Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Physiological Parameters
2.2.1. Chlorophyll Contents
2.2.2. Hydrogen Peroxide (H2O2)
2.2.3. Ascorbic Acid (AsA)
2.2.4. Malondialdehyde (MDA)
2.2.5. Total Phenolics
2.2.6. Glycinebetaine (GB)
2.2.7. Proline
2.2.8. Antioxidant Enzymes
2.2.9. Statistical Analysis
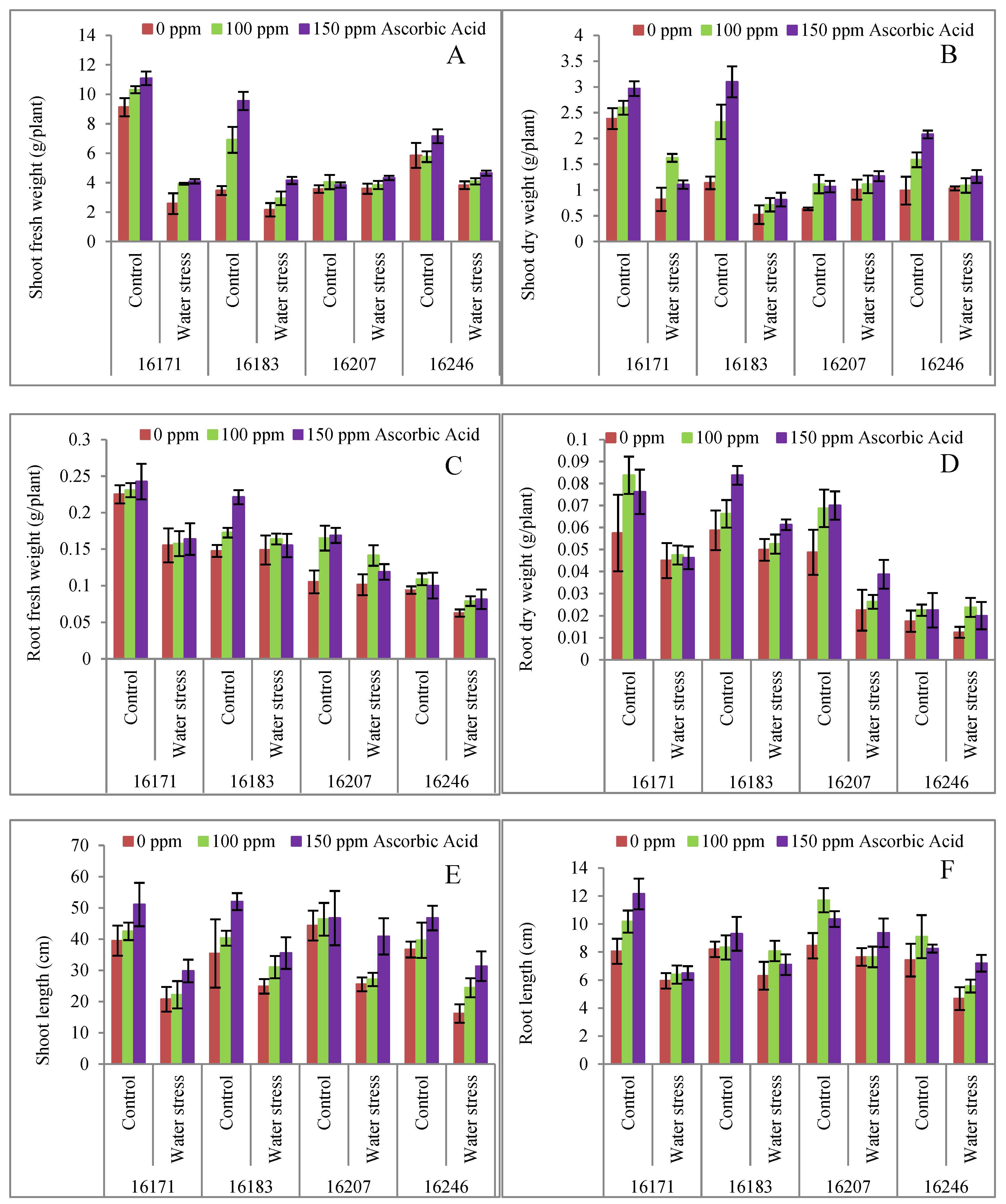
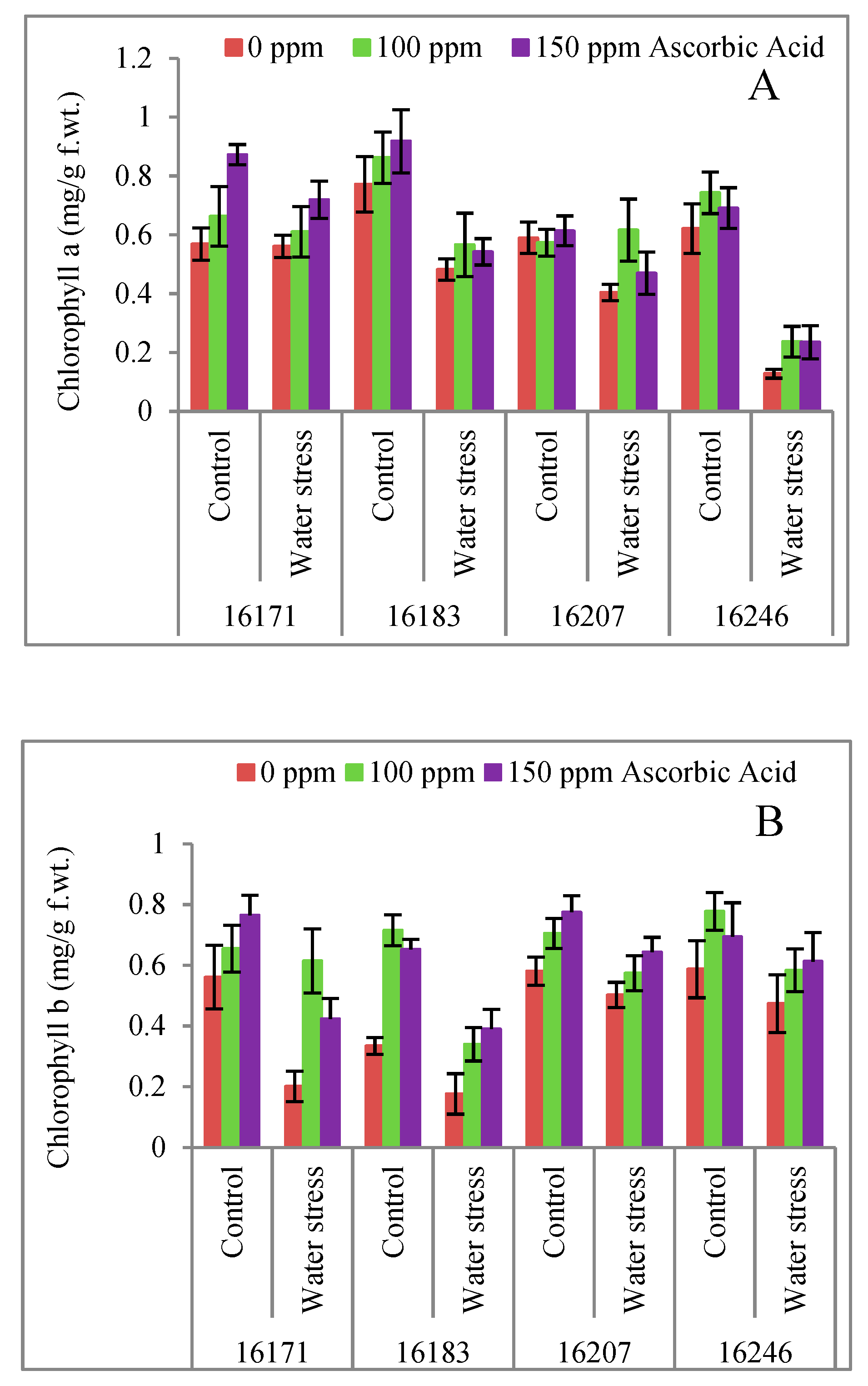
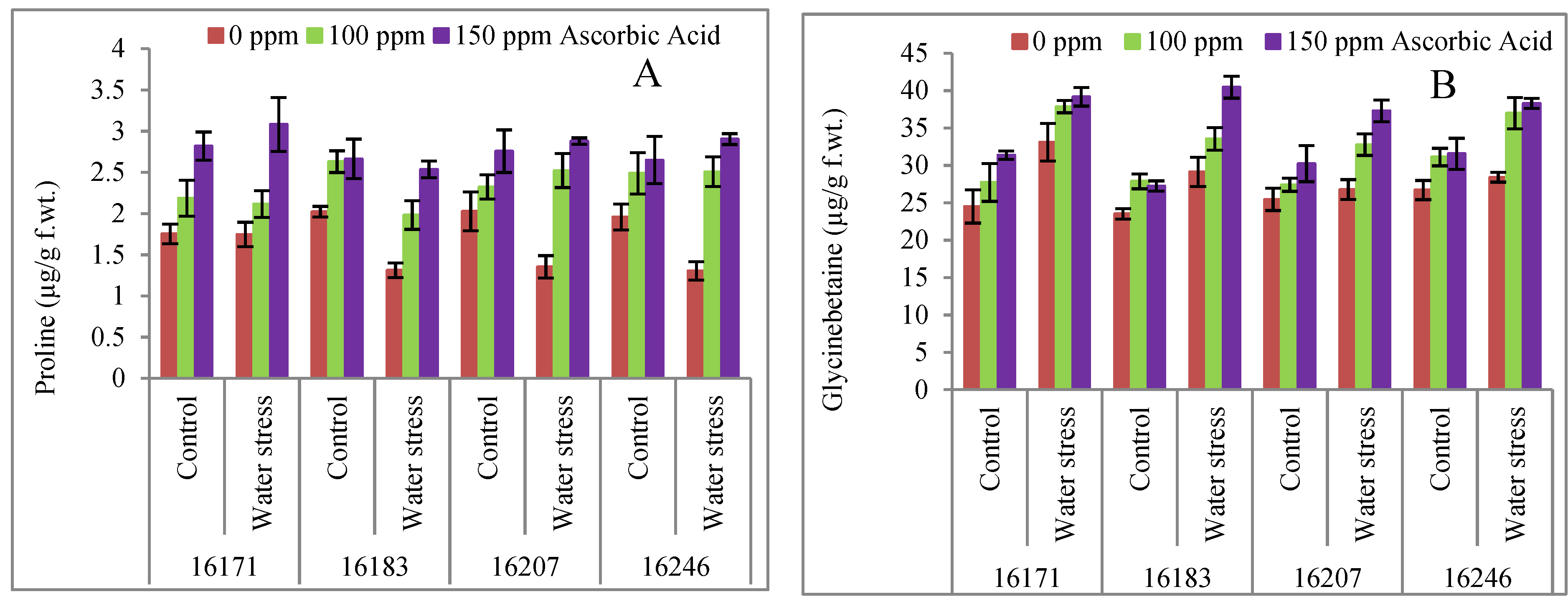
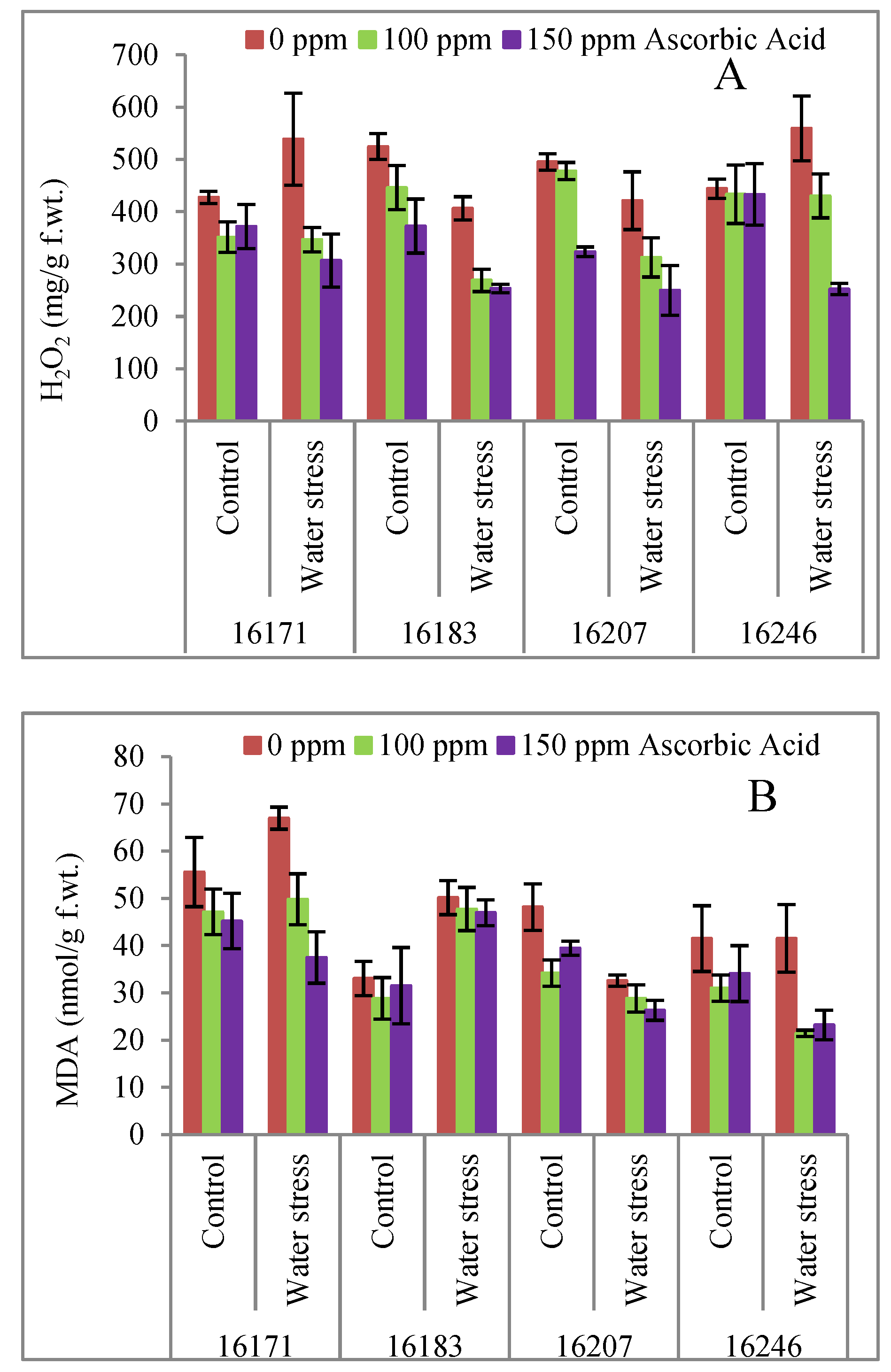
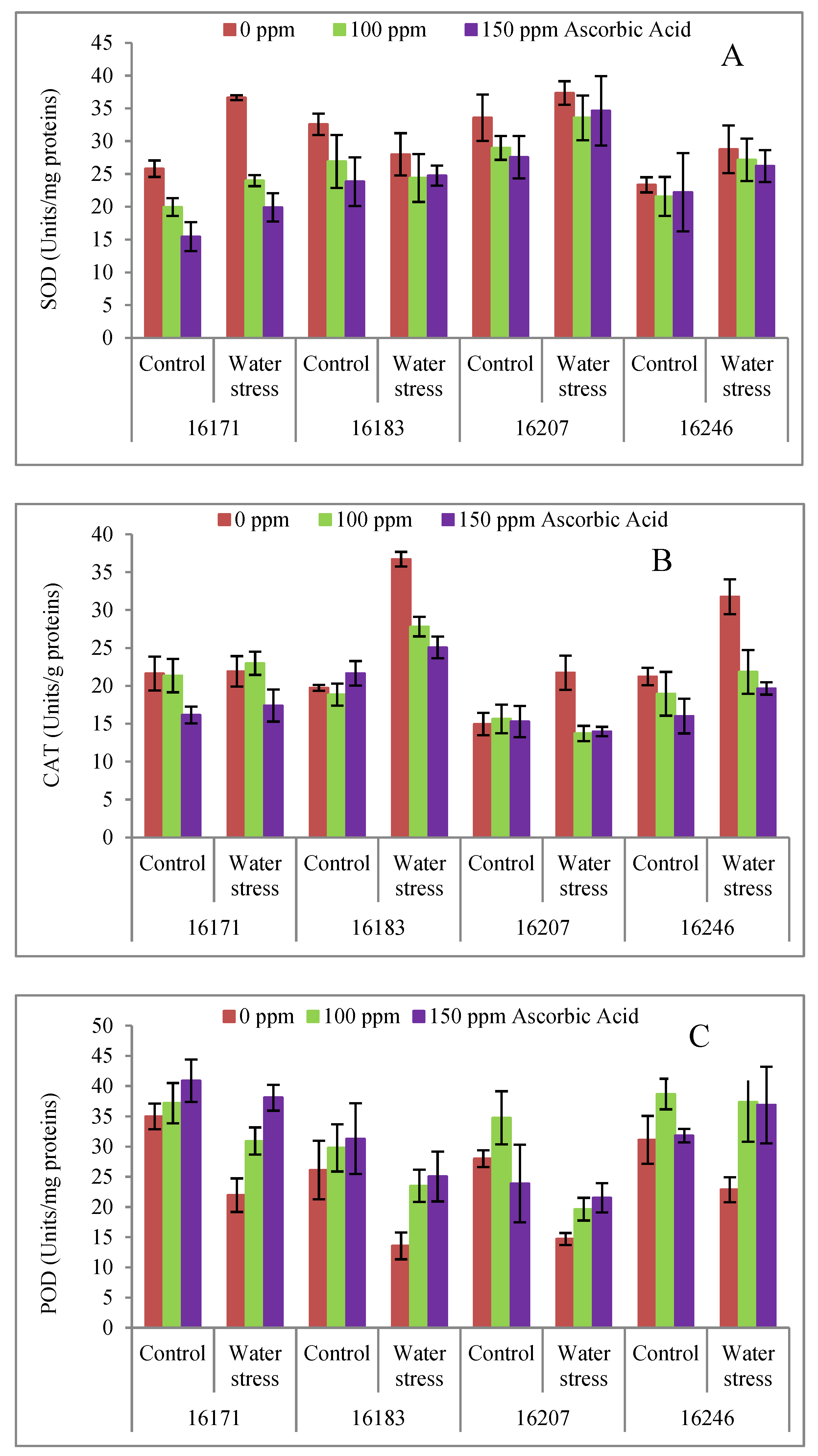
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2015, 36, 3. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Liu, Y.; Ishaq, M.; Shah, T.; Ilyas, A.; Din, I. Climate change and its impact on the yield of major food crops: Evidence from Pakistan. Foods 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Masih, I.; Maskey, S.; Mussá, F.E.F.; Trambauer, P. A review of droughts on the African continent: A geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 2014, 18, 3635–3649. [Google Scholar] [CrossRef]
- Praba, M.L.; Cairns, J.E.; Babu, R.C.; Lafitte, H.R. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop Sci. 2009, 195, 30–46. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef]
- Zafari, M.; Ebadi, A. Effects of water strass and brassinosteroid (24-epibrassinolide) on changes of some amino acids and pigments in safflower (Cartamus tinctorius L.). J. Curr. Res. Sci. 2016, 1, 711. [Google Scholar]
- Kosar, F.; Akram, N.A.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Eslam, B.P. Evaluation of physiological traits for improving ater deficit tolerance in spring safflower. J. Agric. Sci. Technol. 2011, 13, 327–338. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Akram, N.A.; Khan, I.; Javed, Z.; Khan, Z.; Mahmood, S.; Ashraf, M.; Shafiq, S.; Naz, H. Modulation in some key biochemical attributes in drought stressed pea (Pisum sativum L.) plants treated with different plant growth regulators. Agrochimica 2019, 62, in press. [Google Scholar]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Asilan, K.S. Effect of ascorbic acid foliar application on yield, yield component and several morphological traits of grain corn under water deficit stress conditions. Not. Sci. Biol. 2010, 2, 45–50. [Google Scholar] [CrossRef]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The potential of natural products for targeting PPARα. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Akram, N.A.; Ashraf, M. Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak. J. Bot. 2016, 48, 877–883. [Google Scholar]
- Javed, S.; Bukhari, S.A.; Ashraf, M.Y.; Mahmood, S.; Iftikhar, T. Effect of salinity on growth, biochemical parameters and fatty acid composition in safflower (Carthamus tinctorius L.). Pak. J. Bot. 2014, 46, 1153–1158. [Google Scholar]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Khan, M.; Naeem, M. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J. Agric. Sci. 2007, 3, 685–695. [Google Scholar]
- Soliman, E.Z.; Mendis, S.; Dissanayake, W.P.; Somasundaram, N.P.; Gunaratne, P.S.; Jayasingne, I.K.; Furberg, C.D. A Polypill for primary prevention of cardiovascular disease: A feasibility study of the World Health Organization. Trials 2011, 12, 3. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- van Rossum, M.W.P.C.; Alberda, M.; van der Plas, L.H.W. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997, 130, 207–216. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1955; pp. 764–775. [Google Scholar] [CrossRef]
- Luck, H. Catalases. In Methods of Enzymatic Analysis; Bregmeyer, H.U., Ed.; Academic: New York, NY, USA, 1974. [Google Scholar]
- Edgerton, M.D. Increasing Crop Productivity to Meet Global Needs for Feed, Food, and Fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid profiling of the leaves of selected African eggplant accessions subjected to drought stress. Food Sci. Nutr. 2017, 5, 113–122. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S. Chlorophyll degradation in horticultural crops. Walailak J. Sci. Technol. 2011, 8, 9–19. [Google Scholar]
- Malekzadeh, P. Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiol. Mol. Biol. Plants 2015, 21, 225–232. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Kordi, S.; Saidi, M.; Ghanbari, F. Induction of drought tolerance in sweet basil (Ocimum basilicum L) by salicylic acid. Int. J. Agric. Food Res. 2013, 2, 18–26. [Google Scholar] [CrossRef]
- Lum, M.; Hanafi, M.; Rafii, Y.; Akmar, A. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Rana, V.; Ram, S.; Nehra, K. Review proline biosynthesis and its role in abiotic stress. Int. J. Agric. Innov. Res. 2017, 6, 2319–2473. [Google Scholar]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol. Rep. 2014, 8, 279–293. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2012, 64, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Halimeh, R.; Mahlagh, G.; Maryam, P.; Pazoki, A. Effect of drought interactions with ascorbate on some biochemical parameters and antioxidant enzymes activities in Dracocephalum moldavica L. Middle East J. Sci. Res. 2013, 13, 522–531. [Google Scholar]
- Khan, T.; Mazid, M.; Mohammad, F. A review of ascorbic acid potentialities against oxidative stress induced in plants. J. Agrobiol. 2011, 28, 97–111. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, M.R.; Mahdiyeh, M. Antioxidative and biochemical responses of wheat to drought stress. J. Agric. Biol. Sci. 2013, 8, 291–301. [Google Scholar]
- Ghorbanli, M.; Gafarabad, M.; Amirkian, T.; Allahverdi, M.B. Investigation of proline, total protein, chlorophyll, ascorbate and dehydroascorbate changes under drought stress in Akria and Mobil tomato cultivars. Iran. J. Plant Physiol. 2013, 3, 651–658. [Google Scholar]
- Hafez, E.; Gharib, H. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int. J. Plant Prod. 2016, 10, 579–596. [Google Scholar]
- Malik, S.; Ashraf, M. Exogenous application of ascorbic acid stimulates growth and photosynthesis of wheat (Triticum aestivum L.) under drought. Soil Environ. 2012, 31, 199–206. [Google Scholar]

| Source of Variations | df | Shoot FW | Shoot DW | Root FW | Root DW | Shoot Length |
|---|---|---|---|---|---|---|
| Cultivars (Cvs) | 3 | 37.02 *** | 3.208 *** | 0.052 *** | 0.008 *** | 163.4 ns |
| Drought (D) | 1 | 221.6 *** | 15.38 *** | 0.034 *** | 0.008 *** | 6118.4 *** |
| Ascorbic acid (AsA) | 2 | 26.99 *** | 3.477 *** | 0.006 *** | 0.001 ** | 1068.5 *** |
| Cvs × D | 3 | 48.17 *** | 4.116 *** | 0.0035 ** | 0.001 ** | 70.42 ns |
| Cv × AsA | 6 | 5.13 *** | 0.283 * | 0.001 ns | 0.0001 ns | 12.46 ns |
| D × AsA | 2 | 2.59 * | 1.125 *** | 0.002 ns | 0.0002 ns | 11.41 ns |
| Cvs × D × AsA | 6 | 2.26 *** | 0.381 ** | 0.0007 ns | 0.0001 ns | 51.76 ns |
| Root Length | Chlorophyll a | Chlorophyll b | Proline | |||
| Cultivars (Cvs) | 3 | 19.07 *** | 0.316 *** | 0.198 *** | 0.07 ns | |
| Drought (D) | 1 | 140.3 *** | 1.417 *** | 0.857 *** | 0.68 * | |
| Ascorbic acid (AsA) | 2 | 24.91 *** | 0.122 ** | 0.397 *** | 9.83 *** | |
| Cvs × D | 3 | 6.39 ns | 0.231 *** | 0.036 ns | 0.32 ns | |
| Cv × AsA | 6 | 0.813 ns | 0.021 ns | 0.017 ns | 0.14 ns | |
| D × AsA | 2 | 2.08 ns | 0.012 ns | 0.002 ns | 0.83 ** | |
| Cvs × D × AsA | 6 | 5.42 ns | 0.011 ns | 0.031 ns | 0.11 ns | |
| GB | Total Phenolics | TSP | AsA | H2O2 | ||
| Cultivars (Cvs) | 3 | 12.68 ns | 67.13 *** | 146,428.5 *** | 1.72 ** | 11,391.5 ns |
| Drought (D) | 1 | 0.10 * | 26.96 ** | 72,409.6 *** | 0.695 ns | 94,925.5 *** |
| Ascorbic acid (AsA) | 2 | 145.5 *** | 20.62 *** | 31,712.8 *** | 45.75 *** | 199,078.7 *** |
| Cvs × D | 3 | 80.41 *** | 0.402 ns | 6380.7 ns | 2.558 *** | 29,751.7 ** |
| Cv × AsA | 6 | 15.55 ns | 4.69 ns | 11,800.3 ** | 0.303 ns | 4309.9 ns |
| D × AsA | 2 | 1.064 ns | 2.22 ns | 977.5 ns | 0.148 ns | 31,651.6 * |
| Cvs × D × AsA | 6 | 4.34 ns | 2.702 ns | 2211.1 ns | 0.336 ns | 12,225.1 ns |
| MDA | CAT | POD | SOD | |||
| Cultivars (Cvs) | 3 | 1545.4 *** | 339.2 *** | 12.56 *** | 379.7 *** | |
| Drought (D) | 1 | 1.802 ns | 470.1 *** | 20.65 *** | 316.5 ** | |
| Ascorbic acid (AsA) | 2 | 1146.7 *** | 253.8 *** | 9.95 *** | 362.6 *** | |
| Cvs × D | 3 | 947.5 *** | 104.3 ** | 1.555 ns | 88.75 ns | |
| Cv × AsA | 6 | 137.5 ns | 25.48 ns | 1.0817 ns | 52.19 ns | |
| D × AsA | 2 | 118.3 ns | 109.1 *** | 3.812 * | 3.153 ns | |
| Cvs × D × AsA | 6 | 66.22 ns | 22.44 ns | 0.335 ns | 16.25 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. https://doi.org/10.3390/plants9010104
Farooq A, Bukhari SA, Akram NA, Ashraf M, Wijaya L, Alyemeni MN, Ahmad P. Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants. 2020; 9(1):104. https://doi.org/10.3390/plants9010104
Chicago/Turabian StyleFarooq, Ayesha, Shazia Anwer Bukhari, Nudrat A. Akram, Muhammad Ashraf, Leonard Wijaya, Mohammed Nasser Alyemeni, and Parvaiz Ahmad. 2020. "Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.)" Plants 9, no. 1: 104. https://doi.org/10.3390/plants9010104
APA StyleFarooq, A., Bukhari, S. A., Akram, N. A., Ashraf, M., Wijaya, L., Alyemeni, M. N., & Ahmad, P. (2020). Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants, 9(1), 104. https://doi.org/10.3390/plants9010104






