Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance
Abstract
:1. Introduction
2. Results
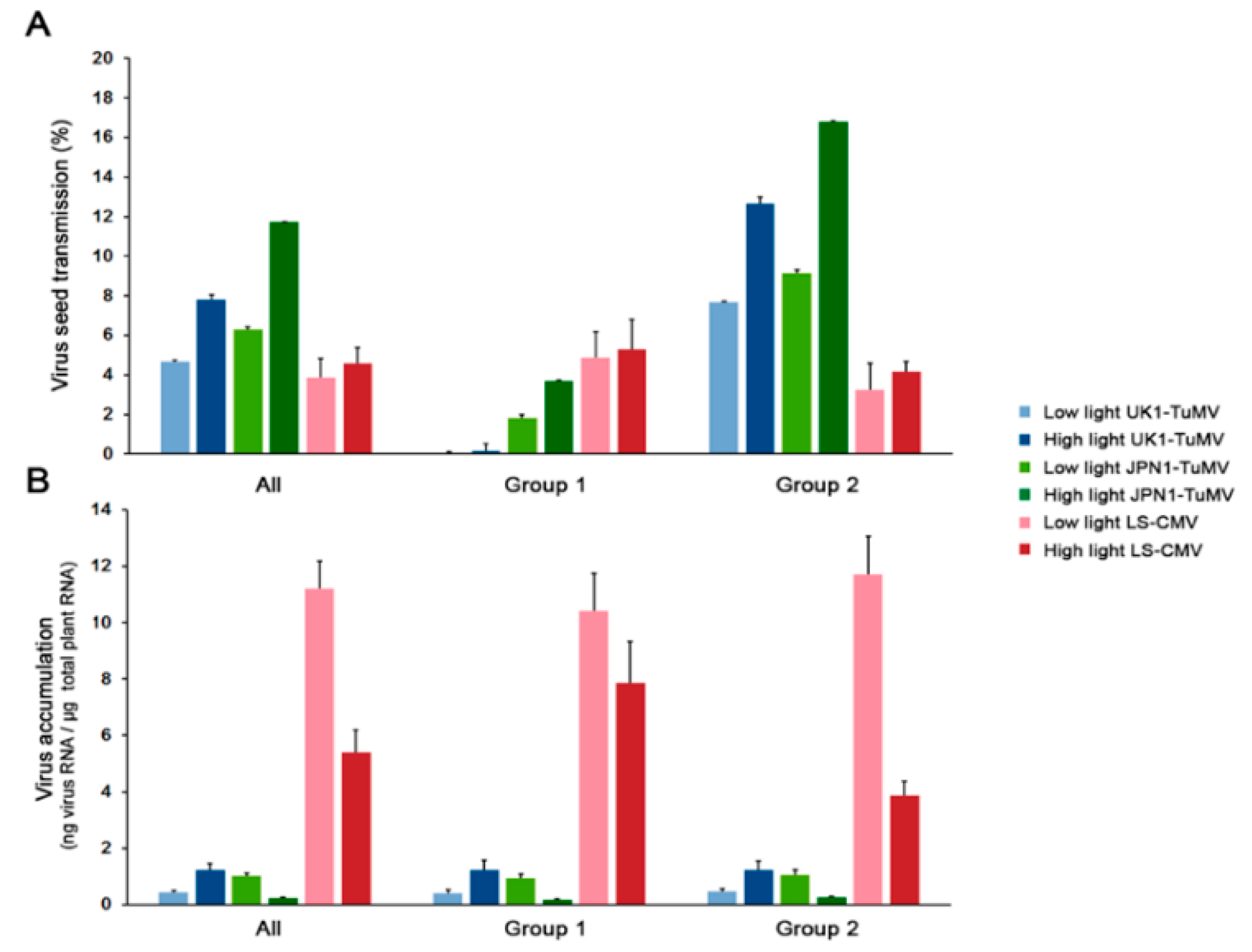
2.1. Effect of Light on CMV and TuMV Seed Transmission in Arabidopsis. 1
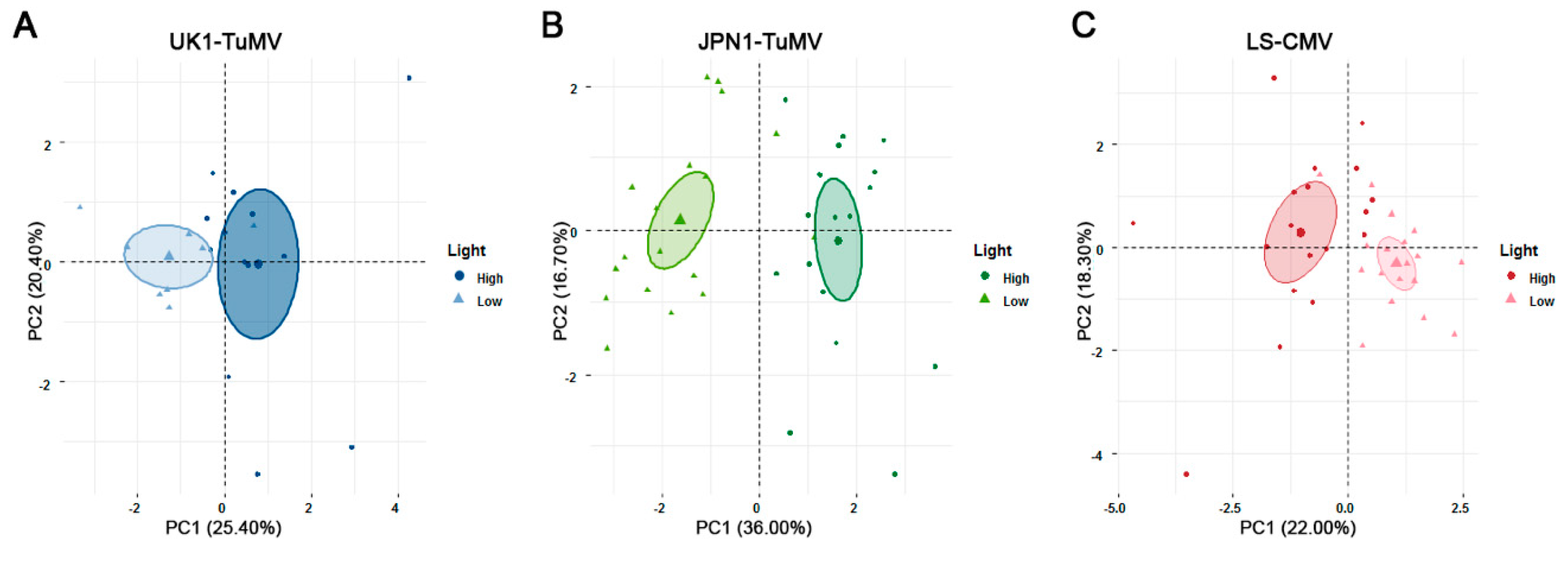
2.2. Effect of Light on Arabidopsis Resistance to CMV and TuMV
2.3. Effect of Light on Arabidopsis Growth, Reproduction and Developmental Schedule upon CMV and TuMV Infection
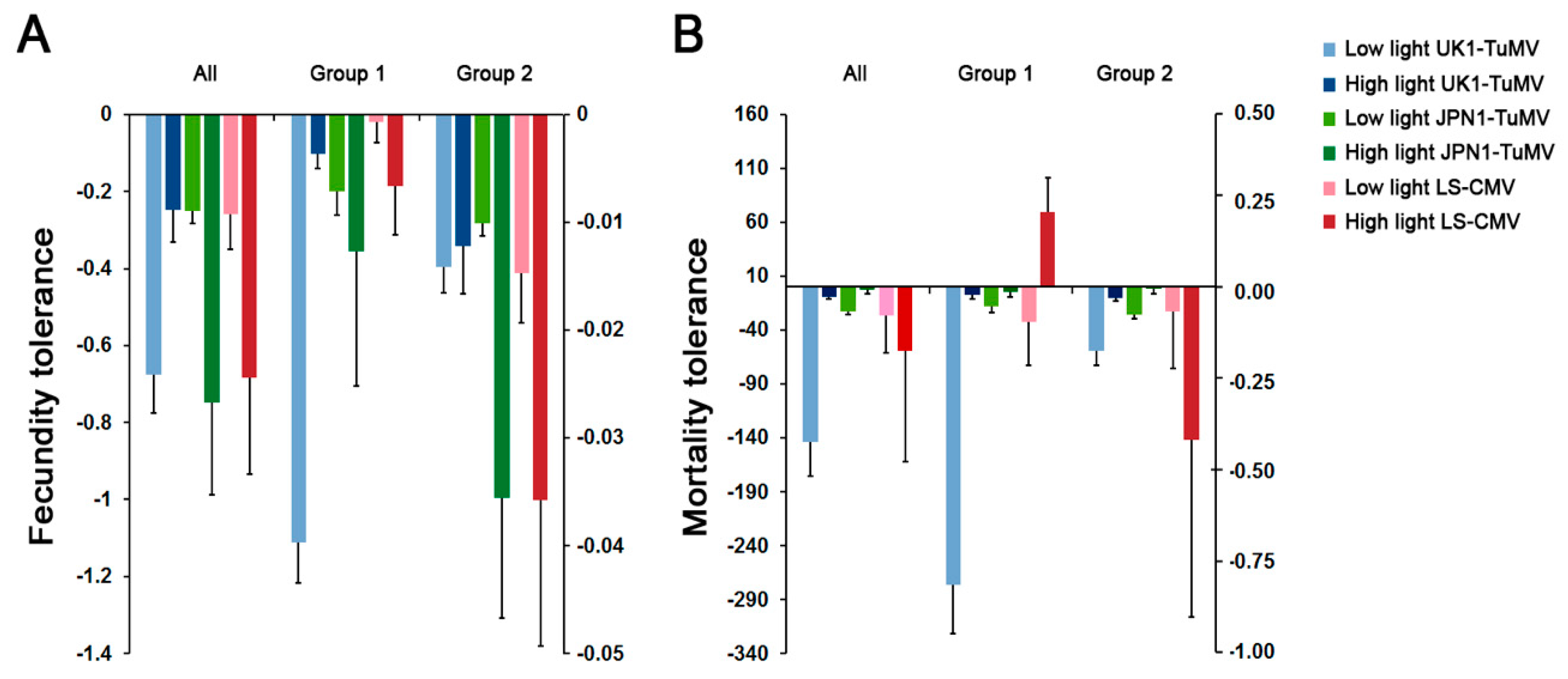
2.4. Effect of Light on Arabidopsis Tolerance to CMV and TuMV
2.5. Relationship between Light Intensity, Virus Seed Transmission and Plant Tolerance to CMV and TuMV
3. Discussion
4. Materials and Methods
4.1. Arabidopsis Accessions and Virus Isolates
4.2. Virus Multiplication
4.3. Effect of Infection on Plant Growth, Reproduction and Development
4.4. Arabidopsis Tolerance
4.5. Efficiency of Virus Seed Transmission
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 747–845. [Google Scholar]
- Schneider, T.; Kaul, C.M.; Pressel, K.G. Possible climate transitions from breakup of stratocumulus decks under greenhouse warming. Nat. Geosci. 2019, 12, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Grimm, N.B.; Chapin, F.S.; Bierwagen, B.; Gonzalez, P.; Groffman, P.M.; Luo, Y.; Melton, F.; Nadelhoffer, K.; Pairis, A.; Raymond, P.A.; et al. The impacts of climate change on ecosystem structure and function. Front. Ecol. Environ. 2013, 11, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.D.; Harris, G.M. Climate change and ecosystem composition across large landscapes. Landscape Ecol. 2017, 32, 195–207. [Google Scholar] [CrossRef]
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Change Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A. Future scenarios for plant virus pathogens as climate change progresses. Adv. Virus Res. 2016, 95, 87–147. [Google Scholar]
- Woolhouse, M.E. Population biology of emerging and re-emerging pathogens. Trends Microbiol. 2002, 10, S3–S7. [Google Scholar] [CrossRef]
- Pagán, I.; García-Arenal, F. Tolerance to plant pathogens: Theory and experimental evidence. Int. J. Mol. Sci. 2018, 19, 810. [Google Scholar] [CrossRef]
- Clarke, D.D. Tolerance of parasites and disease in plants and its significance in host-parasite interactions. Adv. Plant. Pathol. 1986, 5, 161–198. [Google Scholar]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Little, T.J.; Shuker, D.M.; Colegrave, N.; Day, T.; Graham, A.L. The coevolution of virulence: Tolerance in perspective. PLoS Pathog. 2010, 6, e1001006. [Google Scholar] [CrossRef]
- Råberg, L. How to live with the enemy: Understanding tolerance to parasites. PLoS Biol. 2014, 12, e1001989. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.; Allende, L.; del Toro, F.J.; Chung, B.-N.; Canto, T.; Tenllado, F. Effects of elevated CO2 and temperature on pathogenicity determinants and virulence of Potato virus X/Potyvirus-associated synergism. Mol. Plant.-Microbe Interact. 2015, 12, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Obrepalska-Steplowska, A.; Renaut, J.; Planchon, S.; Przybylska, A.; Wieczorek, P.; Barylski, J.; Palukaitis, P. Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front. Plant. Sci. 2015, 6, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Toro, F.J.; Rakhshandehroob, F.; Larruy, B.; Aguilar, E.; Tenllado, F.; Canto, T. Effects of simultaneously elevated temperature and CO2 levels on Nicotiana benthamiana and its infection by different positive-sense RNA viruses are cumulative and virus type-specific. Virology 2017, 511, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Canto, T.; Palukaitis, P. Novel N gene-associated, temperature-independent resistance to the movement of Tobacco mosaic virus vectors neutralized by a Cucumber mosaic virus RNA1 transgene. J. Virol. 2002, 76, 12908–12916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Singh, J.; Li, D.; Qu, F. Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires dcl2, AGO2, and HEN1. J. Virol. 2012, 86, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Chandra-Shekara, A.C.; Gupte, M.; Navarre, D.; Raina, S.; Raina, R.; Klessig, D.; Kachroo, P. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant. J. 2006, 45, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant. Biol. 2014, 65, 15.1–15.29. [Google Scholar] [CrossRef]
- Bergès, S.E.; Vile, D.; Vazquez-Rovere, C.; Blanc, S.; Yvon, M.; Bédiée, A.; Rolland, G.; Dauzat, M.; van Munster, M. Interactions between drought and plant genotype change epidemiological traits of Cauliflower mosaic virus. Front. Plant. Sci. 2018, 9, 703. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Hall, S.R.; Becker, C.; Cáceres, C.E. Parasitic castration: A perspective from a model of dynamic energy budgets. Integr. Comp. Biol. 2007, 47, 295–309. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Halliday, K.J. Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Hily, J.M.; Poulicard, N.; Mora, M.A.; Pagán, I.; García-Arenal, F. Environment and host genotype determine the outcome of a plant–virus interaction: From antagonism to mutualism. New Phytol. 2016, 209, 812–822. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Metcalf, C.J.E.; Walter, K.S.; Wesolowski, A.; Buckee, C.O.; Shevliakova, E.; Tatem, A.J.; Boos, W.R.; Weinberger, D.M.; Pitzer, V.E. Identifying climate drivers of infectious disease dynamics: Recent advances and challenges ahead. Proc. R Soc. B 2017, 284, 20170901. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Kennelly, M.; O’Mara, J.; Rivard, C.; Miller, G.L.; Smith, D. Introduction to abiotic disorders in plants. Plant. Health Instr. 2012, 10, 1094. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef]
- Diaz, B.M.; Fereres, A. Life table and population parameters of Nasonovia ribisnigri (Homoptera: Aphididae) at different constant temperatures. Environ. Entomol. 2005, 34, 527–534. [Google Scholar] [CrossRef]
- Culbreath, A.K.; Srinivasan, R. Epidemiology of spotted wilt disease of peanut caused by Tomato spotted wilt virus in the southeastern US. Virus Res. 2011, 159, 101–109. [Google Scholar] [CrossRef]
- van Munster, M.; Yvon, M.; Vile, D.; Dader, B.; Fereres, A.; Blanc, S. Water deficit enhances the transmission of plant viruses by insect vectors. PLoS ONE 2017, 12, e0174398. [Google Scholar] [CrossRef]
- Sastry, K.S. Seed-Borne Plant Virus Diseases; Springer: New Delhi, India, 2013. [Google Scholar]
- Simmons, H.E.; Munkvold, G.P. Seed transmission in the Potyviridae. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Gullino, M.L., Munkvold, G.P., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 3–15. [Google Scholar]
- Anderson, R.M.; May, R.M. Coevolution of hosts and parasites. Parasitology 1982, 85, 411–426. [Google Scholar] [CrossRef]
- Lipsitch, M.; Nowak, M.A.; Ebert, D.; May, R.M. The population dynamics of vertically and horizontally transmitted parasites. Proc. R Soc. Lond. B 1995, 260, 321–327. [Google Scholar]
- Lipsitch, M.; Siller, S.; Nowak, M.A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 1996, 50, 1729–1741. [Google Scholar] [CrossRef]
- Cobos, A.; Montes, N.; López-Herranz, M.; Gil-Valle, M.; Pagán, I. Within-host multiplication and speed of colonization as infection traits associated with plant virus vertical transmission. J. Virol. 2019. under second review with minor changes. [Google Scholar]
- Pagán, I.; Fraile, A.; Fernández-Fueyo, E.; Montes, N.; Alonso-Blanco, C.; García-Arenal, F. Arabidopsis thaliana as a model for the study of plant–virus co-evolution. Philos. Trans. R Soc. Lond. B 2010, 365, 1983–1995. [Google Scholar] [CrossRef]
- Pagán, I.; Alonso-Blanco, C.; García-Arenal, F. Host responses in life-history traits and tolerance to virus infection in Arabidopsis thaliana. PLoS Pathog. 2008, 4, e1000124. [Google Scholar] [CrossRef]
- Shukla, A.; Pagán, I.; García-Arenal, F. Effective tolerance based on resource reallocation is a virus-specific defence in Arabidopsis thaliana. Mol. Plant. Pathol. 2018, 19, 1454–1465. [Google Scholar] [CrossRef]
- Montes, N.; Alonso-Blanco, C.; García-Arenal, F. Cucumber mosaic virus infection as a potential selective pressure on Arabidopsis thaliana populations. PLoS Pathog. 2019, 15, e1007810. [Google Scholar] [CrossRef]
- Pagán, I.; Montes, N.; Milgroom, M.G.; García-Arenal, F. Vertical transmission selects for reduced virulence in a plant virus and for increased resistance in the host. PLoS Pathog. 2014, 10, e1004293. [Google Scholar] [CrossRef]
- Pagán, I. Movement between plants: Vertical transmission. In Cucumber Mosaic Virus; Palukaitis, P., García-Arenal, F., Eds.; APS Press: Washington, USA, 2019; pp. 185–198. [Google Scholar]
- Alizon, S.; Hurford, A.; Mideo, N.; Van Baalen, M. Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. J. Evol. Biol. 2009, 2, 245–259. [Google Scholar] [CrossRef]
- Fereres, A. Insect vectors as drivers of plant virus emergence. Curr. Opin. Virol. 2015, 10, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Bos, L. Seed-borne viruses. In Plant Health and Quarantine in International Transfer of Genetic Resources; Hewitt, W.B., Chiarappa, L., Eds.; CRC Press: Cleveland, OH, USA, 1977; pp. 36–39. [Google Scholar]
- Albrechtsen, S.E. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Maule, A.J.; Wang, D. Seed transmission of plant viruses: A lesson in biological complexity. Trends Microbiol. 1996, 4, 153–158. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Proudlove, W. Further studies on cucumber mosaic virus infection of narrow-leafed lupin (Lupinus angustifolius): Seed-borne infection, aphid transmission, spread and effects on grain yield. Ann. Appl. Biol. 1991, 118, 319–329. [Google Scholar] [CrossRef]
- Sánchez, F.; Manrique, P.; Mansilla, C.; Lunello, P.; Wang, X.; Rodrigo, G.; López-González, S.; Jenner, C.; González-Melendi, P.; Elena, S.F.; et al. Viral strain-specific differential alterations in Arabidopsis developmental patterns. Mol. Plant.–Microbe Interact. 2015, 28, 1304–1315. [Google Scholar] [CrossRef]
- Montes, N.; Vijayan, V.; Pagán, I. Trade-offs between host tolerances to different pathogens in plant-virus interactions. Virus Evol. 2019. Submitted. [Google Scholar]
- Wang, D.; Maule, A.J. A model for seed transmission of a plant virus: Genetic and structural analyses of pea embryo invasion by pea seed-borne mosaic virus. Plant. Cell 1994, 6, 777–787. [Google Scholar] [CrossRef]
- Hull, R. Plant Virology, 5th ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Jenner, C.E.; Walsh, J.A. Pathotypic variation in Turnip mosaic virus with special reference to European isolates. Plant. Patho.l 1996, 45, 848–856. [Google Scholar] [CrossRef]
- Bonnet, J.; Fraile, A.; Sacristán, S.; Malpica, J.M.; García-Arenal, F. Role of recombination in the evolution of natural populations of Cucumber mosaic virus, a tripartite RNA plant virus. Virology 2005, 332, 359–368. [Google Scholar] [CrossRef]
- Rizzo, T.M.; Palukaitis, P. Construction of full-length cDNA clones of Cucumber mosaic virus RNAs 1, 2 and 3: Generation of infectious RNA transcripts. Mol. Gen. Genet. 1990, 222, 249–256. [Google Scholar] [CrossRef]
- Zhang, L.; Hanada, K.; Palukaitis, P. Mapping local and systemic symptom determinants of cucumber mosaic cucumovirus in tobacco. J. Gen. Virol. 1994, 75, 3185–3191. [Google Scholar] [CrossRef]
- Sánchez, F.; Martínez-Herrera, D.; Aguilar, I.; Ponz, F. Infectivity of turnip mosaic potyvirus cDNA clones and transcripts on the systemic host Arabidopsis thaliana and local lesion hosts. Virus Res. 1998, 55, 207–219. [Google Scholar] [CrossRef]
- Sharbel, T.F.; Haubold, B.; Mitchell-Olds, T. Genetic isolation by distance in Arabidopsis thaliana: Biogeography and postglacial colonization of Europe. Mol. Ecol. 2000, 9, 2109–2118. [Google Scholar] [CrossRef]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage–based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant. Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]
- Lunello, P.; Mansilla, C.; Sánchez, F.; Ponz, F. A developmentally linked, dramatic, and transient loss of virus from roots of Arabidopsis thaliana plants infected by either of two RNA viruses. Mol. Plant.-Microbe Interact. 2007, 20, 1589–1595. [Google Scholar] [CrossRef]
- Hily, J.M.; García, A.; Moreno, A.; Plaza, M.; Wilkinson, M.D.; Fereres, A.; Fraile, A.; García-Arenal, F. The relationship between host lifespan and pathogen reservoir potential: An analysis in the system Arabidopsis thaliana-Cucumber mosaic virus. PLoS Pathog. 2014, 10, e1004492. [Google Scholar] [CrossRef]
- Thompson, K.; Stewart, A.J.A. The measurement and meaning of reproductive effort in plants. Am. Nat. 1981, 117, 205–211. [Google Scholar] [CrossRef]
- Simmons, H.E.; Holmes, E.C.; Gildow, F.E.; Bothe-Goralczyk, M.A.; Stephenson, A.G. Experimental verification of seed transmission in Zucchini yellow mosaic virus. Plant. Dis. 2011, 95, 751–754. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Gower, J.C. The use of a multiple-transfer method in plant virus transmission studies: Some statistical points arising in the analysis results. Ann. Appl. Biol. 1960, 48, 75–83. [Google Scholar] [CrossRef]
- Belgorodski, N.; Greiner, M.; Tolksdorf, K.; Schueller, K. rriskDistributions: Fitting Distributions to Given Data or Known Quantiles. R Package Version 2.1.2. 2015. Available online: https://CRAN.R-project.org/package=rriskDistributions (accessed on 26 August 2018).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R-Package Version 3.1-140. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 26 August 2018).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.5. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 26 August 2018).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project.org/ (accessed on 26 August 2018).

| Accession | Origin | Allometric Group |
|---|---|---|
| An-1 | Amberes (Belgium) | Group 2 |
| Bay-0 | Bayreuth (Germany) | Group 2 |
| Cad-0 | Candelario (Spain) | Group 1 |
| Cdm-0 | Caldas de Miravete (Spain) | Group 1 |
| Cen-1 | Centenera (Spain) | Group 2 |
| Col-0 | Columbia (Unknown) | Group 2 |
| Cum-0 | Cumbres Mayores (Spain) | Group 1 |
| Cvi | Cape Verde Islands | Group 2 |
| Fei-0 | Santa María da Feira (Portugal) | Group 2 |
| Kas-0 | Kashmir (India) | Group 1 |
| Kas-2 | Kashmir (India) | Group 1 |
| Kyo-1 | Kyoto (Japan) | Group 1 |
| Ler | Landsberg (Poland) | Group 2 |
| Ll-0 | Llagostera (Spain) | Group 1 |
| Mer-0 | Mérida (Spain) | Group 2 |
| Pro-0 | Proaza (Spain) | Group 2 |
| Shak | Shakdara (Tadjikistan) | Group 2 |
| Ver-5 | Verin (Spain) | Group 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, N.; Pagán, I. Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance. Plants 2019, 8, 304. https://doi.org/10.3390/plants8090304
Montes N, Pagán I. Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance. Plants. 2019; 8(9):304. https://doi.org/10.3390/plants8090304
Chicago/Turabian StyleMontes, Nuria, and Israel Pagán. 2019. "Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance" Plants 8, no. 9: 304. https://doi.org/10.3390/plants8090304
APA StyleMontes, N., & Pagán, I. (2019). Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance. Plants, 8(9), 304. https://doi.org/10.3390/plants8090304






