The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro
Abstract
1. Introduction
2. Results and Discussion
2.1. Distribution of Metals in the Sediment and the Plants
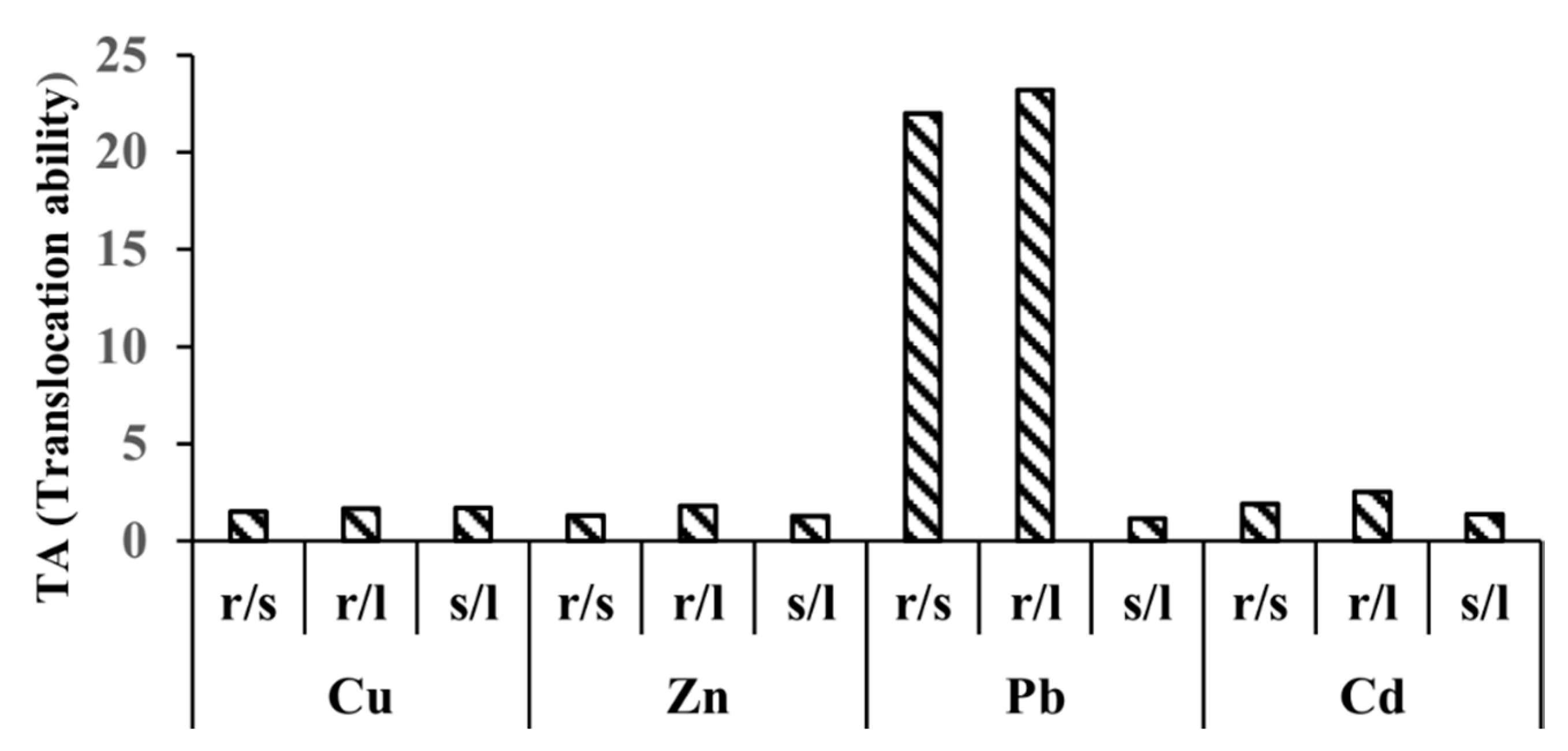
2.2. Translocation Ability (TA)
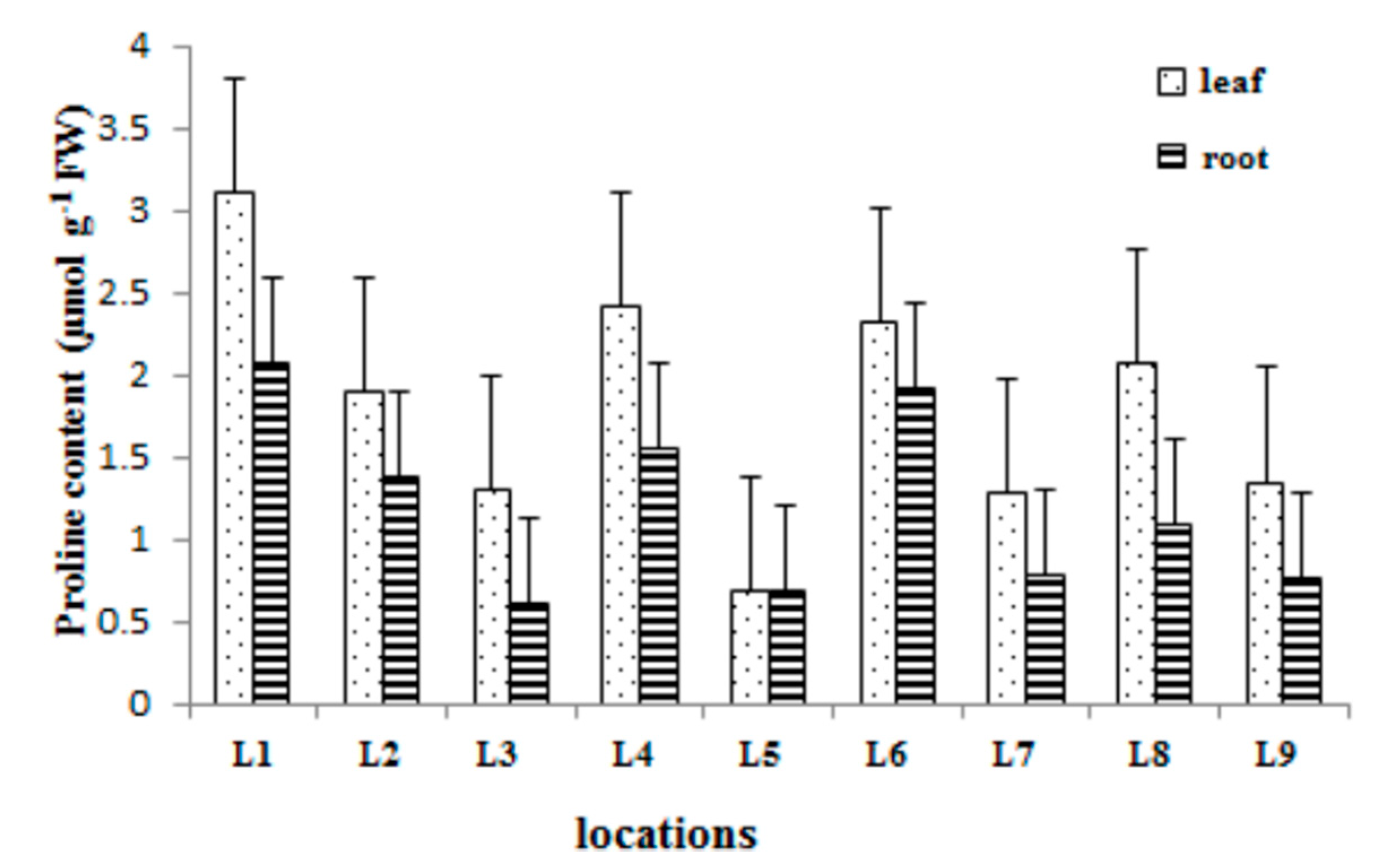
2.3. Proline Content
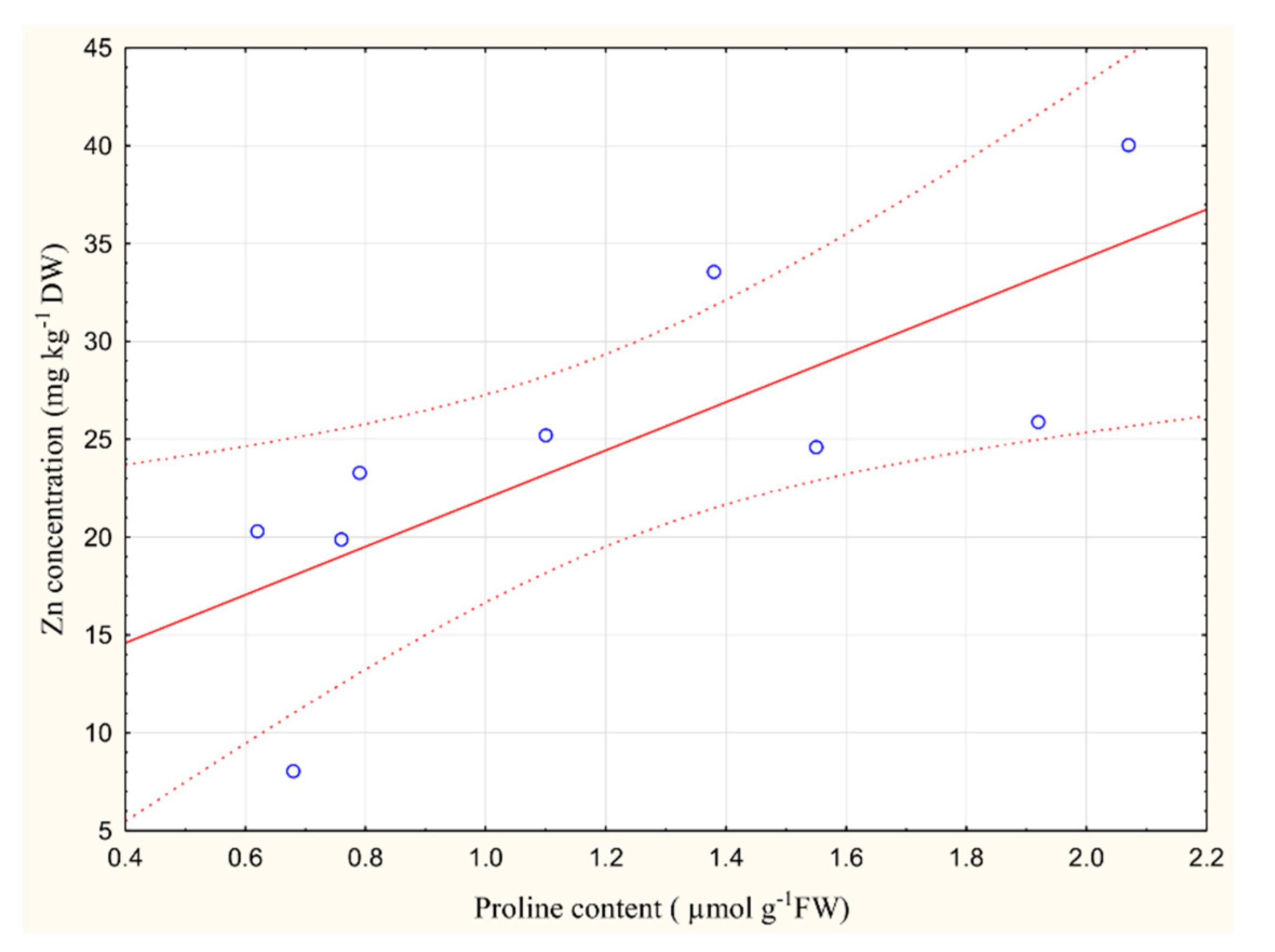
2.4. The Relationship between Metal Content in the Plant and Proline Accumulation
2.5. Chlorophyll Content
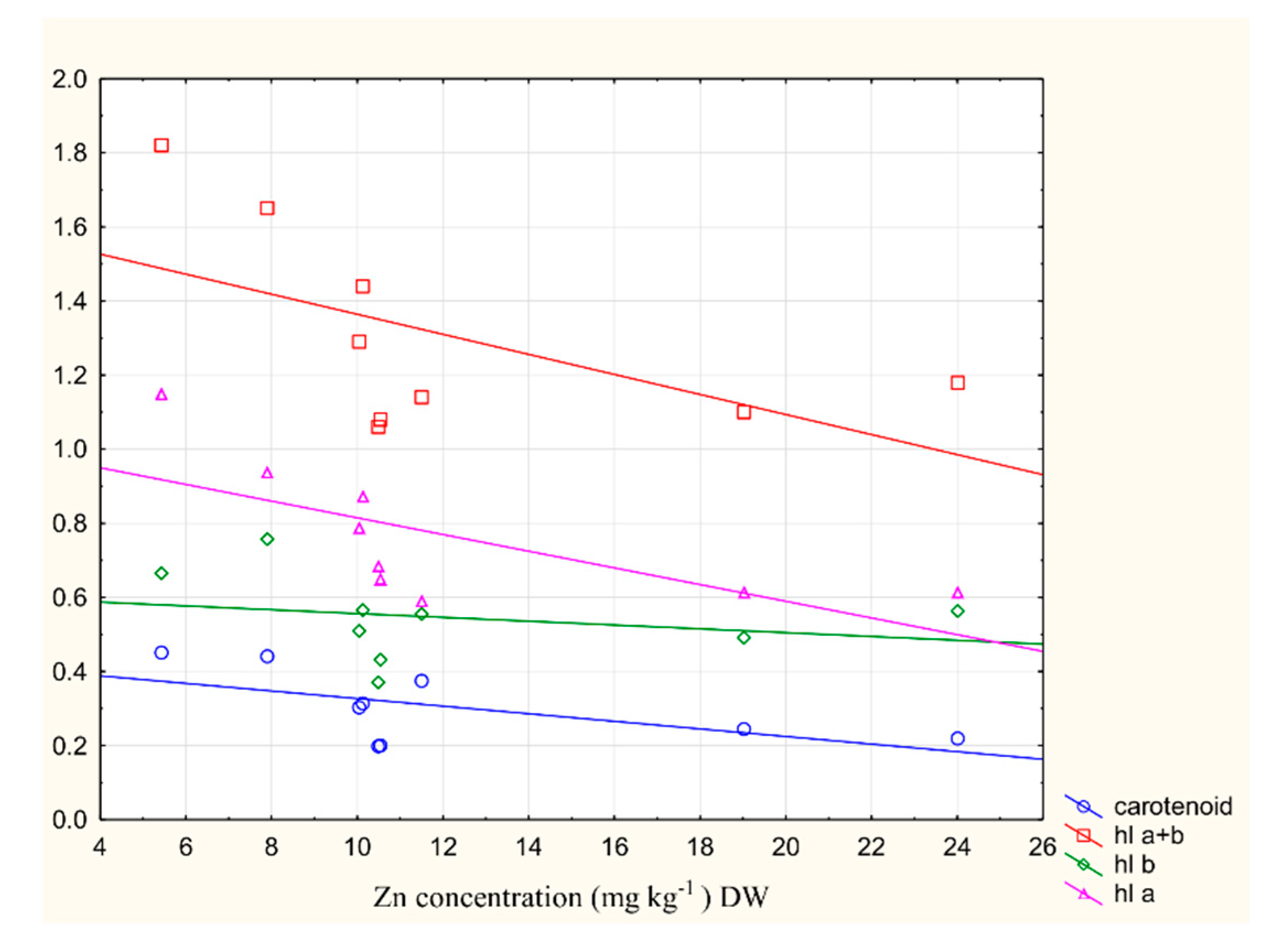
2.6. The Relationship between Metal Content and Chlorophyll Content in the Plant
3. Materials and Methods
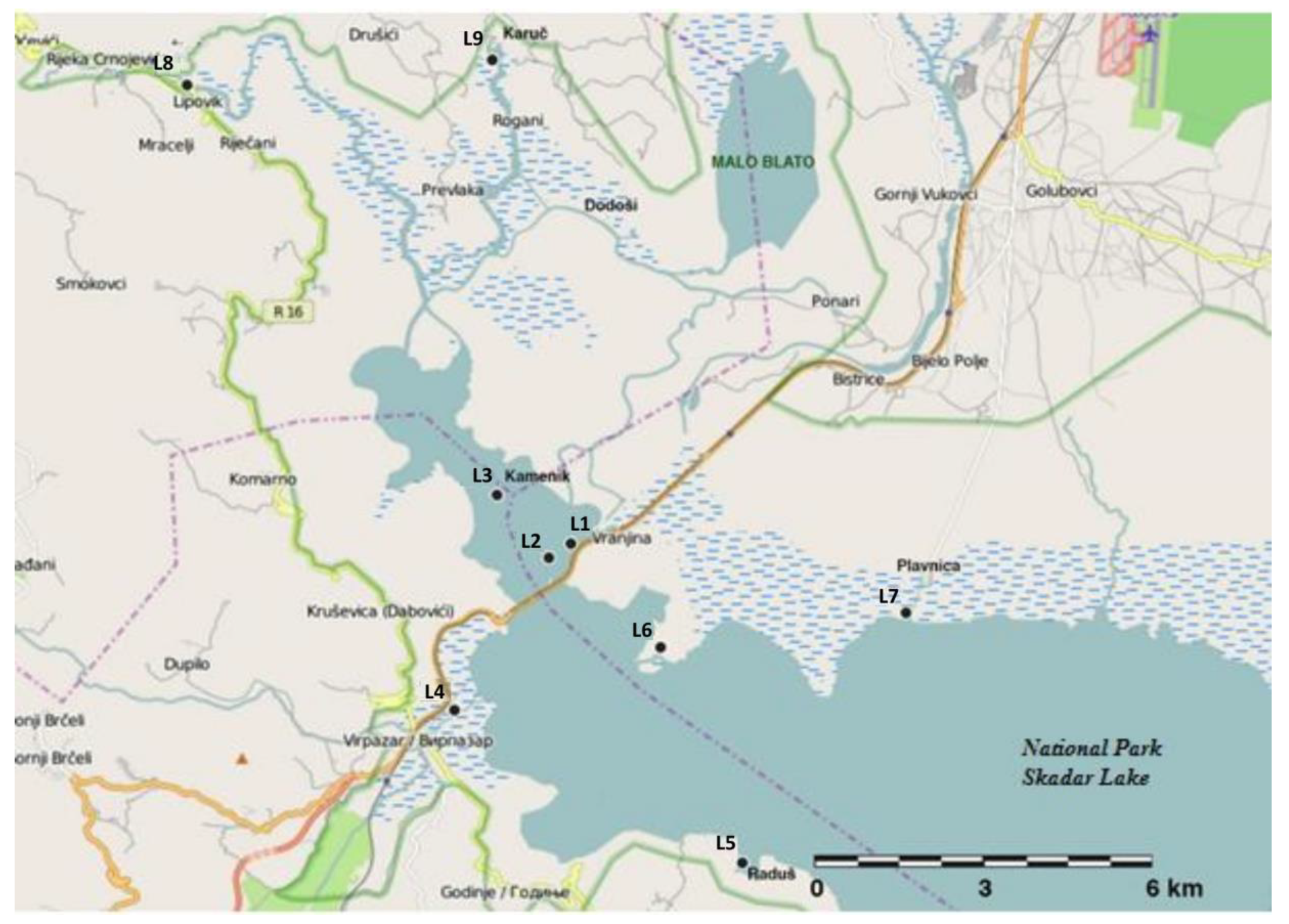
3.1. Sample Collection
3.2. Metal Analysis
3.3. Calculation of Translocation Ability
3.4. Determination of Proline
3.5. Photosynthetic Pigment Determination in Leaves
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular echanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- An, Y.J.; Kim, Y.M.; Kwon, T.I.; Jeong, S.W. Combined effects of copper, cadmium, and lead upon Cucumis sativus growth and bioaccumulation. Sci. Total Environ. 2004, 326, 85–93. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil End Environ. 2008, 54, 262–270. [Google Scholar] [CrossRef]
- Crowder, A. Acidification of metal and macrophytes. Environ. Pollut. 1991, 71, 171–203. [Google Scholar] [CrossRef]
- Nayek, S.; Gupta, S.; Saha, R. Effects of metal stress on biochemical response of some aquatic macrophytes growing along an industrial waste discharge channel. J. Plant Interact. 2010, 5, 91–99. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 17, 1–19. [Google Scholar] [CrossRef]
- Pandey, N.; Patkak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef]
- Pongrac, P.; Zhao, F.J.; Razinger, J.; Zrimec, A.; Regvar, M. Physiological responses to Cd and Zn in two Cd/Zn hyperaccumulating Thlapsi species. Environ. Exp. Bot. 2009, 66, 479–486. [Google Scholar] [CrossRef]
- Nadgorska-Socha, A.; Ptasinski, B.; Kita, A. Heavy metal bioaccumulation and antioxidative responses in Cardominopsis arenosa and Plantago lanceolate leaves from metalliferous and non- metalliferous sites.a filed study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolinska, B.; Leszczynska, J.; Fotopouls, V. Influnece of Heavy metals (Ni, Cu and Zn) on Nitro-Oxidative Stress Responses, Proteome Regulation and Allergen Productio in Basil (Ocimum basilicum L.) Plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef]
- Singh, T.N.; Aspinall, D.; Paleg, L.G.; Boggess, S.F. Stress metabolism II. Changes in proline concentration in excised plant tissues. Aust. J. Biol. Sci. 1973, 26, 57–63. [Google Scholar] [CrossRef]
- Bassi, R.; Sharma, S.S. Proline accumulation in wheat seedlings exposed to zinc and copper. Phytochemistry 1993, 33, 1339–1342. [Google Scholar] [CrossRef]
- Bhupinder, D.; Sharmila, P.; Saradhi, P. Hidrophytes lack potential to exhibit Cadmium stress induced enhancement in lipid peroxidation and accumulation of Proline. Aquat. Toxicol. 2003, 66, 141–147. [Google Scholar]
- Kumar, M.; Chikara, S.; Chand, M.K.; Bhatnagar, A.K. Accumulation of lead, cadmium, zinc and copper in the edible aquatic plants Trapa bispinosa Roxb. and Nelumbo nucifera Gaertn. Bull. Environ. Contam. Toxicol. 2002, 69, 649–654. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.; Singh, R.P. The suitability of Trapa natans L. for phytoremediation of inorganic contaminants from the aquatic. Ecol. Eng. 2015, 93, 39–42. [Google Scholar]
- Kumar, V.; Chopra, A.K. Phytoremediation potential of water caltrop (Trapa natans L.) Using municipal wastewater of activated sludge process based municipal wastewater treatment plant. Environ. Technol. 2017, 39, 12–23. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.L.; Yuceer, A.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochem. 2003, 39, 179–183. [Google Scholar] [CrossRef]
- Lesagea, E.; Mundia, C.; Rousseaub, D.P.L.; Van de Moortela, A.M.K.; Lainga, G.D.; Meersa, E. Sorption of Co, Cu, Ni and Zn from industrial effluents by the submerged aquatic macrophyte Myriophyllum spicatum L. Ecol. Eng. 2007, 30, 320–325. [Google Scholar] [CrossRef]
- Rai, P.K.; Tripathi, B.D. Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from G.B. Pant Sagar of Singrauli Industrial region, India. Environ. Onitoring Assess. 2009, 148, 75–84. [Google Scholar] [CrossRef]
- Stešević, D.; Feiler, U.; Šundić, D.; Mijović, S.; Erdinger, L.; Seiler, T.M.; Heininger, P.; Hollert, H. Application of a new sediment contact test with Myriophyllum aquaticum and of the aquatic Lemna test to assess the sediment quality of Lake Skadar. J. Soils Sediments 2007, 7, 342–349. [Google Scholar] [CrossRef]
- Kastratović, V.; Krivokapić, S.; Đurović, D.; Blagojević, N. Sesonal changes in metal accumulation and distribution in the organs of Phragmites australis (common reed) from Lake Skadar, Montenegro. J. Serb. Chem. Soc. 2013, 78, 1241–1258. [Google Scholar] [CrossRef]
- Kastratović, V.; Krivokapić, S.; Bigović, M.; Đurović, D.; Blagojević, N. Bioaccumulation and translocation of heavy metals by Ceratophyllum demersum from the Skadar Lake, Montenegro. J. Serb. Chem. Soc. 2014, 79, 1445–1460. [Google Scholar] [CrossRef]
- Kastratović, V.; Bigović, M.; Jaćimović, Ž.; Kosović, M.; Đurović, D.; Krivokapić, S. Levels and distribution of cobalt and nickel in the aquatic macrophytes found in Skadar Lake, Montenegro. Environ. Sci. Pollut. Res. 2018, 25, 26823–26830. [Google Scholar] [CrossRef] [PubMed]
- Vemić, M.; Rousseau, D.; Du Laing, G.; Lens, P. Distribution and fate of metals in the Montenegrian part of Skadar Lake. Int. J. Sediment Res. 2014, 29, 357–367. [Google Scholar] [CrossRef]
- Petrović, D.; Jančić, D.; Furdek, M.; Mikac, N. Aquatic plant Trapa natans L., as bioindicator of trace metal contamination in freshwater lake (Skadar Lake, Montenegro). Acta Bot. Croat. 2016, 75, 236–243. [Google Scholar] [CrossRef]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evalution of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Schreck, E.; Foucault, Y.; Sarret, G.; Sobanska, S.; Cecillon, L.; Castrec-Rouelle, M.; Uzu, G.; Dumat, C. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012, 427–428, 253–262. [Google Scholar] [CrossRef]
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A. Heavy metals in aquatic plants and sesiments from water systems in Macedonia, Greece. Ecotoxicol. Environ. Saf. 1995, 32, 73–80. [Google Scholar] [CrossRef]
- Hoseinizadeh, G.R.; Azarpour, E.; Motamed, M.K.; Ziaeidoustan, H.; Moraditocheae, M.; Bozorgi, H.R. Heavy metals phyttoremediation Management via Organs of Aquatic plants of Anzali International Lagoon (Iran). World Appl. Sci. J. 2011, 14, 711–715. [Google Scholar]
- Baldantoni, D.; Alfani, A.; Di Tommasi, P.; Bartoli, G.; De Santo, A.V. Assessment of macro and microelement accumulation capability of two aquatic plants. Environ. Pollut. 2004, 130, 149–156. [Google Scholar] [CrossRef]
- Mazej, Z.; Germ, M. Trace element accumulation and distribution in four aquatic macrophytes. Chemosphere 2009, 74, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Naresh Bharagava, R.; Kumar, V.; Singh, A.; Dhusia, N.; More, N. Role of macrophytes in heavy metal removal through rhizo-filtration in aquatic ecosystem. Eur. J. Biotechnol. Biosci. 2016, 4, 15–20. [Google Scholar]
- Dinkar, N.; Nagajyothi, P.C.; Suresh, S.; Damodharam, T.; Suresh, C. Cadmium induced changes on proline, antioxidant enzymes, nitrate and nitrite reductases in Arachis hypogaea L. J. Environ. Biol. 2009, 30, 289–294. [Google Scholar]
- Handique, G.K.; Handique, A.K. Proline accumulation i lemongrass (Cymbopogon flexuosus Stapf.) due to heavy metal stress. J. Environ. Biol. 2009, 30, 299–302. [Google Scholar] [PubMed]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chang, C.C. Effects of Copper and Cadmium on Heavy Metal Polluted Waterbody Restoration by Duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Walker, P.L. Ecophysiology of metal uptake by tolerant plants: Heavy metal tolerance in plants. In Evolutionary Aspects; Shaw, A.J., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 155–177. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll letale and der sub-mikroskopishe formweschselder plastiden. Exp. Cell Res. 1957, 12, 427. [Google Scholar] [CrossRef]
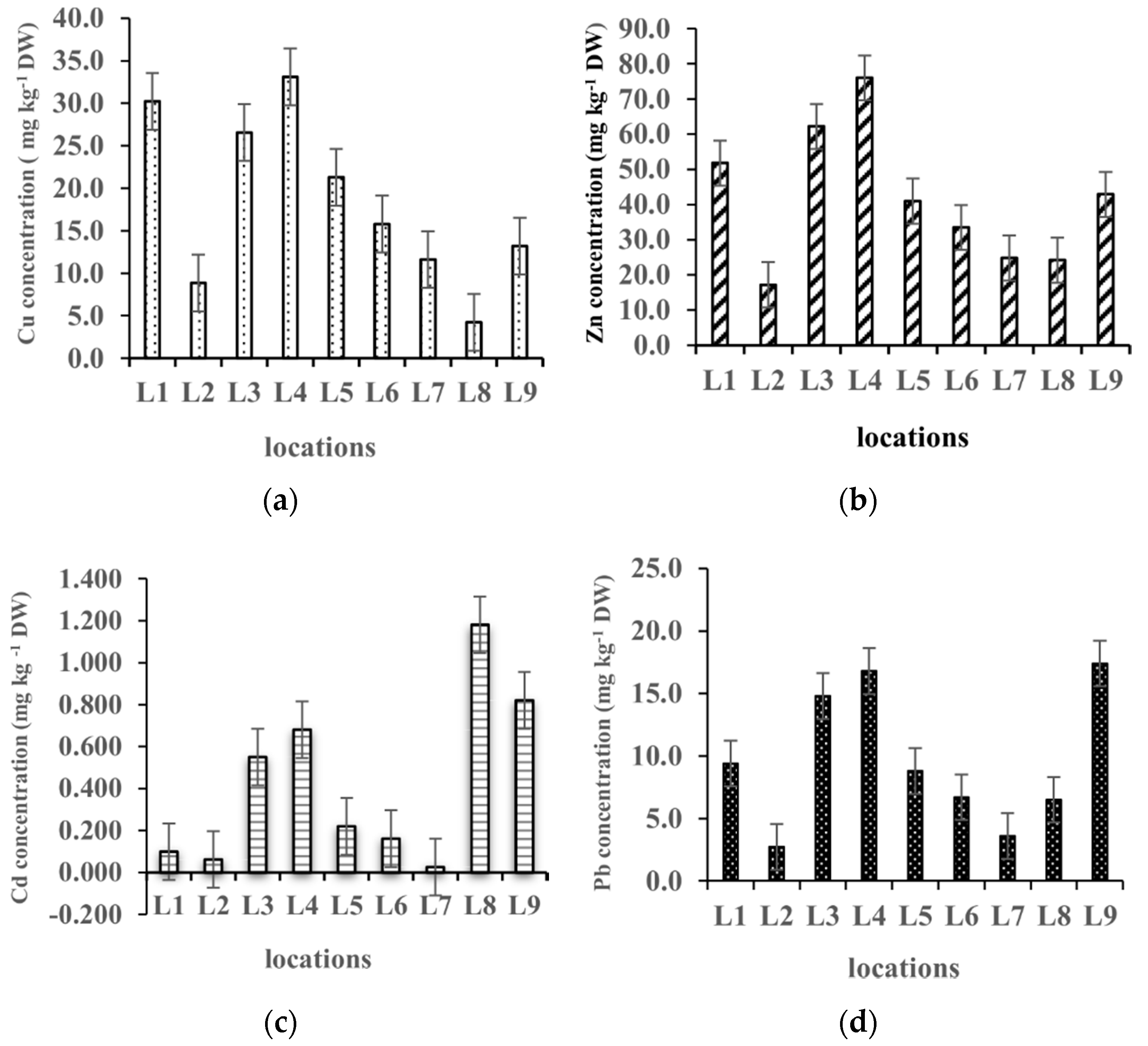



| Metal | Min–Max (mg kg−1) | Average ± SD | CV (%) | |
|---|---|---|---|---|
| Cu | root | 2.62–5.03 | 3.949 ± 0.700 a | 17.73 |
| stem | 1.82–4.66 | 2.906 ± 0.980 ab | 33.72 | |
| leaf | 1.79–3.67 | 2.473 ± 0.620 b | 25.07 | |
| Zn | root | 8.03–42.03 | 24.53 ± 8.925 a | 36.38 |
| stem | 9.66–26.02 | 18.04 ± 4.906 ab | 27.20 | |
| leaf | 5.43–24.31 | 12.11 ± 5.759 b | 47.56 | |
| Cd | root | 0.066–1.12 | 0.210 ± 0.320 ac | 152.38 |
| stem | 0.042–0.373 | 0.092 ± 0.101 ab | 109.78 | |
| leaf | 0.032–0.287 | 0.073 ± 0.075 b | 102.74 | |
| Pb | root | 0.030–3.684 | 0.113 ± 0.123 a | 108.85 |
| stem | 0.060–0.396 | 0.079 ± 0.069 b | 87.34 | |
| leaf | 0.060–0.396 | 1.194 ± 1.177 b | 98.58 | |
| Location | Description | Latitude and Longitude |
|---|---|---|
| L1 | Inflow at the right branch of the River Morača | 42°27′70″ N; 19°12′32″ E |
| L2 | Small lake at the right branch of the River Morača | 42°27′57″ N; 19°12′15″ E |
| L3 | Kamenik | 42°28′54″ N; 19°10′ 25″ E |
| L4 | Milovića Bay | 42°26′ 22″ N; 19°10′77″ E |
| L5 | Underwater spring at Raduš, | 42°22′79″ N; 19°12′94″ E |
| L6 | Inflow at the left branch of the River Morača | 42°25′84″ N; 19°13′46″ E |
| L7 | Inflow of the River Plavnica | 42°26′55″ N; 19°19′80″ E |
| L8 | Crnojevića River | 42°35′23″ N; 19°03′99″ E |
| L9 | Karuč | 42°35′81″ N; 19°10′71″ E |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic, D.; Krivokapic, S. The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants 2020, 9, 1287. https://doi.org/10.3390/plants9101287
Petrovic D, Krivokapic S. The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants. 2020; 9(10):1287. https://doi.org/10.3390/plants9101287
Chicago/Turabian StylePetrovic, Dragana, and Sladjana Krivokapic. 2020. "The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro" Plants 9, no. 10: 1287. https://doi.org/10.3390/plants9101287
APA StylePetrovic, D., & Krivokapic, S. (2020). The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants, 9(10), 1287. https://doi.org/10.3390/plants9101287






