A Convenient Plant-Based Detection System to Monitor Androgenic Compound in the Environment
Abstract
:1. Introduction
2. Results and Discussion
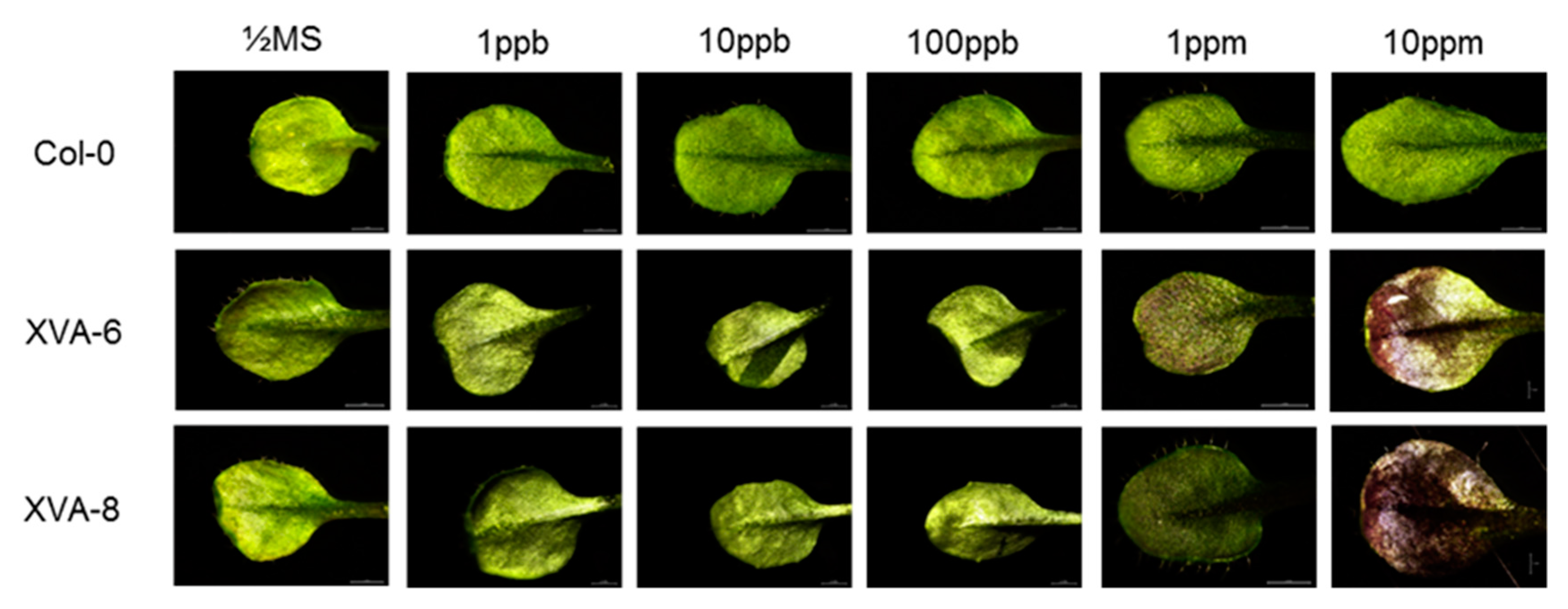
2.1. Preliminary Evaluation of the Transgenic Arabidopsis for DHT Detection
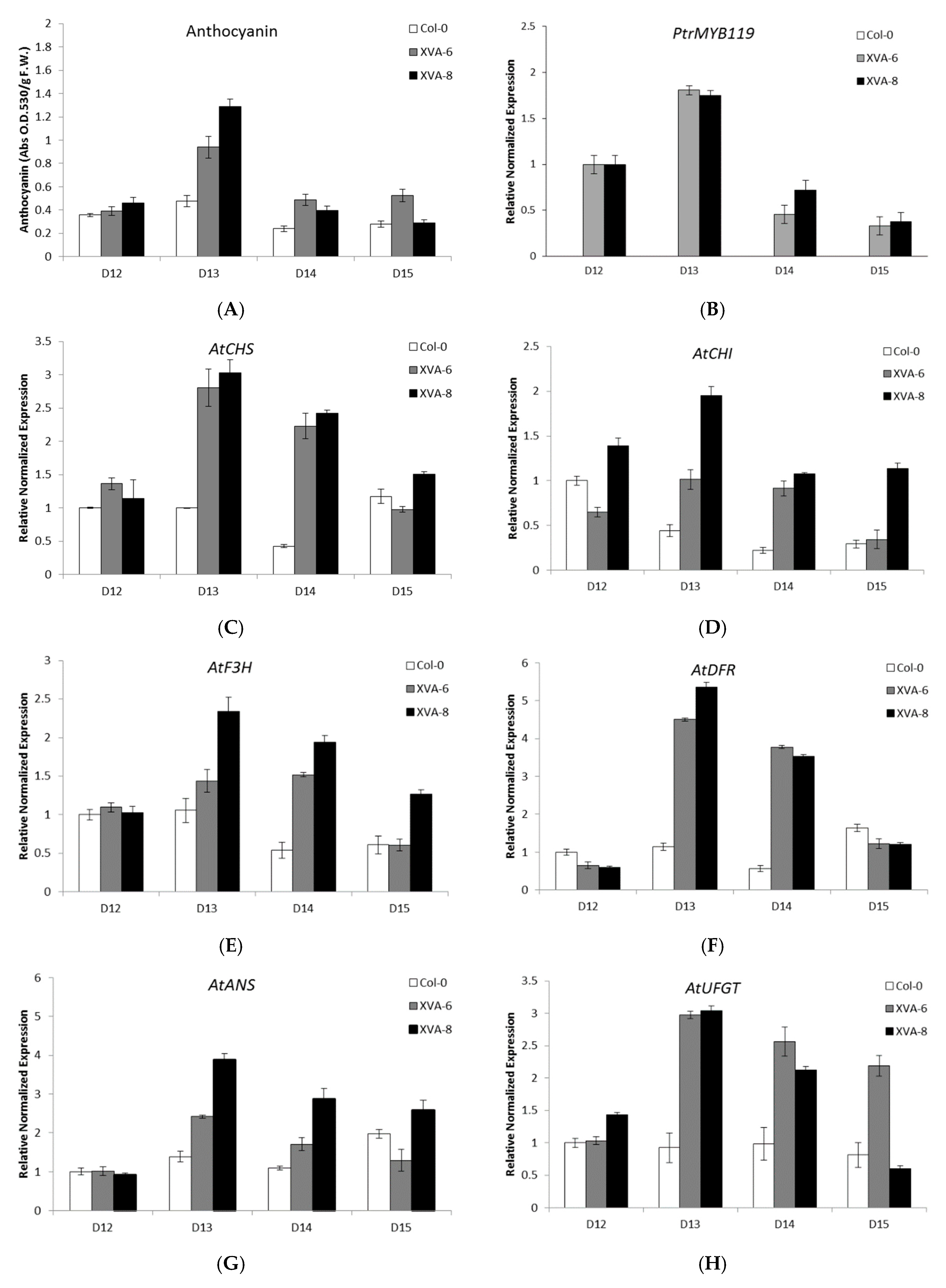
2.2. Anthocyanin Production in Transgenic Arabidopsis Is Influenced by Expression Levels of PtrMYB119 and Anthocyanin Biosynthesis Genes after DHT Treatment
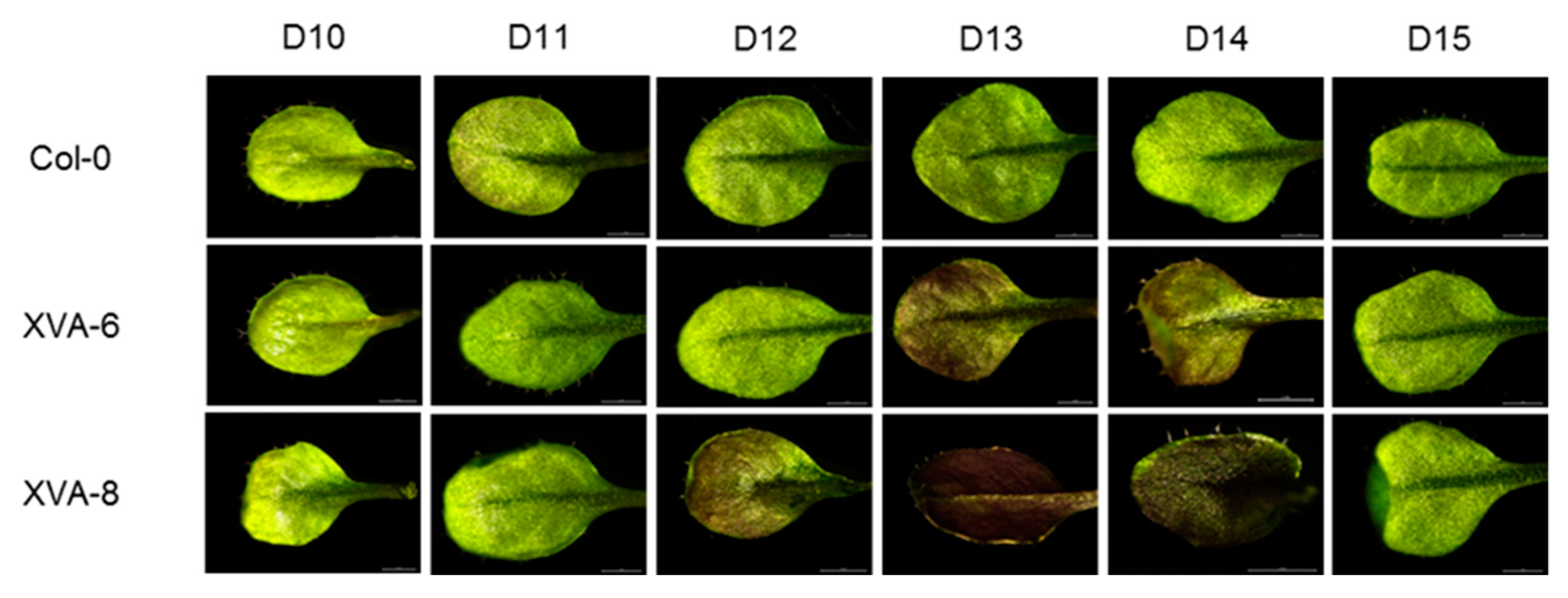
2.3. Time-Dependent Anthocyanin Synthesis in Response to DHT in TRANSGENIC ARABIDOPSIS
3. Materials and Methods
3.1. Plant Material, Growth Condition, and Chemical Treatments
3.2. Plasmid Construction and Plant Transformation
3.3. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
3.4. Anthocyanin Measurement
3.5. Light Microscopy Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine Disruptors Leading to Obesity and Related Diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wojtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Zlatnik, M.G. Endocrine-Disrupting Chemicals and Reproductive Health. J. Midwifery Womens Health 2016, 61, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Preciados, M.; Yoo, C.; Roy, D. Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases. Int. J. Mol. Sci. 2016, 17, 2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Hwang, K.A.; Choi, K.C. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol. Cell. Endocrinol. 2017, 457, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.M.; Knight, L.A.; D’Souza, D.L.; Kolok, A.S. Comparing the effects of atrazine and an environmentally relevant mixture on estrogen-responsive gene expression in the northern leopard frog and the fathead minnow. Environ. Toxicol. Chem. 2018, 37, 1182–1188. [Google Scholar] [CrossRef]
- Montagnini, B.G.; Pernoncine, K.V.; Borges, L.I.; Costa, N.O.; Moreira, E.G.; Anselmo-Franci, J.A.; Kiss, A.C.I.; Gerardin, D.C.C. Investigation of the potential effects of triclosan as an endocrine disruptor in female rats: Uterotrophic assay and two-generation study. Toxicology 2018, 410, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Loder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K. Ubiquity of bisphenol A in the atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Furhacker, M.; Scharf, S.; Weber, H. Bisphenol A: Emissions from point sources. Chemosphere 2000, 41, 751–756. [Google Scholar] [CrossRef]
- Meesters, R.J.; Schroder, H.F. Simultaneous determination of 4-nonylphenol and bisphenol A in sewage sludge. Anal. Chem. 2002, 74, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Berlioz-Barbier, A.; Vauchez, A.; Wiest, L.; Baudot, R.; Vulliet, E.; Cren-Olive, C. Multi-residue analysis of emerging pollutants in sediment using QuEChERS-based extraction followed by LC-MS/MS analysis. Anal. Bioanal. Chem. 2014, 406, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Magi, E.; Di Carro, M.; Liscio, C. Passive sampling and stir bar sorptive extraction for the determination of endocrine-disrupting compounds in water by GC-MS. Anal. Bioanal. Chem. 2010, 397, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.K.; Shanmugam, G.; Sampath, S.; Larsson, D.G.; Ramaswamy, B.R. GC-MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicol. Environ. Saf. 2014, 99, 13–20. [Google Scholar] [CrossRef]
- Heppell, S.A.; Denslow, N.D.; Folmar, L.C.; Sullivan, C.V. Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ. Health Perspect. 1995, 103, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.; van Aerle, R.; Brack, S.; Tyler, C.R.; Segner, H. Development and validation of a homologous zebrafish (Danio rerio Hamilton-Buchanan) vitellogenin enzyme-linked immunosorbent assay (ELISA) and its application for studies on estrogenic chemicals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 129, 217–232. [Google Scholar] [CrossRef]
- Markell, L.K.; Mingoia, R.T.; Peterson, H.M.; Yao, J.; Waters, S.M.; Finn, J.P.; Nabb, D.L.; Han, X. Endocrine disruption screening by protein and gene expression of vitellogenin in freshly isolated and cryopreserved rainbow trout hepatocytes. Chem. Res. Toxicol. 2014, 27, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Katsiadaki, I.; Morris, S.; Squires, C.; Hurst, M.R.; James, J.D.; Scott, A.P. Use of the three-spined stickleback (Gasterosteus aculeatus) as a sensitive in vivo test for detection of environmental antiandrogens. Environ. Health Perspect. 2006, 114, 115–121. [Google Scholar] [CrossRef]
- Katsiadaki, I.; Scott, A.P.; Hurst, M.R.; Matthiessen, P.; Mayer, I. Detection of environmental androgens: A novel method based on enzyme-linked immunosorbent assay of spiggin, the stickleback (Gasterosteus aculeatus) glue protein. Environ. Toxicol. Chem. 2002, 21, 1946–1954. [Google Scholar] [CrossRef]
- Sanchez, W.; Goin, C.; Brion, F.; Olsson, P.E.; Goksoyr, A.; Porcher, J.M. A new ELISA for the three-spined stickleback (Gasterosteus aculeatus L.) spiggin, using antibodies against synthetic peptide. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Urbatzka, R.; van Cauwenberge, A.; Maggioni, S.; Vigano, L.; Mandich, A.; Benfenati, E.; Lutz, I.; Kloas, W. Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere 2007, 67, 1080–1087. [Google Scholar] [CrossRef]
- Weiss, J.M.; Hamers, T.; Thomas, K.V.; van der Linden, S.; Leonards, P.E.; Lamoree, M.H. Masking effect of anti-androgens on androgenic activity in European river sediment unveiled by effect-directed analysis. Anal. Bioanal. Chem. 2009, 394, 1385–1397. [Google Scholar] [CrossRef] [Green Version]
- Tojo, T.; Tsuda, K.; Wada, T.S.; Yamazaki, K. A simple and extremely sensitive system for detecting estrogenic activity using transgenic Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2006, 64, 106–114. [Google Scholar] [CrossRef]
- Inui, H.; Sasaki, H.; Chua, N.H.; Ohkawa, H. Bioassay of estrogenic compounds in transgenic Arabidopsis plants carrying a recombinant human estrogen receptor gene and a GFP reporter gene. Transgenic. Res. 2009, 18, 899–909. [Google Scholar] [CrossRef]
- Kim, D.; Bahmani, R.; Ko, J.H.; Hwang, S. Development of bisphenol A (BPA)-sensing indicator Arabidopsis thaliana which synthesizes anthocyanin in response to BPA in leaves. Ecotoxicol. Environ. Saf. 2019, 170, 627–634. [Google Scholar] [CrossRef]
- Pitz, H.S.; Trevisan, A.C.; Cardoso, F.R.; Pereira, A.; Moreira, E.L.; de Pra, M.A.; Mazzarino, L.; Veleirinho, M.B.; Yunes, R.A.; do Valle, R.M.; et al. Assessment of In Vitro Biological Activities of Anthocyanins-Rich Plant Species Based on Plinia cauliflora Study Model. Methods Mol. Biol. 2016, 1391, 65–80. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Cho, J.S.; Nguyen, V.P.; Jeon, H.W.; Kim, M.H.; Eom, S.H.; Lim, Y.J.; Kim, W.C.; Park, E.J.; Choi, Y.I.; Ko, J.H. Overexpression of PtrMYB119, a R2R3-MYB transcription factor from Populus trichocarpa, promotes anthocyanin production in hybrid poplar. Tree Physiol. 2016, 36, 1162–1176. [Google Scholar] [CrossRef]
- Slomczynska, M. Xenoestrogens: mechanisms of action and some detection studies. Pol. J. Vet. Sci. 2008, 11, 263–269. [Google Scholar]
- Tao, L.; Shiwei, J.; Yang, F.X.; Yang, H.; Ying, X. An enzyme-linked immunosorbent assay for rare minnow (Gobiocypris rarus) vitellogenin and comparison of vitellogenin responses in rare minnow and zebrafish (Danio rerio). Sci. Total Environ. 2006, 364, 284–294. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.G.; Kim, J.A.; Hwang, S. The Density and Length of Root Hairs Are Enhanced in Response to Cadmium and Arsenic by Modulating Gene Expressions Involved in Fate Determination and Morphogenesis of Root Hairs in Arabidopsis. Front. Plant Sci. 2016, 7, 1763. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Niu, Q.W.; Chua, N.H. Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000, 24, 265–273. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.; Lee, B.D.; Hwang, S. Over-expression of tobacco UBC1 encoding a ubiquitin-conjugating enzyme increases cadmium tolerance by activating the 20S/26S proteasome and by decreasing Cd accumulation and oxidative stress in tobacco (Nicotiana tabacum). Plant Mol. Biol. 2017, 94, 433–451. [Google Scholar] [CrossRef]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type transcription factor, aba-inducible bhlh-type transcription factor/ja-associated myc2-like1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-G.; Bahmani, R.; Ko, J.-H.; Hwang, S. A Convenient Plant-Based Detection System to Monitor Androgenic Compound in the Environment. Plants 2019, 8, 266. https://doi.org/10.3390/plants8080266
Kim D-G, Bahmani R, Ko J-H, Hwang S. A Convenient Plant-Based Detection System to Monitor Androgenic Compound in the Environment. Plants. 2019; 8(8):266. https://doi.org/10.3390/plants8080266
Chicago/Turabian StyleKim, Dong-Gwan, Ramin Bahmani, Jae-Heung Ko, and Seongbin Hwang. 2019. "A Convenient Plant-Based Detection System to Monitor Androgenic Compound in the Environment" Plants 8, no. 8: 266. https://doi.org/10.3390/plants8080266
APA StyleKim, D.-G., Bahmani, R., Ko, J.-H., & Hwang, S. (2019). A Convenient Plant-Based Detection System to Monitor Androgenic Compound in the Environment. Plants, 8(8), 266. https://doi.org/10.3390/plants8080266







