Settling for Less: Do Statoliths Modulate Gravity Perception?
Abstract
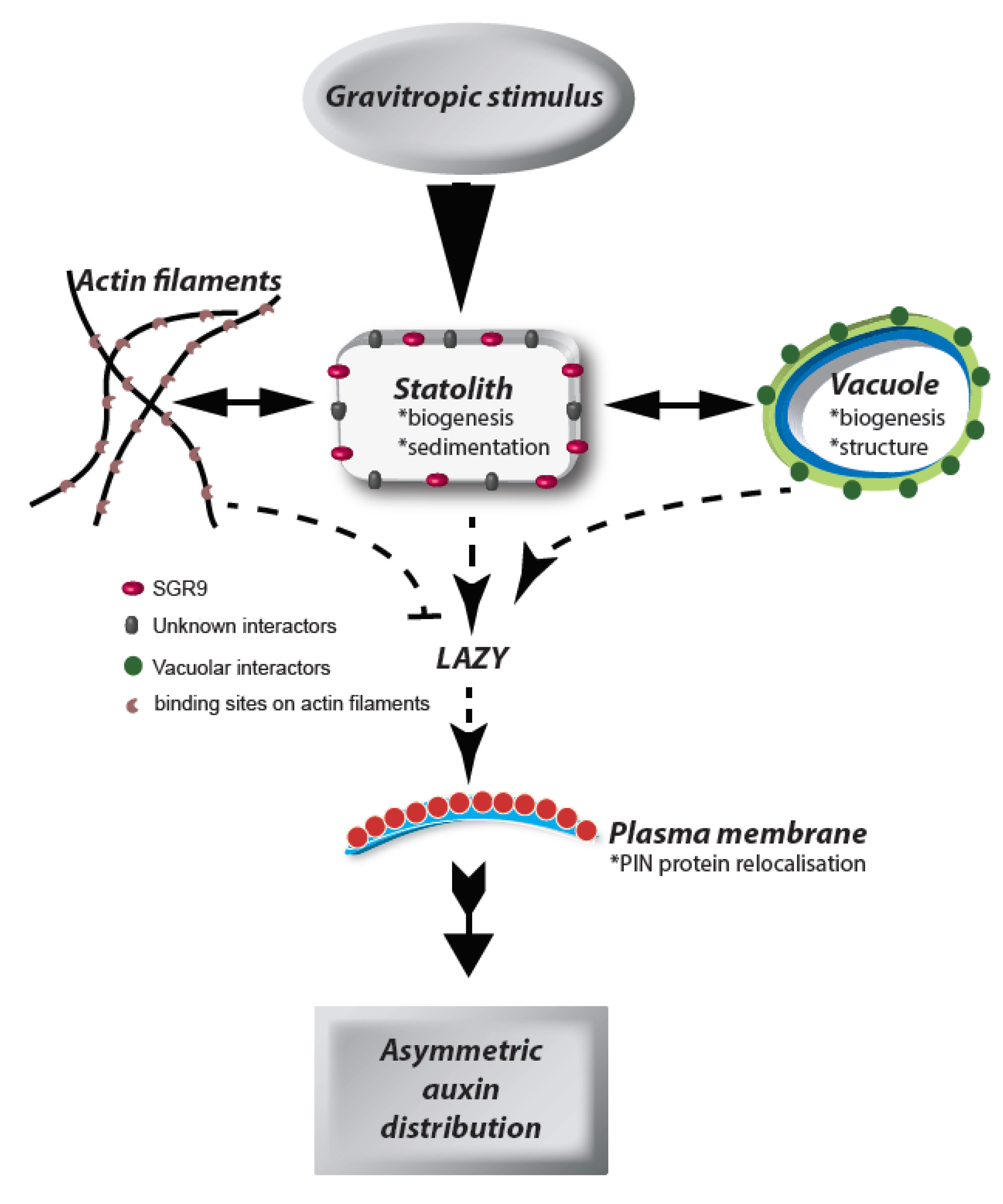
1. Introduction
2. Auxin as a Mobile Signal
3. The Statolith
4. Statoliths and the Vacuolar Membrane
5. Statolith Movement
6. Outlook
Funding
Conflicts of Interest
References
- Nakamura, M.; Nishimura, T.; Morita, M.T. Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot. 2019, 70, 3495–3506. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; Marchant, A.; Green, H.G.; May, S.T.; Ward, S.P.; Millner, P.A.; Walker, A.R.; Schulz, B.; Feldmann, K.A. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 1996, 273, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Ottenschlager, I.; Wolff, P.; Wolverton, C.; Bhalerao, R.P.; Sandberg, G.; Ishikawa, H.; Evans, M.; Palme, K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Walker, L.M.; Young, J.C.; Sonawala, A.; Timpte, C.; Estelle, M.; Reed, J.W. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000, 123, 563–573. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Aloni, R.; Langhans, M.; Aloni, E.; Ullrich, C.I. Role of cytokinin in the regulation of root gravitropism. Planta 2004, 220, 177–182. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Vaseva, I.; Vissenberg, K.; Van Der Straeten, D. Ethylene in vegetative development: A tale with a riddle. New Phytol. 2012, 194, 895–909. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.; Tian, L.; Liu, L.; Huang, M.; Xu, X.; Song, G.; Wu, P.; Sato, S.; Jiang, H.; et al. The LjLAZY3 gene plays a distinct role in the positive root gravitropism in Lotus japonicus. J. Exp. Bot 2019. [Google Scholar] [CrossRef]
- Park, C.H.; Seo, C.; Park, Y.J.; Youn, J.H.; Roh, J.; Moon, J.; Kim, S.K. BES1 directly binds to the promoter of the ACC oxidase 1 gene to regulate gravitropic response in the roots of Arabidopsis thaliana. Plant Signal. Behav. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nziengui, H.; Lasok, H.; Kochersperger, P.; Ruperti, B.; Rébeillé, F.; Palme, K.; Ditengou, F.A. Root Gravitropism Is Regulated by a Crosstalk between para-Aminobenzoic Acid, Ethylene, and Auxin. Plant Physiol. 2018, 178, 1370–1389. [Google Scholar] [CrossRef] [PubMed]
- Rakusova, H.; Gallego-Bartolome, J.; Vanstraelen, M.; Robert, H.S.; Alabadi, D.; Blazquez, M.A.; Benkova, E.; Friml, J. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J. 2011, 67, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Driss-Ecole, D.; Lefranc, A.; Perbal, G. A polarized cell: The root statocyte. Physiol. Plant. 2003, 118, 305–312. [Google Scholar] [CrossRef]
- Mays, R.W.; Beck, K.A.; James Nelson, W. Organization and function of the cytoskeleton in polarized epithelial cells: A component of the protein sorting machinery. Curr. Opin. Cell Biol. 1994, 6, 16–24. [Google Scholar] [CrossRef]
- Edelmann, H.G. Graviperception in maize plants: Is amyloplast sedimentation a red herring? Protoplasma 2018, 255, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Strauch, S.M.; Lebert, M. Disproval of the Starch-Amyloplast Hypothesis? Trends Plant Sci. 2019, 24, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Blancaflor, E.B. The cytoskeleton and gravitropism in higher plants. J. Plant Growth Regul. 2002, 21, 120–136. [Google Scholar] [CrossRef]
- Morita, M.T. Directional gravity sensing in gravitropism. Annu Rev. Plant Biol 2010, 61, 705–720. [Google Scholar] [CrossRef]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenberg, K.; Swarup, R. New insights into root gravitropic signalling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef]
- White, R.G.; Sack, F.D. Actin Microfilaments in the Presumptive Statocytes of Grass Coleoptiles. J. Cell Biol. 1986, 103, A110. [Google Scholar]
- Sack, F.D.; Leopold, A.C. Cytoplasmic Streaming Affects Gravity-Induced Amyloplast Sedimentation in Maize Coleoptiles. Planta 1985, 164, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sack, F.D.; Suyemoto, M.M.; Leopold, A.C. Amyloplast Sedimentation and Organelle Saltation in Living Corn Columella Cells. Am. J. Bot. 1986, 73, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Ma, X.; Yang, Z.; Pang, C.; Yu, J.; Wang, G.; Friml, J.; Xiao, G. Auxin-mediated statolith production for root gravitropism. New Phytol. 2019, 224, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, G.; Wang, X.; Zhang, X.; Friml, J. Evolution of fast root gravitropism in seed plants. Nat. Commun. 2019, 10, 3480. [Google Scholar] [CrossRef]
- Saito, C.; Morita, M.T.; Kato, T.; Tasaka, M. Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 2005, 17, 548–558. [Google Scholar] [CrossRef]
- Morita, M.T.; Kato, T.; Nagafusa, K.; Saito, C.; Ueda, T.; Nakano, A.; Tasaka, M. Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 2002, 14, 47–56. [Google Scholar] [CrossRef]
- Bérut, A.; Chauvet, H.; Legué, V.; Moulia, B.; Pouliquen, O.; Forterre, Y. Gravisensors in plant cells behave like an active granular liquid. Proc. Natl. Acad. Sci. USA 2018, 115, 5123–5128. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Hertel, R.; Sack, F.D. Amyloplasts Are Necessary for Full Gravitropic Sensitivity in Roots of Arabidopsis-Thaliana. Planta 1989, 177, 198–206. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Wright, J.B.; Caspar, T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol. Plant. 1996, 97, 237–244. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef]
- Weise, S.E.; Kiss, J.Z. Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int. J. Plant Sci. 1999, 160, 521–527. [Google Scholar] [CrossRef]
- Toyota, M.; Ikeda, N.; Sawai-Toyota, S.; Kato, T.; Gilroy, S.; Tasaka, M.; Morita, M.T. Amyloplast displacement is necessary for gravisensing in Arabidopsis shoots as revealed by a centrifuge microscope. Plant J. 2013, 76, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Toyota, M.; Tasaka, M.; Morita, M.T. An Arabidopsis E3 Ligase, SHOOT GRAVITROPISM9, Modulates the Interaction between Statoliths and F-Actin in Gravity Sensing. Plant Cell 2011, 23, 1830–1848. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Wisniewska, J.; Benkova, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Vehn, J.; Ding, Z.; Jones, A.R.; Tasaka, M.; Morita, M.T.; Friml, J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22344–22349. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Sonoda, H.; Mizoguchi, T.; Aoki, J.; Arai, H.; Nagahama, M.; Tagaya, M.; Tani, K. A novel phospholipase A(1) with sequence homology to a mammalian Sec23p-interacting protein, p125. J. Biol. Chem. 2002, 277, 11329–11335. [Google Scholar] [CrossRef]
- Shimoi, W.; Ezawa, I.; Nakamoto, K.; Uesaki, S.; Gabreski, G.; Aridor, M.; Yamamoto, A.; Nagahama, M.; Tagaya, M.; Tani, K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J. Biol. Chem. 2005, 280, 10141–10148. [Google Scholar] [CrossRef]
- Tani, K.; Mizoguchi, T.; Iwamatsu, A.; Hatsuzawa, K.; Tagaya, M. P125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J. Biol. Chem. 1999, 274, 20505–20512. [Google Scholar] [CrossRef]
- Nakamura, M.; Nishimura, T.; Morita, M.T. Bridging the gap between amyloplasts and directional auxin transport in plant gravitropism. Curr. Opin. Plant Biol. 2019, 52, 54–60. [Google Scholar] [CrossRef]
- Taniguchi, M.; Furutani, M.; Nishimura, T.; Nakamura, M.; Fushita, T.; Iijima, K.; Baba, K.; Tanaka, H.; Toyota, M.; Tasaka, M.; et al. The Arabidopsis LAZY1 Family Plays a Key Role in Gravity Signaling within Statocytes and in Branch Angle Control of Roots and Shoots. Plant Cell 2017, 29, 1984–1999. [Google Scholar] [CrossRef]
- Yoshihara, T.; Spalding, E.P. LAZY Genes Mediate the Effects of Gravity on Auxin Gradients and Plant Architecture. Plant Physiol. 2017, 175, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Romano, L.E.; De Pascale, S.; Mele, G.; Gargiulo, L.; Aronne, G. Chemotropic vs Hydrotropic Stimuli for Root Growth Orientation in Microgravity. Front. Plant Sci. 2019, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Ampudia, C.S.; Julkowska, M.M.; Darwish, E.; Gandullo, J.; Korver, R.A.; Brunoud, G.; Haring, M.A.; Munnik, T.; Vernoux, T.; Testerink, C. Halotropism is a response of plant roots to avoid a saline environment. Curr. Biol. 2013, 23, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yamazaki, Y.; Kobayashi, A.; Higashitani, A.; Takahashi, H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol. 2003, 132, 805–810. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, W.; Hu, H.; Li, B.; Wang, Y.; Zhao, Y.; Li, K.; Liu, M.; Li, X. Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. Plant Physiol. 2008, 146, 178–188. [Google Scholar] [CrossRef]
- Bizet, F.; Pereda-Loth, V.; Chauvet, H.; Gerard, J.; Eche, B.; Girousse, C.; Courtade, M.; Perbal, G.; Legue, V. Both gravistimulation onset and removal trigger an increase of cytoplasmic free calcium in statocytes of roots grown in microgravity. Sci. Rep. 2018, 8, 11442. [Google Scholar] [CrossRef]
- Wendt, M.; Sievers, A. The polarity of statocytes and the gravisensitivity of roots are dependent on the concentration of calcium in statocytes. Plant Cell Physiol. 1989, 30, 929–932. [Google Scholar] [CrossRef]
- Staves, M.P. Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta 1997, 203, S79–S84. [Google Scholar] [CrossRef]
- Hodick, D.; Buchen, B.; Sievers, A. Statolith positioning by microfilaments in Chara rhizoids and protonemata. Adv. Space Res. Ser. 1998, 21, 1183–1189. [Google Scholar] [CrossRef]
- Braun, M. Gravitropism in tip-growing cells. Planta 1997, 203, S11–S19. [Google Scholar] [CrossRef]
- Wayne, R.; Staves, M.P. A down to earth model of gravisensing or Newton’s Law of Gravitation from the apple’s perspective. Physiol. Plant. 1996, 98, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Czapek, F. Untersuchungen über Geotropismus; Jahrbuch der Wissenschaftlichen Botanik; Gebrüder Borntraeger: Berlin, Germany, 1895; Volume 27, pp. 243–339. [Google Scholar]
- Chebli, Y.; Geitmann, A. Gravity research on plants: Use of single-cell experimental models. Front. Plant Sci. 2011, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, D.; Zhuang, F.Y. The force induced by organelles’ weight in the microfilament is in the range of 0.1–1 pN. Acta Astronaut. 2008, 63, 923–928. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditengou, F.A.; Teale, W.D.; Palme, K. Settling for Less: Do Statoliths Modulate Gravity Perception? Plants 2020, 9, 121. https://doi.org/10.3390/plants9010121
Ditengou FA, Teale WD, Palme K. Settling for Less: Do Statoliths Modulate Gravity Perception? Plants. 2020; 9(1):121. https://doi.org/10.3390/plants9010121
Chicago/Turabian StyleDitengou, Franck Anicet, William David Teale, and Klaus Palme. 2020. "Settling for Less: Do Statoliths Modulate Gravity Perception?" Plants 9, no. 1: 121. https://doi.org/10.3390/plants9010121
APA StyleDitengou, F. A., Teale, W. D., & Palme, K. (2020). Settling for Less: Do Statoliths Modulate Gravity Perception? Plants, 9(1), 121. https://doi.org/10.3390/plants9010121





