Clothing the Emperor: Dynamic Root–Shoot Allocation Trajectories in Relation to Whole-Plant Growth Rate and in Response to Temperature
Abstract
1. Introduction
- Root and shoot growth each has a sigmoid trajectory with time. Growth is initially slow, followed by a linear or exponential phase of faster growth. Growth rate falls gradually, eventually approaching zero when there is no further net increase in biomass, typical of annuals, but many species, including perennials, grow like this for at least part of their lives [14,15]. Biomass increase is halted by factors including self-shading, inter-root competition, resource depletion, crowding, tissue turnover, transitioning from vegetative to reproductive growth, determinate development, dormancy, photoperiodic downregulation of metabolism, senescence and numerous environmental and biotic constraints, depending on species and circumstances.
- An ontogenetic response of root–shoot allocation to the environment is defined as a deviation in allocation following a change in environmental conditions compared with allocation measured in control plants. In most root–shoot allocation studies, plants are subjected to different but temporally static environmental conditions. Often, no temporal information about allocation is collected, so it is not always clear what constitutes ontogenetic drift in allocation as distinct from a genuine response. But if some plants are subjected to a specific treatment at a defined time, and if growth is measured repeatedly before and after that change, and compared with controls, it is possible to say definitively if allocation responds to that treatment; temporal changes in allocation in control plants then reflect ontogenetic drift [16,17,18,19,20,21,22].
- A biomass allocation response to the environment can occur only if there is a differential change in rates of biomass production between root and shoot [1]. That is not to say there can be no response at all without biomass change, but it will be confined to adjustments in physiological processes such as specific rates of photosynthesis, respiration, water uptake, nutrient capture and so on, which do not necessarily involve the production of new biomass; such processes are obviously important but are not considered here [23,24].
2. Materials and Methods
2.1. Experimental
2.2. Data Analysis
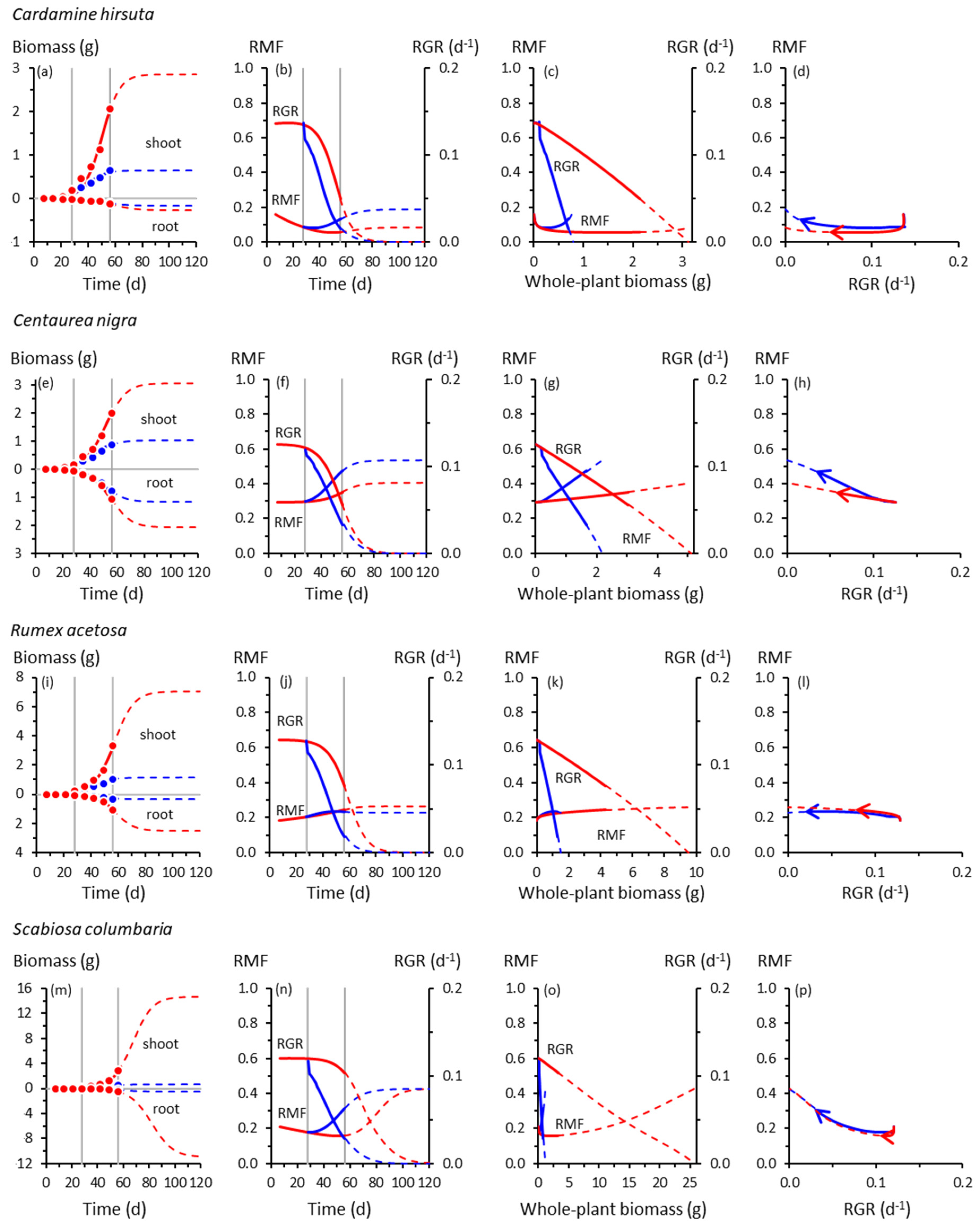
3. Results
3.1. Root and Shoot Growth
3.2. Root and Shoot Biomass Allocation
3.3. Co-Variation between Allocation and Whole-Plant Growth Rate
4. Discussion
4.1. Response of Root–Shoot Allocation to Cooling
4.2. Co-Variation between Allocation and Whole-Plant Growth Rate
4.3. Compensating for Something?
4.4. Growing Fast or Slow
4.5. Experimental Designs and Analytical Approaches
4.6. Sigmoid Growth Can Constrain Ontogenetic Allocation—and Its Interpretation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brouwer, R. Nutritive influences on the distribution of dry matter in the plant. Neth. J. Agric. Sci. 1962, 10, 399–408. [Google Scholar]
- Davidson, R.L. Effect of root/leaf temperature differentials on root/shoot ratios in some pasture grasses and clover. Ann. Bot. 1969, 33, 561–569. [Google Scholar] [CrossRef]
- Reich, P.B. Do plants increase resource acquisition potential in the face of resource shortfalls, and if so, how? New Phytol. 2018, 219, 1142–1144. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Reich, P.B. Root–shoot relations: Optimality in acclimation and adaptation or the “Emperor’s New Clothes”? In Plant Roots: The Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 205–220. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 19, 30–50. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Robinson, D. Scaling the depths: Below-ground allocation in plants, forests and biomes. Funct. Ecol. 2004, 18, 290–295. [Google Scholar] [CrossRef]
- Evans, G.C. The Quantitative Analysis of Plant Growth; Blackwell Scientific Publications: Oxford, UK, 1972; ISBN 0-632-06130-8. [Google Scholar]
- Farrar, J.; Gunn, S. Allocation: Allometry, acclimation—And alchemy? In Inherent Variations in Plant Growth: Physiological Mechanisms and Ecological Consequences; Lambers, H., Poorter, H., van Vuuren, M.M.I., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 183–198. ISBN 90-73348-96-X. [Google Scholar]
- Rustad, L.E. From transient to steady-state response of ecosystems to atmospheric CO2-enrichment and global climate change: Conceptual challenges and need for an integrated approach. Plant Ecol. 2006, 182, 43–62. [Google Scholar] [CrossRef]
- Trinder, C.; Brooker, R.; Davidson, H.; Robinson, D. Dynamic trajectories of growth and nitrogen capture by competing plants. New Phytol. 2012, 193, 948–958. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves. The Functional Approach to Plant Growth Analysis; Edward Arnold: London, UK, 1982; ISBN 0-7131-2844-5. [Google Scholar]
- Deng, J.; Ran, J.; Wang, Z.; Fan, Z.; Wang, G.; Jia, M.; Liu, J.; Wang, Y.; Liu, J.; Brown, J.H. Models and tests of optimal density and maximal yield for crop plants. Proc. Natl. Acad. Sci. USA 2012, 109, 15823–15828. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Rorison, I.H. A comparison of the responses of Lolium perenne L., Holcus lanatus L. and Deschampsia flexuosa (L.) Trin. to a localised supply of nitrogen. New Phytol. 1983, 94, 263–273. [Google Scholar] [CrossRef]
- Caloin, M.; Clément, B.; Herrmann, S. Regrowth kinetics of Dactylis glomerata following defoliation. Ann. Bot. 1990, 66, 397–405. [Google Scholar] [CrossRef]
- Caloin, M.; Clément, B.; Herrmann, S. Regrowth kinetics of Dactylis glomerata following root excision. Ann. Bot. 1991, 68, 435–440. [Google Scholar] [CrossRef]
- Gedroc, J.J.; McConnaughay, K.D.M.; Coleman, J.S. Plasticity in root/shoot partitioning: Optimal, ontogenetic, or both? Funct. Ecol. 1996, 10, 44–50. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Can plants track changes in nutrient availability via changes in biomass partitioning? Plant Soil 1998, 202, 201–209. [Google Scholar] [CrossRef]
- Shipley, B. Plasticity in relative growth rate and its components following a change in irradiance. Plant Cell Environ. 2000, 23, 1207–1216. [Google Scholar] [CrossRef]
- Useche, A.; Shipley, B. Interspecific correlates of plasticity in relative growth rate following a decrease in nitrogen availability. Ann. Bot. 2010, 105, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Loveys, B.R.; Atkinson, L.J.; Pons, T.L. Phenotypic plasticity and growth temperature: Understanding interspecific variability. J. Exp. Bot. 2006, 57, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Lohier, T.; Jabot, F.; Meziane, D.; Shipley, B.; Reich, P.B.; Deffuant, G. Explaining ontogenetic shifts in root–shoot scaling with transient dynamics. Ann. Bot. 2014, 114, 513–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterkin, J.H. Plant Growth and Nitrogen Nutrition in Relation to Temperature. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 1981. [Google Scholar]
- Paine, C.E.T.; Marthews, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2012, 3, 245–256. [Google Scholar] [CrossRef]
- Rosenbaum, B.; Raatz, M.; Weithoff, G.; Fussmann, G.F.; Gaedke, U. Estimating parameters from multiple time series of population dynamics using Bayesian inference. Front. Ecol. Evol. 2019, 6, 234. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology. A Functional Approach to Common British Species; Unwin, Hyman: London, UK, 1988; ISBN 0-04-581028-1. [Google Scholar]
- Stace, C.A. Field Flora of The British Isles; Cambridge University Press: Cambridge, UK, 1999; ISBN 0-521-65315-0. [Google Scholar]
- Rorison, I.H. A double shell plant growth cabinet. New Phytol. 1964, 63, 358–362. [Google Scholar] [CrossRef]
- Booth, R.E.; Mackey, J.M.L.; Rorison, I.H.; Spencer, R.E.; Gupta, P.L.; Hunt, R. ISP germination and rooting environments: Sand, compost and solution culture. In Methods in Comparative Plant Ecology. A Laboratory Manual; Hendry, G.A.F., Grime, J.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 19–24. ISBN 0-412-46230-3. [Google Scholar]
- Hunt, R.; Parsons, I.T. A computer program for deriving growth-functions in plant growth-analysis. J. Appl. Ecol. 1974, 11, 297–307. [Google Scholar] [CrossRef]
- Conn, P.B.; Johnson, D.S.; Boveng, P.L. On extrapolating past the range of observed data when making statistical predictions in ecology. PLoS ONE 2015, 10, e0141416. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Allometry; University of Chicago Press: Chicago, IL, USA, 1994; ISBN 0-226-58081-4. [Google Scholar]
- Hunt, R.; Nicholls, A.O.; Fathy, S.A. Growth and root–shoot partitioning in eighteen British grasses. Oikos 1987, 50, 53–59. [Google Scholar] [CrossRef]
- Shipley, B.; Peters, R.H. A test of the Tilman model of plant strategies: Relative growth rate and biomass partitioning. Am. Nat. 1990, 136, 39–153. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Ericsson, T. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 168-169, 205–214. [Google Scholar] [CrossRef]
- Glimskar, A.; Ericsson, T. Relative nitrogen limitation at steady-state nutrition as a determinant of plasticity in five grassland plant species. Ann. Bot. 1999, 84, 413–420. [Google Scholar] [CrossRef][Green Version]
- Ruts, T.; Matsubara, S.; Walter, A. Synchronous high-resolution phenotyping of leaf and root growth in Nicotiana tabacum over 24-h periods with GROWMAP-plant. Plant Methods 2013, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Smith, D.D.; Savage, V.M.; Enquist, B.J.; McCulloh, K.A.; Reich, P.B.; Bentley, L.P.; von Allmen, E.I. A species-level model for metabolic scaling in trees. I. Exploring boundaries to scaling space within and across species. Funct. Ecol. 2012, 26, 1054–1065. [Google Scholar] [CrossRef]
- Thornley, J.H.M. Modelling shoot:root relations: The only way forward? Ann. Bot. 1998, 81, 165–171. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Díaz-Pérez, C.; Martínez-Trujillo, M.; Del Río, R.E.; Campos-García, J.; López-Bucioa, J. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 7253–7258. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; George, G.M.; Ince, Y.C.; Dankwa-Egli, B.; Hersch, M.; Zeeman, S.C.; Fankhauser, C. Changes in resource partitioning between and within organs support growth adjustment to neighbour proximity in Brassicaceae seedlings. Proc. Natl. Acad. Sci. USA 2018, 115, E9953–E9961. [Google Scholar] [CrossRef] [PubMed]
- Kachi, N.; Rorison, I.H. Optimal partitioning between root and shoot in plants with contrasted growth rates in response to nitrogen availability and temperature. Funct. Ecol. 1989, 3, 549–559. [Google Scholar] [CrossRef]
- Van der Werf, A.; Visser, A.J.; Schieving, F.; Lambers, H. Evidence for optimal partitioning of biomass and nitrogen at a range of nitrogen availabilities for a fast- and slow-growing species. Funct. Ecol. 1993, 7, 63–74. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Coleman, J.S.; Bazzaz, F.A.; McConnaughay, K.D.M. Biomass allocation in old-field annual species grown in elevated CO2 environments: No evidence for optimal partitioning. Glob. Chang. Biol. 2000, 6, 855–863. [Google Scholar] [CrossRef]
- Trugman, A.T.; Detto, M.; Bartlett, M.K.; Medvigy, D.; Anderegg, W.R.L.; Schwalm, C.; Schaffer, B.; Pacala, S.W. Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 2018, 21, 1552–1560. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes and Ecosystem Properties; John Wiley and Sons: Chichester, UK, 2001; ISBN 0-471-49601-4. [Google Scholar]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter, A.H. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc. R. Soc. Lond. B 1999, 266, 431–435. [Google Scholar] [CrossRef]
- Rorison, I.H.; Peterkin, J.H.; Clarkson, D.T. Nitrogen source, temperature and the growth of herbaceous plants. In Nitrogen as an Ecological Factor; Lee, J.A., McNeill, S., Rorison, I.H., Eds.; Blackwell Scientific Publications: Oxford, UK, 1981; pp. 189–209. ISBN 0-632-01074-6. [Google Scholar]
- Coleman, J.S.; McConnaughay, K.D.M.; Ackerley, D.D. Interpreting phenotypic variation in plants. Trends Ecol. Evol. 1994, 9, 187–191. [Google Scholar] [CrossRef]
- Wright, S.D.; McConnaughay, K.D.M. Interpreting phenotypic plasticity: The importance of ontogeny. Plant Species Biol. 2002, 17, 119–131. [Google Scholar] [CrossRef]
- Fisher, R.A.; Koven, C.D.; Anderegg, W.R.L.; Christoffersen, B.O.; Dietze, M.C.; Farrior, C.E.; Holm, J.A.; Hurtt, G.C.; Knox, R.G.; Lawrence, P.J.; et al. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob. Chang. Biol. 2018, 24, 35–54. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, D.; Peterkin, J.H. Clothing the Emperor: Dynamic Root–Shoot Allocation Trajectories in Relation to Whole-Plant Growth Rate and in Response to Temperature. Plants 2019, 8, 212. https://doi.org/10.3390/plants8070212
Robinson D, Peterkin JH. Clothing the Emperor: Dynamic Root–Shoot Allocation Trajectories in Relation to Whole-Plant Growth Rate and in Response to Temperature. Plants. 2019; 8(7):212. https://doi.org/10.3390/plants8070212
Chicago/Turabian StyleRobinson, David, and John Henry Peterkin. 2019. "Clothing the Emperor: Dynamic Root–Shoot Allocation Trajectories in Relation to Whole-Plant Growth Rate and in Response to Temperature" Plants 8, no. 7: 212. https://doi.org/10.3390/plants8070212
APA StyleRobinson, D., & Peterkin, J. H. (2019). Clothing the Emperor: Dynamic Root–Shoot Allocation Trajectories in Relation to Whole-Plant Growth Rate and in Response to Temperature. Plants, 8(7), 212. https://doi.org/10.3390/plants8070212




