) genotypes for their salt tolerance. Pak. J. Biol. Sci. 2005, 8, 433–438. [Google Scholar]
- Jamil, M.; Lee, C.C.; Rehman, S.U.; Lee, D.B.; Ashraf, M.; Rha, E.S. Salinity (NaCl) tolerance of Brassica species at germination and early seedling growth. Electron. J. Environ. Agric. Food Chem. 2005, 4, 970–976. [Google Scholar]
- Jamil, M.; Lee, D.B.; Yung, K.Y.; Ashraf, M.; Lee, S.C.; Rha, E.S. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. J. Cent. Eur. Agric. 2006, 7, 273–281. [Google Scholar]
- An, P.; Inanaga, S.; Li, X.; Shimizu, H.; Tanimoto, E. Root characteristics in salt tolerance. Root Res. 2003, 12, 125–132. [Google Scholar] [CrossRef]
- Schleiff, U.; Muscolo, A. Fresh look at plant salt tolerance as affected by dynamics at the soil/root-interface using Leek and Rape as model crops. Eur. J. Plant Sci. Biotechnol. 2011, 5, 27–32. [Google Scholar]
- Schiefelbein, J.W. Constructing a plant cell: The genetic control of root hair development. Plant Physiol. 2000, 124, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Ewens, M.; Leigh, R.A. The effect of nutrient solution composition on the length of root hairs of wheat (Triticum aestivum L.). J. Exp. Bot. 1985, 36, 713–724. [Google Scholar] [CrossRef]
- Cao, X.F.; Linstead, P.; Berger, F.; Kieber, J.; Dolan, L. Differential ethylene sensitivity of epidermal cells is involved in the establishment of cell pattern in the Arabidopsis root. Physiol. Plant. 1999, 106, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Schikora, A.; Schmidt, W. Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability. Plant Physiol. 2001, 125, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Jones, D.L. Through form to function: Root hair development and nutrient uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.G.; Brown, K.M.; Lynch, J.P. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Müller, M.; Schmidt, W. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol. 2004, 134, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Srivastava, P.S.; Iqbal, M. Structural changes in root and shoot of Bacopa monniera in response to salt stress. J Plant Biol. 1999, 42, 222. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Saha, P.S. Morphology of lateral roots of twelve rice cultivars of Bangladesh: Dimension increase and diameter reduction in progressive root branching at the vegetative stage. Plant Root 2015, 9, 34–42. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Matthew, C.; Uddin, M.J.; Bayazid, K.N. Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomere level in wheat. J. Exp. Bot. 2016, 67, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Li, J.; Xiong, X.; Chen, Y.; Yin, X.; Feng, D. The response of mulberry trees after seedling hardening to summer drought in the hydro-fluctuation belt of Three Gorges Reservoir Areas. Environ. Sci. Pollut. Res. 2013, 20, 7103–7111. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Arreola, J.; Vicente, M.J.; Martinez-Sanchez, J.J. Nursery irrigation regimes affect the seedling characteristics of Silene vulgaris as they relate to potential performance following transplanting into semi-arid conditions. J. Hort. Sci. Biotechnol. 2008, 83, 15–22. [Google Scholar] [CrossRef]
- Hogland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N. Morphological and physio-biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J. Plant Interact. 2009, 4, 67–80. [Google Scholar]
- Reddy, M.P.; Vora, A.B. Changes in pigment composition, Hill reaction activity and saccharides metabolism in Bajra (Pennisetum typhoides S & H) leaves under NaCl salinity. Photosynthetica 1986, 20, 50–55. [Google Scholar]
- Munns, R.; Schachtman, D.P.; Condon, A.G. The significance of a two-phase growth response to salinity in wheat and barley. Funct. Plant Biol. 1995, 22, 561–569. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aktas, H.; Abak, K.; Cakmak, I. Determination of screening techniques to salinity tolerance in tomatoes and investigation of genotype responses. Plant Sci. 2002, 163, 695–703. [Google Scholar] [CrossRef]
- Meloni, D.A.; Gulotta, M.R.; Martínez, C.A.; Oliva, M.A. The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz. J. Plant Physiol. 2004, 16, 39–46. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.A.; Ali, F.Z.M.; Abou Hadid, A.F. Cell and plant responses to NaCl in Zea mays L. cultivars differing in salt tolerance. Gen. Appl. Plant Physiol. 2005, 31, 29–41. [Google Scholar]
- Dhingra, H.R.; Sharma, P.K. Reproductive performance of pea (Pisum sativum L.) under saline conditions. Ind. J. Plant Physiol. 1992, 35, 198. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: I. Response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci. 2004, 44, 797–805. [Google Scholar] [CrossRef]
- Bernstein, N.; Kafkafi, U. Root growth under salinity stress. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, Y., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 787–805. [Google Scholar]
- Bell, D.L.; Sultan, S.E. Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. Am. J. Bot. 1999, 86, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 2011, 342, 117–128. [Google Scholar] [CrossRef]
- Jones, M.P. Genetic analysis of salt tolerance in mangrove swamp rice. Rice Genet. 1985, 411–422. [Google Scholar] [CrossRef]
- Zhu, J.K.; Liu, J.; Xiong, L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1192. [Google Scholar] [CrossRef]
- Jbir, N.; Chaibi, W.; Ammar, S.; Jemmali, A.; Ayadi, A. Root growth and lignification of two wheat species differing in their sensitivity to NaCl, in response to salt stress. C. R. Acad. Sci. 2001, 324, 863–868. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- West, G.; Inzé, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.R.; Lynch, J.P. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Caradus, J.R. Selection for root hair length in white clover (Trifolium repens L.). Euphytica 1979, 28, 489–494. [Google Scholar] [CrossRef]
- Haling, R.E.; Richardson, A.E.; Culvenor, R.A.; Lambers, H.; Simpson, R.J. Root morphology, root-hair development and rhizosheath formation on perennial grass seedlings is influenced by soil acidity. Plant Soil 2010, 335, 457–468. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Uddin, M.J.; Afrin, S.; Paul, P.R. Genotypic variations in root traits of wheat varieties at phytomer level. J. Bangladesh Agric. Univ. 2014, 12, 45–54. [Google Scholar] [CrossRef]
- Noreen, Z.; Ashraf, M. Inter-accessional variation for salt tolerance in pea (Pisum sativum L.) at germination and screening stage. Pak. J. Bot. 2007, 39, 2075–2085. [Google Scholar]
- Malamy, J.E.; Benfey, P.N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 1997, 124, 33–44. [Google Scholar]
- Nibau, C.; Gibbs, D.J.; Coates, J.C. Branching out in new directions: The control of root architecture by lateral root formation. New Phytol. 2008, 179, 595–614. [Google Scholar] [CrossRef]
- Fitter, A.H. An architectural approach to the comparative ecology of plant-root systems. New Phytol. 1987, 106, 61–77. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhang, W.; Liu, Y.; Gao, Y.; Wang, X.; Yan, J.; Li, J. Genetic analysis of seedling root traits reveals the association of root trait with other agronomic traits in maize. BMC Plant Biol. 2018, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, F.; Pagès, L. Apical diameter and branching density affect lateral root elongation rates in banana. Environ. Exp. Bot. 2007, 59, 243–251. [Google Scholar] [CrossRef]
- Lecompte, F.; Pagès, L.; Ozier-Lafontaine, H. Patterns of variability in the diameter of lateral roots in the banana root system. New Phytol. 2005, 167, 841–850. [Google Scholar] [CrossRef] [PubMed]







| Score | Leaves | Flowers | Siliquae |
|---|---|---|---|
| 1 | Normal color and growth | Healthy and of normal color, blossoming | Normal color and growth |
| 3 | Nearly normal conditions, but leaf tip discoloration and wilting have started | Bud does not blossom properly, opened bud has started shrinking | Nearly normal, but slight discoloration has started |
| 5 | The leaf has rolled, most of the leaf has discolored and started to dry | Petal compacted or twisted; young bud has started to die instead of blossoming | No further growth or very slow growth, discolored |
| 7 | The leaf is mostly dry and totally discolored | unopened flower bud has died, open flower has dried | Growth totally ceased, drying |
| 9 | The leaf is dead or near death | Most of the bud is dead or near death | Siliquae dead or near death |
| Source of Variation | df | Lateral Root Traits | Root Hair Traits | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First Order | Second Order | Third Order | On First Order | On Third Order | |||||
| Diameter | Density | Diameter | Density | Length | Diameter | Density | Length | ||
| Treatments (T) | 1 | 0.08 | 0.47 | 0.0006 | 0.75 | 0.58 *** | 5.39 | 330.31 | 0.05 * |
| Varieties (V) | 1 | 0.14 * | 123.49 *** | 0.0165 ** | 4.57 * | 0.00 | 61.56 * | 535.39 * | 0.11 ** |
| T × V | 1 | 0.17 * | 3.75 | 0.0024 | 4.27 * | 0.06 | 98.56 ** | 0.16 | 0.00 |
| Error | 60 | 0.03 | 2.99 | 0.0022 | 1.03 | 0.05 | 9.14 | 130.96 | 0.01 |
| Variable | PC1 | PC2 | PC3 | PC4 | ||
|---|---|---|---|---|---|---|
| Lateral roots | 1st order | Diameter | 0.284 | −0.354 | 0.504 | 0.225 |
| 2nd order | Density | 0.424 | 0.194 | 0.16 | 0.125 | |
| 3rd order | Length | 0.36 | 0.493 | −0.114 | −0.112 | |
| 3rd order | Diameter | −0.289 | −0.172 | 0.224 | −0.286 | |
| 3rd order | Density | 0.416 | 0.082 | −0.131 | 0.257 | |
| Root hairs on | 1st order | Length | 0.3 | −0.456 | −0.048 | −0.502 |
| 1st order | Diameter | 0.057 | 0.119 | 0.381 | −0.546 | |
| 1st order | Density | 0.283 | −0.068 | −0.55 | −0.392 | |
| 3rd order | Length | 0.405 | −0.044 | 0.331 | −0.018 | |
| 3rd order | Diameter | −0.131 | 0.574 | 0.283 | −0.262 | |
| % Variation explained | 23.1 | 14.7 | 13.4 | 11.1 | ||
| P-value | < 0.001 | 4.9 | 41.2 | 19.2 | ||
| Mean PC scores with standard error | ||||||
| BARI Sarisha-8. Control | 0.61 ± 0.36 ab | 0.35 ± 0.34 a | 0.3 ± 0.46 a | 0.23 ± 0.18 ab | ||
| Binasarisha-5. Control | −1.55 ± 0.28 c | 0.32 ± 0.25 a | −0.05 ± 0.16 a | −0.02 ± 0.26 ab | ||
| BARI Sarisha-8. Treated | 1.17 ± 0.27 ab | 0.003 ± 0.31 ab | −0.36 ± 0.21 a | 0.25 ± 0.34 ab | ||
| Binasarisha-5. Treated | −0.11 ± 0.24 b | −0.86 ± 0.36 b | 0.27 ± 0.47 a | −0.58 ± 0.23 b | ||
| Lateral Root Traits | Root Hair Traits | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Order | Second Order | Third Order | On First Order | On Third Order | |||||||||
| Diameter | Length | Density | Length | Diameter | Density | Length | Diameter | Density | Length | Diameter | |||
| Lateral | 2nd order | Length | 0.20 | ||||||||||
| 2nd order | Density | 0.23 | 0.13 | ||||||||||
| 3rd order | Length | 0.02 | −0.15 | 0.39 ** | |||||||||
| 3rd order | Diameter | −0.06 | −0.03 | −0.14 | −0.30 * | ||||||||
| 3rd order | Density | 0.20 | 0.37 ** | 0.31 * | 0.35 ** | −0.27 * | |||||||
| Root hairs on | 1st order | Length | 0.23 | 0.20 | 0.05 | 0.02 | 0.00 | 0.11 | |||||
| 1st order | Diameter | 0.09 | −0.22 | −0.12 | 0.10 | −0.02 | −0.02 | 0.15 | |||||
| 1st order | Density | −0.14 | −0.09 | 0.11 | 0.23 | −0.12 | 0.20 | 0.35 ** | −0.08 | ||||
| 3rd order | Length | 0.32 * | 0.01 | 0.32 * | 0.09 | −0.15 | 0.14 | 0.27 * | 0.01 | 0.09 | |||
| 3rd order | Diameter | −0.23 | −0.39 ** | 0.02 | 0.08 | 0.15 | −0.15 | −0.25 | 0.13 | −0.15 | 0.12 | ||
| 3rd order | Density | 0.24 | 0.12 | 0.11 | 0.09 | −0.24 | 0.24 | 0.27 * | 0.25 | 0.32 * | 0.18 | −0.18 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus. Plants 2019, 8, 192. https://doi.org/10.3390/plants8070192
Arif MR, Islam MT, Robin AHK. Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus. Plants. 2019; 8(7):192. https://doi.org/10.3390/plants8070192
Chicago/Turabian StyleArif, Mohammad Rashid, M. Thoihidul Islam, and Arif Hasan Khan Robin. 2019. "Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus" Plants 8, no. 7: 192. https://doi.org/10.3390/plants8070192
APA StyleArif, M. R., Islam, M. T., & Robin, A. H. K. (2019). Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus. Plants, 8(7), 192. https://doi.org/10.3390/plants8070192