Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean
Abstract
:1. Introduction
2. Results
2.1. Identification of 33 GmWOX Genes and Their Physicochemical Properties
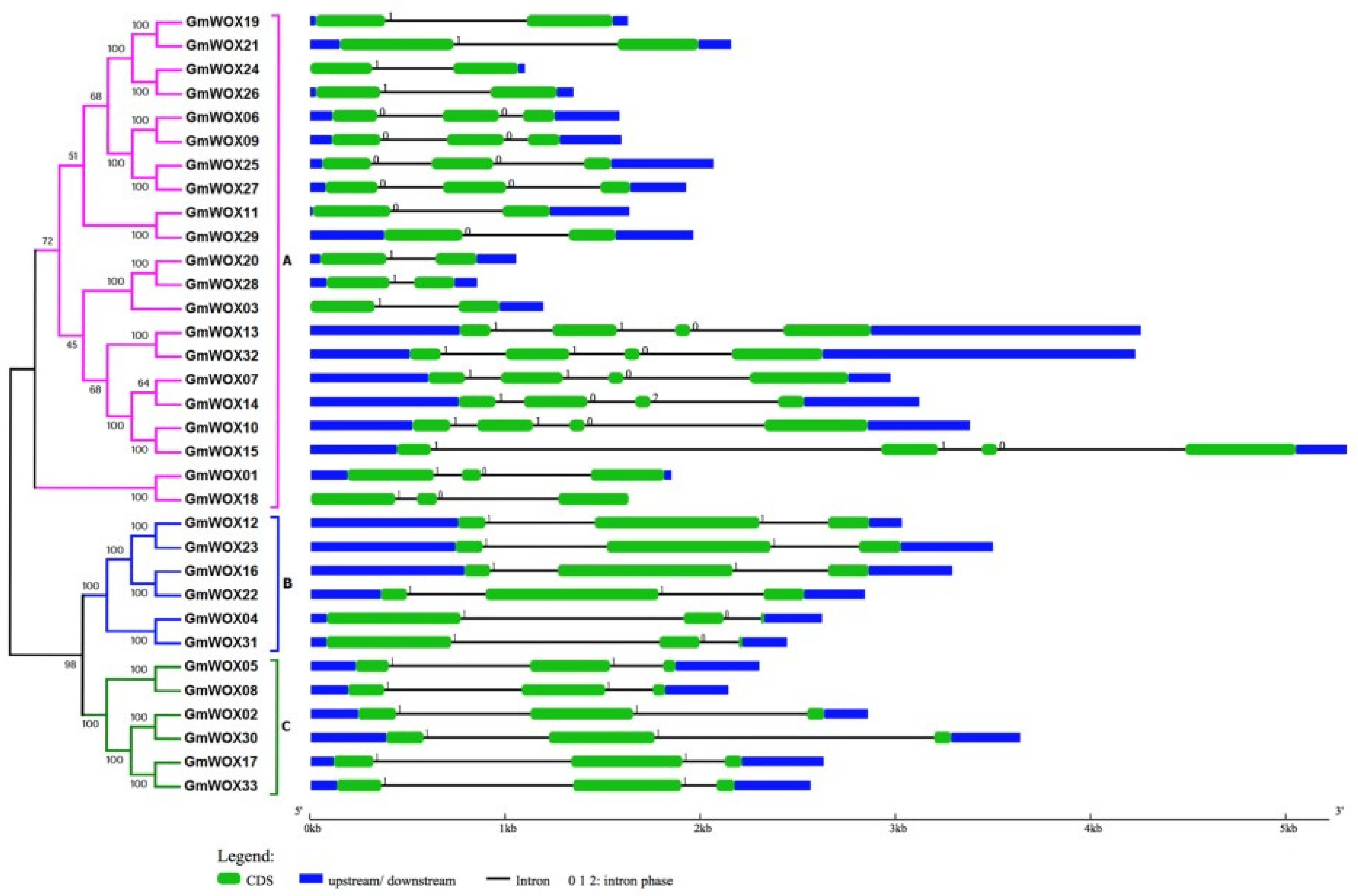
2.2. Phylogenetic Analysis and Structural Predication of the Soybean WOXs
2.3. Chromosomal Location, Conserved Motifs and Cis-Element Analysis of GmWOXs
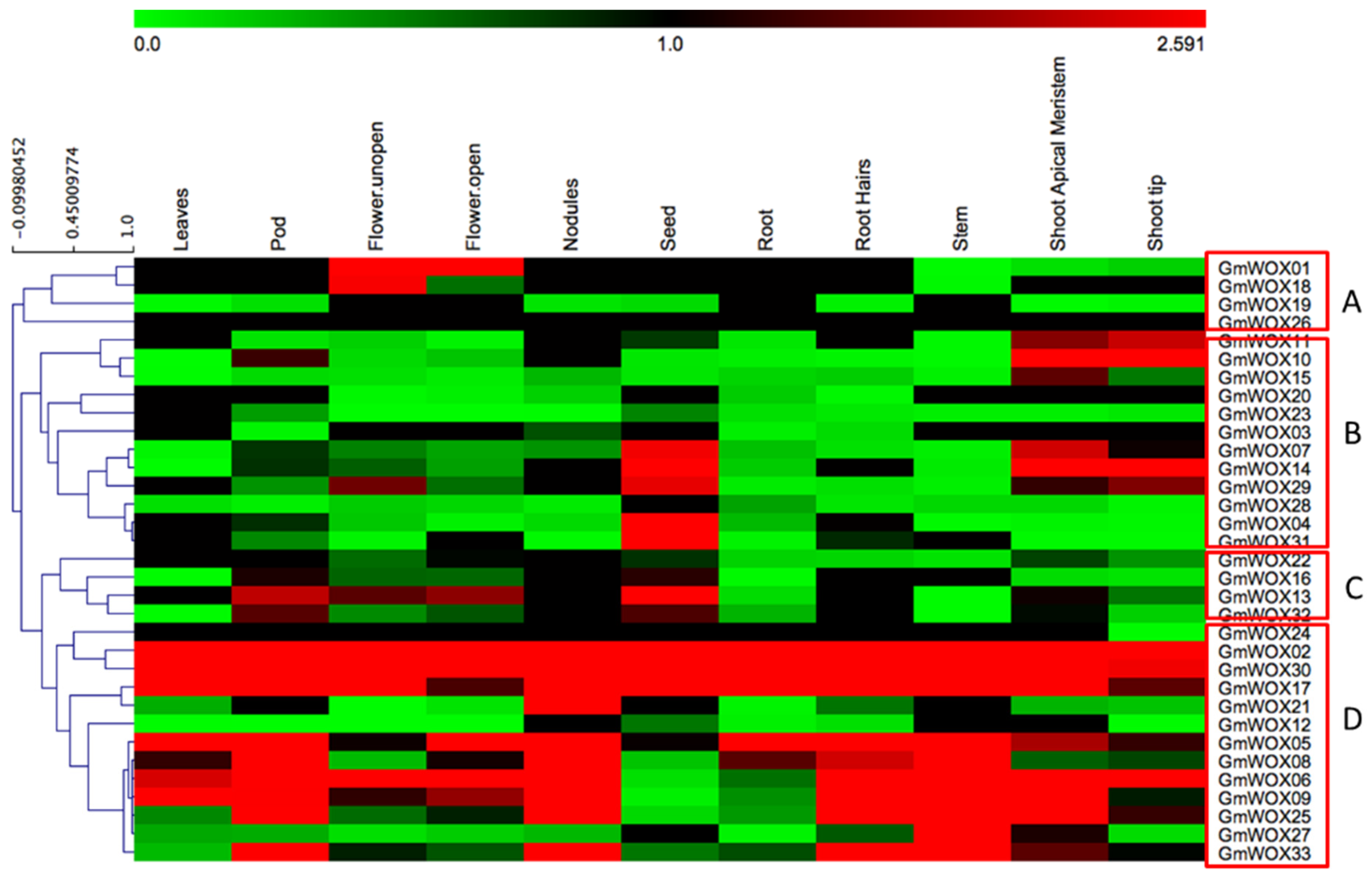
2.4. Expression Profile of GmWOXs in Soybean
2.5. GmWOXs and Soybean Growth and Development
2.6. Phenotypes of GmWOX18 Transgenic Soybean
3. Discussion
3.1. Expression and Phylogenetic Analysis of the Soybean WOX Gene Family
3.2. WOX Transcription Factors Regulate Plant Growth and Development and Participate in the Abiotic Stress Response
3.3. The Function of WOX Genes in Plant Regeneration
4. Materials and Methods
4.1. Gene identification
4.2. Phylogenetic Tree Construction
4.3. GmWOX Gene Structure and Conserved Motif
4.4. Chromosomal Location and Gene Duplication
4.5. Tissue-Specific Expression Profile of GmWOXs
4.6. Plant Materials and Treatments
4.7. Overexpression of GmWOX18 in Soybean
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kamiya, N.; Nagasaki, H.; Morikami, A.; Sato, Y.; Matsuoka, M. Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J. Cell Mol. Biol. 2003, 35, 429–441. [Google Scholar] [CrossRef]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jurgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Haecker, A.; Gross-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Mayer, K.F.; Berger, J.; Jurgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [PubMed]
- Lin, H.; Niu, L.; McHale, N.A.; Ohme-Takagi, M.; Mysore, K.S.; Tadege, M. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc. Natl. Acad. Sci. USA 2013, 110, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhong, S.H.; Cui, X.F.; Li, J.; He, Z.H. Characterization of temperature-sensitive mutants reveals a role for receptor-like kinase SCRAMBLED/STRUBBELIG in coordinating cell proliferation and differentiation during Arabidopsis leaf development. Plant J. Cell Mol. Biol. 2012, 72, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zong, J.; Liu, J.; Yin, J.; Zhang, D. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 2010, 52, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Bouchabke-Coussa, O.; Obellianne, M.; Linderme, D.; Montes, E.; Maia-Grondard, A.; Vilaine, F.; Pannetier, C. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 2013, 32, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Y.; Ding, Y.; Wu, J.; Wang, P.; Yu, Y.; Wei, X.; Wang, Y.; Zhang, C.; Li, F.; et al. Effects of GhWUS from upland cotton (Gossypium hirsutum L.) on somatic embryogenesis and shoot regeneration. Plant Sci. Int. J. Exp. Plant Biol. 2018, 270, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, R.; Qin, G.; Chen, Z.; Gu, H.; Qu, L.J. Over-expression of WOX1 leads to defects in meristem development and polyamine homeostasis in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, H.; Rikirsch, E.; Hermann, M.; Ueda, M.; Laux, T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell 2008, 14, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Ji, J.; Kelsey, E.; Ohtsu, K.; Schnable, P.S.; Scanlon, M.J. Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol. 2009, 149, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Segatto, A.L.; Thompson, C.E.; Freitas, L.B. Contribution of WUSCHEL-related homeobox (WOX) genes to identify the phylogenetic relationships among Petunia species. Genet. Mol. Biol. 2016, 39, 658–664. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.J.; Hwang, J.Y.; Kim, G.T.; Paek, N.C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef]
- Dai, M.; Hu, Y.; Zhao, Y.; Liu, H.; Zhou, D.X. A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development. Plant Physiol. 2007, 144, 380–390. [Google Scholar] [CrossRef]
- Xu, M.; Xie, W.; Huang, M. Two WUSCHEL-related HOMEOBOX genes, PeWOX11a and PeWOX11b, are involved in adventitious root formation of poplar. Physiol. Plant. 2015, 155, 446–456. [Google Scholar] [CrossRef]
- Gonzali, S.; Novi, G.; Loreti, E.; Paolicchi, F.; Poggi, A.; Alpi, A.; Perata, P. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2005, 44, 633–645. [Google Scholar] [CrossRef]
- Park, S.O.; Zheng, Z.; Oppenheimer, D.G.; Hauser, B.A. The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development 2005, 132, 841–849. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, H.; Lee, B.H.; Damsz, B.; Cheng, S.; Stirm, V.; Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A. An Arabidopsis homeodomain transcription factor gene, HOS9, mediates cold tolerance through a CBF-independent pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 9873–9878. [Google Scholar] [CrossRef]
- Kong, D.; Hao, Y.; Cui, H. The WUSCHEL Related Homeobox Protein WOX7 Regulates the Sugar Response of Lateral Root Development in Arabidopsis thaliana. Mol. Plant 2016, 9, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lie, C.; Kelsom, C.; Wu, X. WOX2 and STIMPY-LIKE/WOX8 promote cotyledon boundary formation in Arabidopsis. Plant J. Cell Mol. Biol. 2012, 72, 674–682. [Google Scholar] [CrossRef]
- Wu, X.; Dabi, T.; Weigel, D. Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr. Biol. CB 2005, 15, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, L. Transcription Factors WOX11/12 Directly Activate WOX5/7 to Promote Root Primordia Initiation and Organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, L.; Zhang, J.; Li, J.; Zheng, H.; Chen, J.; Lu, M. WUSCHEL-related Homeobox genes in Populus tomentosa: Diversified expression patterns and a functional similarity in adventitious root formation. BMC Genom. 2014, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Qin, P.; Prasad, K.; Hu, Y.; Xu, L. The WOX11-LBD16 Pathway Promotes Pluripotency Acquisition in Callus Cells During De Novo Shoot Regeneration in Tissue Culture. Plant Cell Physiol. 2018, 59, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, Y.; Toffano-Nioche, C.; Claisse, G.; Thareau, V.; Morin, H.; Laufs, P.; Moreau, H.; Kreis, M.; Lecharny, A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 2008, 8, 291. [Google Scholar] [CrossRef]
- Romera-Branchat, M.; Ripoll, J.J.; Yanofsky, M.F.; Pelaz, S. The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J. Cell Mol. Biol. 2013, 73, 37–49. [Google Scholar] [CrossRef]
- Etchells, J.P.; Provost, C.M.; Mishra, L.; Turner, S.R. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 2013, 140, 2224–2234. [Google Scholar] [CrossRef]
- Denis, E.; Kbiri, N.; Mary, V.; Claisse, G.; Conde, E.S.N.; Kreis, M.; Deveaux, Y. WOX14 promotes bioactive gibberellin synthesis and vascular cell differentiation in Arabidopsis. Plant J. Cell Mol. Biol. 2017, 90, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Y.; Zhu, N.; Zhao, Y. The rice WUSCHEL-related homeobox genes are involved in reproductive organ development, hormone signaling and abiotic stress response. Gene 2014, 549, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Minuto, M.; Boccacci, P.; Perrone, I.; Vallania, R.; Gribaudo, I. Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J. Exp. Bot. 2011, 62, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Palovaara, J.; Hakman, I. Conifer WOX-related homeodomain transcription factors, developmental consideration and expression dynamic of WOX2 during Picea abies somatic embryogenesis. Plant Mol. Biol. 2008, 66, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.A.; Kapulnik, Y. Plant isoflavonoids, pathogens and symbionts. Trends Microbiol. 1995, 3, 58–64. [Google Scholar] [CrossRef]
- Tang, F.; Chen, N.; Zhao, M.; Wang, Y.; He, R.; Peng, X.; Shen, S. Identification and Functional Divergence Analysis of WOX Gene Family in Paper Mulberry. Int. J. Mol. Sci. 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Bueno, N.; Canas, R.A.; Avila, C.; Canovas, F.M.; Ordas, R.J. Analysis of the WUSCHEL-RELATED HOMEOBOX gene family in Pinus pinaster: New insights into the gene family evolution. Plant Physiol. Biochem. PPB 2018, 123, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Liu, M.M.; Ran, F.; Guo, P.C.; Ke, Y.Z.; Wu, Y.W.; Wen, J.; Li, P.F.; Li, J.N.; Du, H. Global Analysis of WOX Transcription Factor Gene Family in Brassica napus Reveals Their Stress- and Hormone-Responsive Patterns. Int. J. Mol. Sci. 2018, 19, 11. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, Q.; Qin, W.; Yang, Z.; Cheng, Y.; Lu, L.; Ge, X.; Zhang, C.; Wu, Z.; Li, F. Genome-wide analysis of WOX genes in upland cotton and their expression pattern under different stresses. BMC Plant Biol. 2017, 17, 113. [Google Scholar] [CrossRef]
- Lian, G.; Ding, Z.; Wang, Q.; Zhang, D.; Xu, J. Origins and evolution of WUSCHEL-related homeobox protein family in plant kingdom. Sci. World J. 2014, 2014, 534140. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jurgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhu, S.S.; Gao, X.Q.; Zhang, X.S. Cytokinin and auxin regulates WUS induction and inflorescence regeneration in vitro in Arabidopsis. Plant Cell Rep. 2010, 29, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Gallois, J.L.; Nora, F.R.; Mizukami, Y.; Sablowski, R. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev. 2004, 18, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solís-Ramos, L.Y.; González-Estrada, T.; Nahuath-Dzib, S.; Zapata-Rodriguez, L.C.; Castaño, E. Overexpression of WUSCHEL in C. chinense causes ectopic morphogenesis. Plant Cell Tissue Organ Cult. 2009, 96, 279–287. [Google Scholar] [CrossRef]
- Arroyo-Herrera, A.; Gonzalez, A.K.; Moo, R.C.; Quiroz-Figueroa, F.R.; Loyola-Vargas, V.M.; Rodriguez-Zapata, L.C.; D’Hondt, C.B.; Suárez-Solís, V.M.; Castaño, E. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult. 2008, 94, 171–180. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Meng, J.L. MapDraw: A microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas 2003, 25, 317–321. [Google Scholar] [PubMed]
- Mar, J.C.; Wells, C.A.; Quackenbush, J. Defining an informativeness metric for clustering gene expression data. Bioinformatics 2011, 27, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Aldrich, D.L.; Valliyodan, B.; Watanabe, Y.; Ha, C.V.; Nishiyama, R.; Guttikonda, S.K.; Quach, T.N.; Gutierrez-Gonzalez, J.J.; Tran, L.S.; et al. Evaluation of candidate reference genes for normalization of quantitative RT-PCR in soybean tissues under various abiotic stress conditions. PloS ONE 2012, 7, e46487. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y. Hypocotyl-based Agrobacterium-mediated transformation of soybean (Glycine max) and application for RNA interference. Plant Cell Rep. 2008, 27, 1177–1184. [Google Scholar] [CrossRef] [PubMed]






| Gene Name | Genome ID | Chromosome | Exons/Introns | Transcript Length (bp) | Amino Acid Length | Molecular Weight (Da) | pI |
|---|---|---|---|---|---|---|---|
| GmWOX01 | Glyma01g37190.2 | Chr01 | 3/2 | 1110 | 296 | 33,266.52 | 7.13 |
| GmWOX02 | Glyma02g10410.1 | Chr02 | 3/2 | 1241 | 262 | 29,686.27 | 5.91 |
| GmWOX03 | Glyma02g42200.1 | Chr02 | 2/2 | 753 | 177 | 20,452.13 | 9.1 |
| GmWOX04 | Glyma03g01000.1 | Chr03 | 3/2 | 1253 | 295 | 32,100.75 | 6.63 |
| GmWOX05 | Glyma04g01830.1 | Chr04 | 3/2 | 1268 | 208 | 23,564.8 | 6.44 |
| GmWOX06 | Glyma04g04310.1 | Chr04 | 3/2 | 1110 | 224 | 25,896.42 | 9.26 |
| GmWOX07 | Glyma05g33850.1 | Chr05 | 4/3 | 1879 | 357 | 40,718.48 | 8.87 |
| GmWOX08 | Glyma06g01940.1 | Chr06 | 3/2 | 1162 | 219 | 24,937.32 | 6.17 |
| GmWOX09 | Glyma06g04470.1 | Chr06 | 3/2 | 1111 | 230 | 26,599.11 | 9.08 |
| GmWOX10 | Glyma07g11372.1 | Chr07 | 4/3 | 2101 | 357 | 40,518.28 | 6.81 |
| GmWOX11 | Glyma07g15710.2 | Chr07 | 2/1 | 1045 | 210 | 24,174.19 | 9.1 |
| GmWOX12 | Glyma07g32430.1 | Chr07 | 3/2 | 1649 | 388 | 43,124.1 | 7.74 |
| GmWOX13 | Glyma07g34425.1 | Chr07 | 4/3 | 2071 | 333 | 37,234.08 | 6.67 |
| GmWOX14 | Glyma08g05831.1 | Chr08 | 4/3 | 2044 | 238 | 27,673.2 | 10.07 |
| GmWOX15 | Glyma09g30831.1 | Chr09 | 4/3 | 1787 | 364 | 40,892.7 | 7.81 |
| GmWOX16 | Glyma10g08030.1 | Chr10 | 3/2 | 2062 | 403 | 43,911.23 | 8.65 |
| GmWOX17 | Glyma10g43580.2 | Chr10 | 3/2 | 1361 | 281 | 31,687.29 | 6.15 |
| GmWOX18 | Glyma11g08091.1 | Chr11 | 3/2 | 870 | 289 | 32,527.58 | 7.8 |
| GmWOX19 | Glyma11g14940.2 | Chr11 | 2/1 | 889 | 261 | 29,224.34 | 5.66 |
| GmWOX20 | Glyma11g34990.1 | Chr11 | 2/1 | 1746 | 400 | 43,763.14 | 8.8 |
| GmWOX21 | Glyma12g06895.1 | Chr12 | 2/1 | 1297 | 327 | 36,678.04 | 6.35 |
| GmWOX22 | Glyma13g21860.2 | Chr13 | 3/2 | 791 | 180 | 20,575.94 | 6.32 |
| GmWOX23 | Glyma13g24150.1 | Chr13 | 3/2 | 1747 | 389 | 43,249.12 | 6.99 |
| GmWOX24 | Glyma13g41000.1 | Chr13 | 2/1 | 673 | 212 | 24,109.08 | 8.93 |
| GmWOX25 | Glyma14g09310.1 | Chr14 | 3/2 | 1270 | 231 | 26,520.89 | 9.03 |
| GmWOX26 | Glyma15g04460.1 | Chr15 | 2/1 | 771 | 219 | 24,907.86 | 7.66 |
| GmWOX27 | Glyma17g35880.2 | Chr17 | 3/2 | 1091 | 244 | 28,079.58 | 9.38 |
| GmWOX28 | Glyma18g03350.1 | Chr18 | 2/1 | 718 | 174 | 20,085.46 | 6.83 |
| GmWOX29 | Glyma18g39520.2 | Chr18 | 2/1 | 1397 | 210 | 24,182.08 | 8.96 |
| GmWOX30 | Glyma18g52491.1 | Chr18 | 3/2 | 1433 | 266 | 30,073.53 | 5.58 |
| GmWOX31 | Glyma19g29660.2 | Chr19 | 3/2 | 1143 | 280 | 30,294.91 | 7.06 |
| GmWOX32 | Glyma20g02161.1 | Chr20 | 4/3 | 1920 | 337 | 37,938.99 | 7.21 |
| GmWOX33 | Glyma20g23220.2 | Chr20 | 3/2 | 1365 | 284 | 32,167.86 | 6.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Zhang, L.; Yang, Y.; Shan, Z.; Zhou, X.-a. Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean. Plants 2019, 8, 215. https://doi.org/10.3390/plants8070215
Hao Q, Zhang L, Yang Y, Shan Z, Zhou X-a. Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean. Plants. 2019; 8(7):215. https://doi.org/10.3390/plants8070215
Chicago/Turabian StyleHao, Qingnan, Ling Zhang, Yanyan Yang, Zhihui Shan, and Xin-an Zhou. 2019. "Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean" Plants 8, no. 7: 215. https://doi.org/10.3390/plants8070215





