Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition
Abstract
:1. Introduction
2. The Importance of Wild Food Plants Today
2.1. Diversity (Geographical Use) and Contribution to Diets
2.1.1. Africa
2.1.2. South America
2.1.3. The Mediterranean
2.1.4. Asia Pacific
2.2. Income Generation
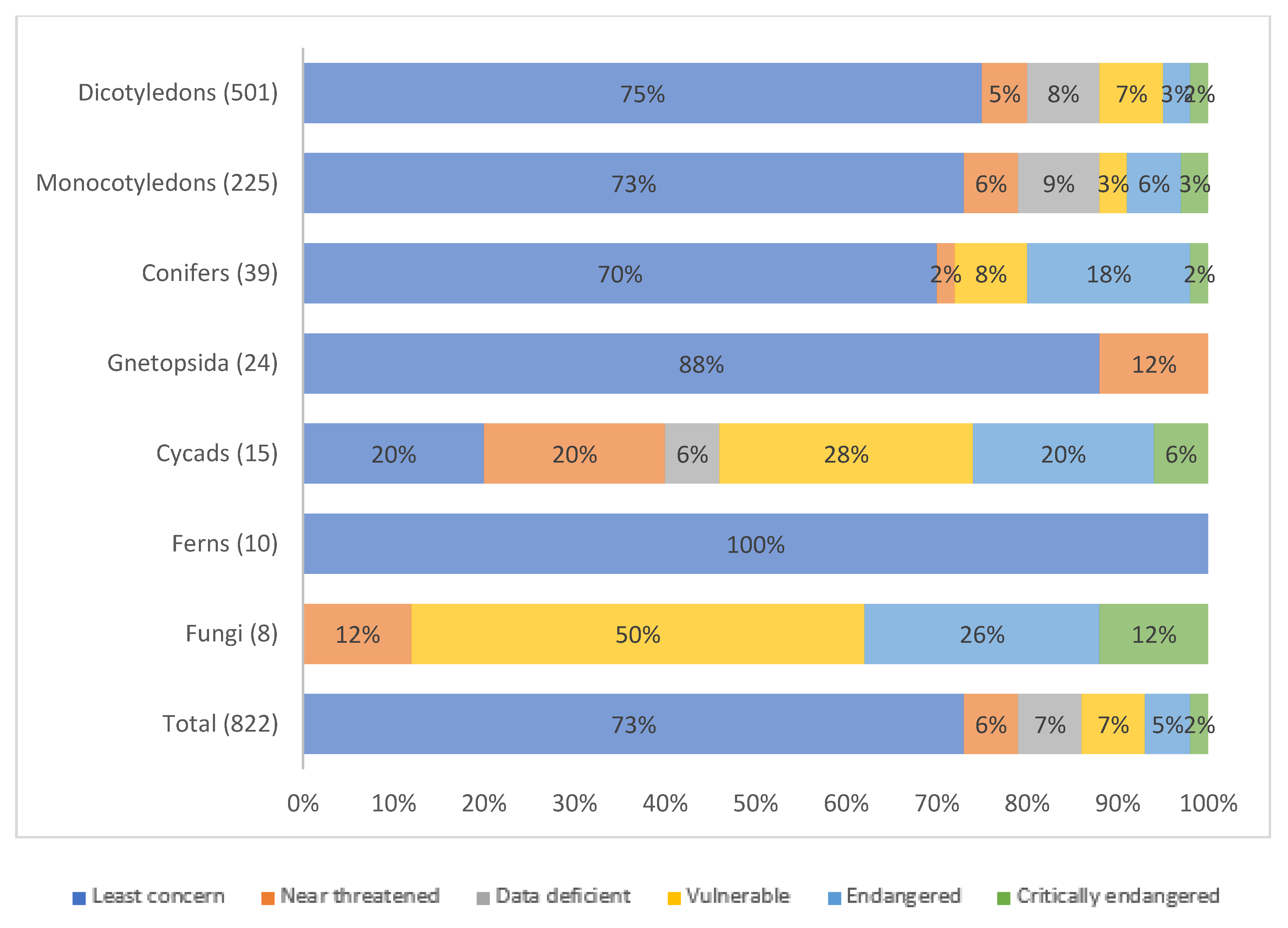
2.3. Threats to WFPs
3. Barriers to the Greater Use of WFPs
- lack of information about the extent of their use and importance in rural economies;
- lack of information, especially statistics, concerning the economic value of WFPs;
- lack of reliable methods for measuring their contribution to farm households and the rural economy;
- lack of information on the sustainability of current harvest levels;
- poorly developed infrastructure and markets for WFPs, with the exception of small number of products (e.g., Açaí berries);
- unevenness of supply;
- lack of quality standards;
- general lack of storage and processing technology;
- availability of substitutes;
- policies and research mostly favoring commodity crops and commercial agriculture.
3.1. Contribution to Nutrition and Diets
3.2. Gathering Grounds, Collection Practices and Use
4. An Integrated Approach for Conserving and Sustainably Using WFPs
4.1. Identify and Prioritize
4.2. The Nutritional Importance of WFPs and Associated Traditional Knowledge
4.3. Collecting, Storing and Maintaining WFP Diversity
4.4. Domestication Programmes and Guidelines for Sustainable Collection
4.5. Strengthening Policies in Support of WFP Conservation and Sustainable Use
4.6. Raising Public Awareness of the Importance of WFPs
4.6.1. Youth
4.6.2. Communities and Households
4.6.3. Policymakers
4.6.4. Broader Audiences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BFN | Biodiversity for Food and Nutrition Project |
| CAP | Community action plans |
| CBD | Convention on Biological Diversity |
| CBO | Community-based organization |
| CIAT | International Center for Tropical Agriculture now part of the Alliance of Bioversity International and CIAT |
| EIARD | European Initiative for Agricultural Research for Development |
| FAD | Food, Agrobiodiversity and Diet Project |
| FAO | Food and Agriculture Organization of the United Nations |
| GEF | Global Environment Facility |
| GPA | Global Plan of Action for Plant Genetic Resources for Food and Agriculture of the FAO |
| IKIAM | Universidad Regional Amazónica - Amazon Regional University (Ecuador) |
| IUCN | International Union for Conservation of Nature |
| MAPA | Ministério da Agricultura, Pecuária e Abastecimento - Ministry of Agriculture, Livestock and Supply (Brazil) |
| NBSAPs | National Biodiversity Strategies and Action Plan (of the CBD) |
| NGO | Non-governmental organization |
| PAA | Programa de Aquisição de Alimentos - Food Procurement Program (Brazil) |
| PNAE | Programa nacional de alimentação escolar - National School Feeding Program (Brazil) |
| PNG | Papua New Guinea |
| PPF | Plantas Paro o Futuro (Plants for the Future Initiative – Brazil) |
| RAE | Retinol Activity Equivalents |
| R&D | Research and Development |
| SDG | Sustainable Development Goal |
| SOWBFA | State of the World’s Biodiversity for Food and Agriculture of the FAO |
| UNEP | UN Environment Programme |
| UPP | Useful Plants Project, Kew |
| WFPs | Wild food plants |
| WLVs | Wild leafy vegetables |
References
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World. The Origin and Spread of Domesticated Plants in South-West Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Sowunmi, M.A. The beginnings of agriculture in West Africa: Botanical evidence. Curr. Anthropol. 1985, 26, 127–129. [Google Scholar] [CrossRef]
- Johnson, A.; Behrens, C.A. Nutritional criteria in machiguenga food production decisions: A linear-programming analysis. Hum. Ecol. 1982, 10, 167–189. [Google Scholar] [CrossRef]
- Heywood, V.H. Use and Potential of Wild Plants in Farm Households; FAO Farm System Management Series; FAO: Rome, Italy, 1999; Volume 15. [Google Scholar]
- Bharucha, Z.; Pretty, J. The roles and values of wild foods in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2913–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, A.; Otero-Arnaiz, A.; Perez-Negron, E.; Valiente-Banuet, A. In situ management and domestication of plants in Mesoamerica. Ann. Bot. 2007, 100, 1101–1115. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Garcia, G.S. Management and motivations to manage “wild” food plants. A case study in a mestizo village in the amazon deforestation frontier. Front. Ecol. Evol. 2017, 5, 127. [Google Scholar] [CrossRef] [Green Version]
- Heywood, V.H. Overview of Agricultural Biodiversity and Its Contribution to Nutrition and Health. In Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health; Fanzo, J., Hunter, D., Borelli, T., Mattei, F., Eds.; Routledge: London, UK, 2013; pp. 35–67. [Google Scholar]
- Commission on Genetic Resources for Food and Agriculture—FAO. Biodiversity for Food and Agriculture—Revised Draft—Needs and Possible Actions CGRFA/NFP-BFA-1/18/2; FAO: Rome, Italy, 2018. [Google Scholar]
- Asprilla-Perea, J.; Díaz-Puente, J.M. Importance of wild foods to household food security in tropical forest areas. Food Secur. 2019, 11, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Bioversity International. Mainstreaming Agrobiodiversity in Sustainable Food Systems: Scientific Foundations for an Agrobiodiversity Index, 1st ed.; Bioversity International: Rome, Italy, 2017. [Google Scholar]
- Broegaard, R.B.; Rasmussen, L.V.; Dawson, N.; Mertz, O.; Vongvisouk, T.; Grogan, K. Wild food collection and nutrition under commercial agriculture expansion in agriculture-forest landscapes. For. Policy Econ. 2017. [Google Scholar] [CrossRef] [Green Version]
- Rowland, D.; Ickowitz, A.; Powell, B.; Nasi, R.; Sunderland, T. Forest foods and healthy diets: Quantifying the contributions. Environ. Conserv. 2017, 44, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Ickowitz, A.; Powell, B.; Rowland, D.; Jones, A.; Sunderland, T. Agricultural intensification, dietary diversity, and markets in the global food security narrative. Glob. Food Sec. 2019, 20, 9–16. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Barata, A.M. The Consumption of Wild Edible Plants. In Wild Plants, Mushrooms and Nuts; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 159–198. [Google Scholar] [CrossRef] [Green Version]
- Ojelel, S.; Mucunguzi, P.; Katuura, E.; Kakudidi, E.K.; Namaganda, M.; Kalema, J. Wild edible plants used by communities in and around selected forest reserves of Teso-Karamoja Region, Uganda. J. Ethnobiol. Ethnomed. 2019, 15, 3. [Google Scholar] [CrossRef]
- Kuhnlein, H.V.; Erasmus, B.; Spigelski, D. Indigenous Peoples’ Food Systems: The Many Dimensions of Culture, Diversity and Environment for Nutrition and Health; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Kuhnlein, H.V. Holding on to Agrobiodiversity: Human Nutrition and Health of Indigenous Peoples. In Routledge Handbook of Agricultural Biodiversity; Hunter, D., Guarino, L., Spillane, C., McKeown, P.C., Eds.; Routledge: London, UK, 2017; p. 692. [Google Scholar]
- United Nations. State of the World’s Indigenous Peoples; United Nations: New York, NY, USA, 2009. [Google Scholar]
- Angelsen, A. Policies for reduced deforestation and their impact on agricultural production. Proc. Natl. Acad. Sci. USA 2010, 107, 19639–19644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, B.; Thilsted, S.H.; Ickowitz, A.; Termote, C.; Sunderland, T.; Herforth, A. Improving diets with wild and cultivated biodiversity from across the landscape. Food Secur. 2015, 7, 535–554. [Google Scholar] [CrossRef] [Green Version]
- Aryal, K.P.; Poudel, S.; Chaudhary, R.P.; Chettri, N.; Chaudhary, P.; Ning, W.; Kotru, R. Diversity and use of wild and non-cultivated edible plants in the western Himalaya. J. Ethnobiol. Ethnomed. 2018, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Boedecker, J.; Termote, C.; Assogbadjo, A.E.; Van Damme, P.; Lachat, C. Dietary contribution of wild edible plants to women’s diets in the buffer zone around the Lama forest, Benin—An underutilized potential. Food Secur. 2014, 6, 833–849. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.; Zhuo, J.; Liu, B.; Long, C. Eating from the wild: Diversity of wild edible plants used by Tibetans in Shangri-La Region, Yunnan, China. J. Ethnobiol. Ethnomed. 2013, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Shumsky, S.A.; Hickey, G.M.; Pelletier, B.; Johns, T. Understanding the contribution of wild edible plants to rural social-ecological resilience in Semi-Arid Kenya. Ecol. Soc. 2014, 19, art34. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Ahmed, S.; Dupuis, V.; Running Crane, M.; Eggers, M.; Pierre, M.; Flagg, K.; Byker Shanks, C. Contribution of wild foods to diet, food security, and cultural values amidst climate change. J. Agric. Food Syst. Community Dev. 2019, 1–24. [Google Scholar] [CrossRef]
- Fernández-Ruiz, V.; Morales, P.; Ruiz-Rodríguez, B.M.; Isasa, E.T. Nutrients and Bioactive Compounds in Wild Fruits Through Different Continents. In Wild Plants, Mushrooms and Nuts; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 263–314. [Google Scholar] [CrossRef]
- Hunter, D.; Borelli, T.; Beltrame, D.M.O.; Oliveira, C.N.S.; Coradin, L.; Wasike, V.W.; Wasilwa, L.; Mwai, J.; Manjella, A.; Samarasinghe, G.W.L.; et al. The potential of neglected and underutilized species for improving diets and nutrition. Planta 2019, 250, 709–729. [Google Scholar] [CrossRef]
- Morales, P.; Herrera, P.G.; González, M.C.M.; Hurtado, M.C.; de Cortes Sánchez Mata, M. Wild Greens As Source of Nutritive and Bioactive Compounds Over the World. In Wild Plants, Mushrooms and Nuts; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 199–261. [Google Scholar] [CrossRef]
- Kinnunen, P.; Guillaume, J.H.A.; Taka, M.; D’Odorico, P.; Siebert, S.; Puma, M.J.; Jalava, M.; Kummu, M. Local food crop production can fulfil demand for less than one-third of the population. Nat. Food 2020, 1, 229–237. [Google Scholar] [CrossRef]
- Padulosi, S.; Mal, B.; King, O.; Gotor, E. Minor millets as a central element for sustainably enhanced incomes, empowerment, and nutrition in rural India. Sustainability 2015, 7, 8904–8933. [Google Scholar] [CrossRef] [Green Version]
- Shackleton, S.; Paumgarten, F.; Kassa, H.; Husselman, M.; Zida, M. Opportunities for enhancing poor women’s socioeconomic empowerment in the value chains of three African Non-Timber Forest Products (NTFPs). Int. For. Rev. 2011, 13, 136–151. [Google Scholar] [CrossRef]
- Torero, M. Without food, there can be no exit from the pandemic. Nature 2020, 580, 588–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HLPE. Food Security and Nutrition: Building a Global Narrative towards 2030; HLPE: Rome, Italy, 2020. [Google Scholar]
- Béné, C. Resilience of local food systems and links to food security—A review of some important concepts in the context of COVID-19 and other shocks. Food Secur. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N. Economic effects of coronavirus outbreak (COVID-19) on the world economy. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- United Nations. Policy Brief: The Impact of COVID-19 on Food Security and Nutrition; United Nations: New York, NY, USA, 2020; p. 23. [Google Scholar]
- Vandebroek, I.; Pieroni, A.; Stepp, J.R.; Hanazaki, N.; Ladio, A.; Alves, R.R.N.; Picking, D.; Delgoda, R.; Maroyi, A.; van Andel, T.; et al. Reshaping the future of ethnobiology research after the COVID-19 pandemic. Nat. Plants 2020, 6, 723–730. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E. Will the COVID-19 pandemic make us reconsider the relevance of short food supply chains and local productions? Trends Food Sci. Technol. 2020, 99, 566–567. [Google Scholar] [CrossRef]
- IPES-Food. COVID-19 and the Crisis in Food Systems: Symptoms, Causes, and Potential Solutions; IPES-Food: Brussels, Belgium, 2020. [Google Scholar]
- Poppick, L. The Effects of COVID-19 Will Ripple through Food Systems. Available online: https://www.scientificamerican.com/article/the-effects-of-covid-19-will-ripple-through-food-systems/ (accessed on 13 July 2020).
- Mururia, D.; Mwale, A. Demand for Indigenous Vegetables Soar as Residents Grapple with COVID-19 Economic Shocks. Available online: https://www.kenyanews.go.ke/demand-for-indigenous-vegetables-soar-as-residents-grapple-with-covid-19-economic-shocks/ (accessed on 13 July 2020).
- Giuliano, G. Coronavirus: From Wild Tobacco New Perspectives in the Treatment of COVID-19. Available online: https://www.enea.it/en/news-enea/news/coronavirus-from-wild-tobacco-new-perspectives-in-the-treatment-of-covid-19 (accessed on 13 July 2020).
- Timoshyna, A.; Ling, X.; Zhang, K. COVID-19—The Role of Wild Plants in Health Treatment and Why Sustainability of Their Trade Matters. Available online: https://www.traffic.org/news/covid-19-the-role-of-wild-plants-in-health-treatment/ (accessed on 13 July 2020).
- FAO. The State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ulian, T.; Diazgranados, M.; Pironon, S.; Padulosi, S.; Davies, L.; Howes, M.-J.; Borrell, J.; Ondo, I.; Perez Escobar, O.A.; Sharrock, S.; et al. Unlocking plant and fungal resources to support food security and promote sustainable agriculture. Plants People Planet 2020. [Google Scholar] [CrossRef]
- Zulu, D.; Ellis, R.H.; Culham, A. Collection, consumption, and sale of Lusala (Dioscorea hirtiflora)—A wild yam—By rural households in southern province, Zambia. Econ. Bot. 2019, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Ulian, T.; Sacandé, M.; Hudson, A.; Mattana, E. Conservation of indigenous plants to support community livelihoods: The MGU—Useful plants project. J. Environ. Plan. Manag. 2017, 60, 668–683. [Google Scholar] [CrossRef]
- Ulian, T.; Flores, C.; Lira, R.; Mamatsharaga, A.; Mogotsi, K.K. Wild Plants for a Sustainable Future: 110 Multipurpose Species, 1st ed.; Ulian, T., Flores, C., Lira, R., Mamatsharaga, A., Mogotsi, K.K., Muthoka, P., Ngwako, S., Nyamongo, D.O., Omondi, W., Sanogo, A.K., et al., Eds.; Kew Publishing: Kew, UK, 2019. [Google Scholar]
- Maundu, P.M.; Njiro, E.I.; Chweya, J.A.; Imungi, J.K.; Seme, E.N. Kenya. In The Biodiversity of Traditional Leafy Vegetables; Chweya, J.A., Eyzaguirre, P.B., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1999; pp. 51–84. [Google Scholar]
- Gotor, E.; Irungu, C. The impact of bioversity international’s African leafy vegetables programme in Kenya. Impact Assess. Proj. Apprais. 2010, 28, 41–55. [Google Scholar] [CrossRef]
- Bioversity International; European Initiative for Agricultural Research for Development. African Leafy Vegetables Come out of the Shade; Bioversity International: Rome, Italy, 2013; p. 6. [Google Scholar]
- Borelli, T.; Gee, E.; Hunter, D. Neglected no more: Reframing the food systems narrative using agricultural biodiversity. In Biodiversity, Food and Nutrition. A New Agenda for Sustainable Food Systems; Hunter, D., Borelli, T., Gee, E., Eds.; Routledge: Oxford, UK, 2020. [Google Scholar]
- Sarfo, J.; Keding, G.B.; Boedecker, J.; Pawelzik, E.; Termote, C. The impact of local agrobiodiversity and food interventions on cost, nutritional adequacy and affordability of women and children’s diet in northern Kenya: A modeling exercise. Front. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Penafiel, D.; Lachat, C.; Espinel, R.; Van Damme, P.; Kolsteren, P. A systematic review on the contributions of edible plant and animal biodiversity to human diets. Ecohealth 2011, 8, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Penafiel, D.; Vanhove, W.; Espinel, R.L.; Van Damme, P. Food biodiversity includes both locally cultivated and wild food species in Guasaganda, Central Ecuador. J. Ethn. Foods 2019, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.C.M.; Araújo de Medeiros, M.F.; Albuquerque, U.P. Biodiverse food plants in the Semiarid Region of Brazil have unknown potential: A systematic review. PLoS ONE 2020, 15, e0230936. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A.; et al. Are neglected plants the food for the future? CRC Crit. Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Dogan, Y.; Baslar, S.; Ay, G.; Mert, H.H. The use of wild edible plants in western and central Anatolia (Turkey). Econ. Bot. 2004, 58, 684–690. [Google Scholar] [CrossRef]
- Taylor, J.; Sarkis, L.; Hani, N.; Abulaila, K.; Ulian, T. Conservation and Sustainable Use of Wild Edible Plants in the Eastern Mediterranean Region. In Role of Mediterranean Forests in the Paris Agreement, Proceedings of the Sixth Mediterranean Forest Week, Brummana, Lebanon, 1–5 April 2019; Mohanna, C., Ed.; Forêt Méditerranéenne: Brummana, Lebanon, 2019; pp. 293–300. [Google Scholar]
- Rivera, D.; Obón, C.; Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean Food Plants—Ethnobotanical Investigations and Historical Development. In Local Mediterranean Food Plants and Nutraceuticals; Heinrich, M., Müller, W.E., Galli, C., Eds.; KARGER: Basel, Switzerland, 2006; pp. 18–74. [Google Scholar] [CrossRef]
- Dogan, Y. Traditionally Used Wild Edible Greens in the Aegean Region of Turkey. Acta Soc. Bot. Pol. 2012, 81, 329–342. [Google Scholar] [CrossRef]
- Dogan, Y.; Ugulu, I.; Durkan, N. Wild edible plants sold in the local markets of Izmir, Turkey. Pak. J. Bot. 2013, 45 (Suppl. 1), 177–184. [Google Scholar]
- Nassif, F.; Tanji, A. Gathered food plants in Morocco: The long forgotten species in ethnobotanical research. Life Sci. Leafl. 2013, 3, 17–54. [Google Scholar]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven Circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.; Ouarghidi, A.; Johns, T.; Ibn Tattou, M.; Eyzaguirre, P. Wild leafy vegetable use and knowledge across multiple sites in Morocco: A case study for transmission of local knowledge? J. Ethnobiol. Ethnomed. 2014, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellatifi, M. The situation of non-wood forest products in Morocco. In Proceedings of the Joint FAO/ECE/ILO Committee on Forest Technology, Management and Training Seminar Proceedings on Harvesting of Non-Wood Forest Products, Menemen-Izmir, Turkey, 2–8 October 2000; Food and Agricultural Organization of The United Nations: Menemen, Turkey, 2000. [Google Scholar]
- Thomson, L.; Doran, J.; Clarke, B. Trees for Life in Oceania: Conservation and Utilisation of Genetic Diversity; ACIAR Mono.; Australian Centre for International Agricultural Research: Canberra, Australia, 2018. [Google Scholar]
- French, B. Food Plants of Papua New Guinea; Australia and Pacific Science Foundation: Sheffield, Tasmania, 1986. [Google Scholar]
- Powell, J.M. Ethnobotany. In New Guinea Vegetation; Paijmans, K., Ed.; Elsevier Scientific Publishing: Amsterdam, The Netherlands, 1976; pp. 106–183. [Google Scholar]
- Bourke, M.R.; Allen, B. Nuts. In Food and Agriculture in Papua New Guinea; Bourke, M., Harwood, T., Eds.; ANU E Press: Canberra, Australia; The Australian National University: Canberra, Australia, 2009; pp. 215–222. [Google Scholar]
- Townsend, P.K. Sago production in a New Guinea economy. Hum. Ecol. 1974, 2, 217–236. [Google Scholar] [CrossRef]
- Roscoe, P. The hunters and gatherers of New Guinea. Curr. Anthropol. 2002, 43, 153–162. [Google Scholar] [CrossRef]
- Government of Niue. The State of Niue’s Biodiversity for Food and Agriculture; Niue Country Report; FAO: Rome, Italy, 2019. [Google Scholar]
- Thaman, R.R. The Evolution of the Fiji Food System. In Food and Nutrition in Fiji: A Historical View; Jansen, A.A.J., Parkinson, S., Robertson, A.F.S., Eds.; University of the South Pacific: Suva, Fiji, 1990; pp. 23–109. [Google Scholar]
- Novaczek, I. A Guide to the Common and Edible Medicinal Sea Plants of the Pacific Islands; Secretariat of the Pacific Community: Suva, Fiji, 2001. [Google Scholar]
- Pawera, L.; Khomsan, A.; Zuhud, E.A.M.; Hunter, D.; Ickowitz, A.; Polesny, Z. Wild food plants and trends in their use: From knowledge and perceptions to drivers of change in West Sumatra, Indonesia. Foods 2020, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Pawera, L.; Lipoeto, N.I.; Khomsan, A.; Zuhud, E.A.D. Food Plants of Minang and Mandailing Communities in Pasaman District, West Sumatra. A Community Guidebook on Biodiversity for Nutrition and Health; Swisscontact: Jakarta, Indonesia, 2018. [Google Scholar]
- Sen, A. Poverty and Famines: An Essay on Entitlement and Deprivation; Clarendon Press: Oxford, UK, 1981. [Google Scholar]
- Shackleton, S.; Cocks, M.; Dold, T.; Kaschula, S.; Mbata, K.; Mickels-Kokwe, G.; von Maltitz, G. Contribution of Non-Wood Forest Products to Livelihoods and Poverty Alleviation. In The Dry Forests and Woodlands of Africa. Managing for Products and Services; Chidumayo, E.N., Gumbo, D.J., Eds.; Routledge: Abingdon, UK, 2010; pp. 93–129. [Google Scholar]
- FAO. The State of the World’s Forests. Forest Pathways to Sustainable Development; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar] [CrossRef]
- Petersen, L.M.; Moll, E.J.; Collins, R.; Hockings, M.T. Development of a compendium of local, wild-harvested species used in the informal economy trade, Cape Town, South Africa. Ecol. Soc. 2012, 17. [Google Scholar] [CrossRef] [Green Version]
- Hudson, A.; Milliken, W.; Timberlake, J.; Giovannini, P.; Fijamo, V.; Massunde, J.; Chipanga, H.; Nivunga, M.; Ulian, T. Natural plant resources for sustainable development: Insights from community use in the Chimanimani trans-frontier conservation area, Mozambique. Hum. Ecol. 2020, 48, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Hickey, G.M.; Pouliot, M.; Smith-Hall, C.; Wunder, S.; Nielsen, M.R. Quantifying the economic contribution of wild food harvests to rural livelihoods: A global-comparative analysis. Food Policy 2016, 62, 122–132. [Google Scholar] [CrossRef]
- Karabak, S. Economic and socio-cultural importance of edible wild species. ANADOLU J. AARI 2017, 27, 26–38. [Google Scholar]
- Morris, C.; Bala, S.; South, G.R.; Lako, J.; Lober, M.; Simos, T. Supply chain and marketing of sea grapes, Caulerpa racemosa (Forsskål) J. Agardh (Chlorophyta: Caulerpaceae) in Fiji, Samoa and Tonga. J. Appl. Phycol. 2014, 26, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Lovrić, M.; Da Re, R.; Vidale, E.; Prokofieva, I.; Wong, J.; Pettenella, D.; Verkerk, P.J.; Mavsar, R. Non-wood forest products in Europe—A quantitative overview. For. Policy Econ. 2020, 116, 102175. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Eduardo, B.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S.; et al. The Global Assessment Report on Report on Biodiversity and Ecosystem Services. Summary for Policymakers; IPBES: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Calvo, E.; Priyadarshi, B.; Shukla, R.; Ferrat, M.; Haughey, E.; et al. Climate Change and Land: IPCC Report; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Royal Botanic Gardens, Kew. State of the World’s Plants; Royal Botanic Gardens, Kew: London, UK, 2016. [Google Scholar]
- Sunderland, T.C.H. Food Security: Why Is Biodiversity Important? Int. For. Rev. 2011, 13, 265–274. [Google Scholar] [CrossRef]
- Kew. Plants under Pressure—A Global Assessment; Royal Botanic Gardens: Kew, UK; Natural History Museum: London, UK, 2016. [Google Scholar]
- Khoury, C.K.; Amariles, D.; Soto, J.S.; Diaz, M.V.; Sotelo, S.; Sosa, C.C.; Ramírez-Villegas, J.; Achicanoy, H.A.; Velásquez-Tibatá, J.; Guarino, L.; et al. Comprehensiveness of conservation of useful wild plants: An operational indicator for biodiversity and sustainable development targets. Ecol. Indic. 2019, 98, 420–429. [Google Scholar] [CrossRef]
- Jarvis, A.; Upadhyaya, H.; Gowda, C.; Aggarwal, P.K.; Fugisaka, S.; Anderson, B. Climate Change and Its Effect on Conservation and Use of Plant Genetic Resources for Food and Agriculture and Associated Biodiversity for Food Security; Thematic Study SoW Report PGRFA; FAO: Rome Italy, 2008; p. 26. [Google Scholar]
- Cooper, M.; Zvoleff, A.; Gonzalez-Roglich, M.; Tusiime, F.; Musumba, M.; Noon, M.; Alele, P.; Nyiratuza, M. Geographic factors predict wild food and nonfood NTFP collection by households across four African countries. For. Policy Econ. 2018, 96, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Belcher, B.; Ruíz-Pérez, M.; Achdiawan, R. Global patterns and trends in the use and management of commercial NTFPs: Implications for livelihoods and conservation. World Dev. 2005, 33, 1435–1452. [Google Scholar] [CrossRef]
- Lamrani-Alaoui, M.; Hassikou, R. Rapid risk assessment to harvesting of wild medicinal and aromatic plant species in Morocco for conservation and sustainable management purposes. Biodivers. Conserv. 2018, 27, 2729–2745. [Google Scholar] [CrossRef]
- Government of Brazil; BFN Project. SiBBr—Information Platform on Brazilian Biodiversity. Available online: https://ferramentas.sibbr.gov.br/ficha/bin/view/FN/ (accessed on 29 July 2020).
- Martinelli, G.; Moraes, M.A. Livro Vermelho da Flora do Brasil, 1st ed.; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Kim, D.-S.; Joo, N. Nutritional composition of Sacha inchi (Plukenetia volubilis L.) as affected by different cooking methods. Int. J. Food Prop. 2019, 22, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- de Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value and farming in the Indo-Pacific Region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Chweya, J.A.; Mnzava, N.A. Cat’s Whiskers. Cleome Gynandra L. Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- FAO/Government of Kenya. Kenya Food Composition Tables; Food and Agricultural Organization of the United Nations: Nairobi, Kenya, 2018. [Google Scholar]
- BFN Project. BFN Species Database. Available online: http://www.b4fn.org/resources/species-database/ (accessed on 29 July 2020).
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- El Abbassi, A.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical characteristics, nutritional properties, and health benefits of Argan oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef]
- Garzuglia, M. Threatened, Endangered and Vulnerable Tree Species: A Comparison between FRA 2005 and the IUCN Red List; Forest Resources Assessment (FRA) Working Paper Series; FRA WP 106/E; FAO: Rome, Italy, 2006. [Google Scholar]
- Wardah; Sambas, E.N.; Ridwan; Ariani, D. Starch product of wild plants species Jalawure (Tacca leontopetaloides L.) Kuntze as the source of food security in the south coastal west Java. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012035. [Google Scholar] [CrossRef] [Green Version]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Tosun, M.; Tosun1, M.; Ercisli, S.; Ozer, H.; Turan, M.; Polat, T.; Ozturk, E.; Padem, H.; Kilicgun, H. Chemical Composition and Antioxidant Activity of Foxtail Lily (Eremurus Spectabilis). Acta Sci. Pol. Hortorum Cultus 2012, 11, 145–153. [Google Scholar]
- Cinar, A.; Tugrul Ay, S.; Ayas, F.; Karabak, S.; Guzelsoy, N.; Ucurum, O. Foxtail Lilly (Eremurus spectabilis M. Bieb.) as priority species of biodiversity for food and nutrition project of Turkey. ANADOLU J. AARI 2017, 27, 69–73. [Google Scholar]
- Choonhahirun, A. Proximate composition and functional properties of Pra (Elateriospermun Tapos Blume) seed flour. Afr. J. Biotechnol. 2010, 9, 5946–5949. [Google Scholar]
- Indonesian Food Composition Data. Available online: https://www.panganku.org/en-EN/tentang_kami (accessed on 14 July 2020).
- Rojas, M.; Lambert, F.; Ramirez-Villegas, J.; Challinor, A.J. Emergence of robust precipitation changes across crop production areas in the 21st century. Proc. Natl. Acad. Sci. USA 2019, 116, 6673–6678. [Google Scholar] [CrossRef] [Green Version]
- Borrell, J.S.; Dodsworth, S.; Forest, F.; Pérez-Escobar, O.A. The climatic challenge: Which plants will people use in the next century? J. Exp. Bot. 2020, 170. [Google Scholar] [CrossRef]
- Anderson, D.; Ford, J.D.; Way, R.G. The impacts of climate and social changes on cloudberry (Bakeapple) picking: A case study from southeastern Labrador. Hum. Ecol. 2018, 46, 849–863. [Google Scholar] [CrossRef] [Green Version]
- FAO. Sixteenth Regular Session of the Commission on Genetic Resources for Food and Agriculture—CGRFA-16/17/Report Rev.1; FAO: Rome, Italy, 2017. [Google Scholar]
- Ingram, V.; Vinceti, B.; van Vliet, N. Wild Plant and Animal Genetic Resources. In Routledge Handbook of Agricultural Biodiversity; Hunter, D., Guarino, L., Spillane, C., McKeown, P.C., Eds.; Routledge: Abingdon, UK, 2017; pp. 65–85. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Convention on Biological Diversity; United Nations: Rio de Janeiro, Brazil, 1992; p. 28. [Google Scholar]
- Tata Ngome, P.I.; Shackleton, C.; Degrande, A.; Tieguhong, J.C. Addressing constraints in promoting wild edible plants’ utilization in household nutrition: Case of the Congo basin forest area. Agric. Food Secur. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jaenicke, H.; Höschle-Zeledon, I. (Eds.) Strategic Framework for Underutilized Plant Species Research and Development, with Special Reference to Asia and the Pacific, and to Sub-Saharan Africa; International Centre for Underutilised Crops, Colombo, Sri Lanka and Global Facilitation Unit for Underutilized Species: Rome, Italy, 2006. [Google Scholar]
- Hawkes, C. Uneven dietary development: Linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Glob. Health 2006, 2. [Google Scholar] [CrossRef] [Green Version]
- Biodiversity, Food and Nutrition. A New Agenda for Sustainable Food Systems, 1st ed.; Hunter, D., Borelli, T., Gee, E., Eds.; Routledge: Oxford, UK, 2020. [Google Scholar]
- Fanzo, J.; Davis, C. Can diets be healthy, sustainable, and equitable? Curr. Obes. Rep. 2019, 8, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Termote, C.; Raneri, J.; Deptford, A.; Cogill, B. Assessing the potential of wild foods to reduce the cost of a nutritionally adequate diet: An example from eastern Baringo District, Kenya. Food Nutr. Bull. 2014, 35, 458–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesbitt, M.; McBurney, R.P.H.; Broin, M.; Beentje, H.J. Linking biodiversity, food and nutrition: The importance of plant identification and nomenclature. J. Food Compos. Anal. 2010, 23, 486–498. [Google Scholar] [CrossRef]
- Kennedy, G.; Lee, W.T.K.; Termote, C.; Charrondière, R.; Tung, J.Y.A. Guidelines on Assessing Biodiverse Foods in Dietary Intake Surveys; Food and Agricultural Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Elmadfa, I.; Meyer, A.L. Importance of food composition data to nutrition and public health. Eur. J. Clin. Nutr. 2010, 64 (Suppl. S3), S4–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Msuya, J.M.; Mamiro, P.; Weinberger, K. Iron, Zinc and β-Carotene nutrient potential of non-cultivated. Indigenous vegetables in Tanzania. Acta Hortic. 2009, 806, 217–222. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO). Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409.e5. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D. Global, regional and national consumption of major food groups in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open 2015, 5, e008705. [Google Scholar] [CrossRef] [Green Version]
- de Bruyn, J.; Msuya, J.; Ferguson, E. Evaluating pictorial charts as a means of collecting participant-recorded data on household dietary diversity in low-literacy communities in Tanzania. Br. J. Nutr. 2019, 122, 1432–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Hattersley, L.; Cogill, B.; Hunter, D.; Kennedy, G. Evidence for the Role of Biodiversity in Supporting Healthy, Diverse Diets and Nutrition. In Biodiversity, Food and Nutrition: A New Agenda for Sustainable Food Systems; Hunter, D., Borelli, T., Gee, E., Eds.; Routledge: Abingdon, UK, 2020. [Google Scholar]
- Shackleton, C.M.; Hurley, P.T.; Dahlberg, A.C.; Emery, M.R.; Nagendra, H. Urban foraging: A ubiquitous human practice overlooked by urban planners, policy, and research. Sustainability 2017, 9, 1884. [Google Scholar] [CrossRef] [Green Version]
- Reyes-García, V.; Menendez-Baceta, G.; Aceituno-Mata, L.; Acosta-Naranjo, R.; Calvet-Mir, L.; Domínguez, P.; Garnatje, T.; Gómez-Baggethun, E.; Molina-Bustamante, M.; Molina, M.; et al. From famine foods to delicatessen: Interpreting trends in the use of wild edible plants through cultural ecosystem services. Ecol. Econ. 2015, 120, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Schulp, C.J.E.; Thuiller, W.; Verburg, P.H. Wild food in Europe: A synthesis of knowledge and data of terrestrial wild food as an ecosystem service. Ecol. Econ. 2014, 105, 292–305. [Google Scholar] [CrossRef]
- Li, D.-Z.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef]
- Commission on Genetic Resources for Food and Agriculture—FAO. The Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture; Food and Agricultural Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- FAO. Voluntary Guidelines for the Conservation and Sustainable Use of Crop Wild Relatives and Wild Food Plants; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Vernooy, R.; Bessette, G.; Otieno, G. (Eds.) Resilient Seed Systems: Handbook, 2nd ed.; Bioversity International: Rome, Italy, 2019. [Google Scholar]
- PAR. Assessing Agrobiodiversity: A Compendium of Methods; Mijatović, D., Hodgkin, T., Eds.; Platform for Agrobiodiversity Research: Rome, Italy, 2018. [Google Scholar]
- Secretariat of the Convention on Biological Diversity (SCBD). Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from Their Utilization to the Convention on Biological Diversity; United Nations: Montreal, QC, Canada, 2011; p. 25. [Google Scholar]
- Bioversity International. Seasonal Calendar of Fruits and Vegetables for a Diversified Diet in Mandla and Dindori Districts, Madhya Pradesh, India; Bioversity International: Rome, Italy; Action for Social Advancement: Madhya Pradesh, India, 2018; p. 1. [Google Scholar]
- Gee, E.; Borelli, T.; Beltrame, D.M.O.; Oliveira, C.N.S.; Coradin, L.; Wasike, V.; Manjella, A.; Samarasinghe, G.; Güner, B.; Tan, A.; et al. The ABC of Mainstreaming Biodiversity for Food and Nutrition. Concepts, Theory and Practice. In Biodiversity Food and Nutrition. A New Agenda for Sustainable Food Systems; Hunter, D., Borelli, T., Gee, E., Eds.; Routledge: Abingdon, UK, 2020. [Google Scholar]
- Boedecker, J.; Odhiambo Odour, F.; Lachat, C.; Van Damme, P.; Kennedy, G.; Termote, C. Participatory farm diversification and nutrition education increase dietary diversity in Western Kenya. Matern. Child Nutr. 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- FAO/INFOODS. FAO/INFOODS Guidelines for Checking Food Composition Data Prior to the Publication of a User Table/Database—Version 1.0; FAO: Rome, Italy, 2012. [Google Scholar]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.M.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local solutions for sustainable food systems: The contribution of orphan crops and wild edible species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Arévalo, I.; Mattana, E.; García, L.; Liu, U.; Lira, R.; Dávila, P.; Hudson, A.; Pritchard, H.W.; Ulian, T. Conserving seeds of useful wild plants in Mexico: Main issues and recommendations. Genet. Resour. Crop Evol. 2017, 64, 1141–1190. [Google Scholar] [CrossRef] [Green Version]
- Sperling, L.; McGuire, S. Fatal gaps in seed security strategy. Food Secur. 2012, 4, 569–579. [Google Scholar] [CrossRef]
- Mattana, E.; Sacande, M.; Abdul Sanogo, K.; Lira, R.; Gomez-Barreiro, P.; Rogledi, M.; Ulian, T. Thermal requirements for seed germination of underutilized Lippia species. S. Afr. J. Bot. 2017, 109, 223–230. [Google Scholar] [CrossRef]
- McCune, L.M. The protection of indigenous peoples’ seed rights during ethnobotanical research. Ethnobiol. Lett. 2018, 9, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Aluso, L.; Nzuki, R. From the Field: How On-Farm Biodiversity Is Transforming Community Livelihoods in Kenya. Available online: https://alliancebioversityciat.org/news_and_blogs/on-farm-biodiversity-transforming-communitys-livelihoods-in-resilient-landscapes/ (accessed on 29 July 2020).
- Mogotsi, K.K.; Ulian, T. Conserving indigenous food plants in Botswana: The case of Morama bean. Samara 2013, 24. [Google Scholar]
- Tan, A.; Adanacıoglu, N.; Karabak, S.; Aykas, L.; Tas, N.; Taylan, T. Biodiversity for food and nutrition: Edible wild plant species of Aegean Region of Turkey. ANADOLU J. AARI 2017, 27, 1–8. [Google Scholar]
- Karik, U. The effect of different harvest dates on the yield and quality of golden thistle (Scolymus hispanicus L.). Turk. J. Feild Crops 2019, 24, 230–236. [Google Scholar] [CrossRef]
- Beltrame, D.M.D.O.; Oliveira, C.N.S.; Borelli, T.; Santiago, R.D.A.C.; Tronco Monego, E.; de Rosso, V.V.; Coradin, L.; Hunter, D. Diversifying institutional food procurement—Opportunities and barriers for integrating biodiversity for food and nutrition in Brazil. Raízes 2016, 36, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T.; et al. A manifesto for the valorization of wild edible plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef]
- Gonsalves, J.; Hunter, D.; Lauridsen, N. School Gardens: Multiple Functions and Multiple Outcomes. In Agrobiodiversity, School Gardens and Healthy Diets. Promoting Biodiversity, Food and Sustainable Nutrition; Hunter, D., Monville-Oro, E., Burgos, B., Rogel, C.N., Calub, B., Gonsalves, J., Lauridsen, N., Eds.; Routledge: Abingdon, UK, 2020; pp. 1–32. [Google Scholar]
- Grazioli, F.; Turdieva, M.; Kettle, C.J. The Role of School Gardens as Conservation Networks for Tree Genetic Resources. In Agrobiodiversity, School Gardens and Healthy Diets: Promoting Biodiversity, Food and Sustainable Nutrition; Hunter, D., Monville-Oro, E., Burgos, B., Rogel, C.N., Calub, B.M., Gonsalves, J., Lauridsen, N., Eds.; Routledge: Abingdon, UK, 2020; p. 302. [Google Scholar]
- Sutcliffe, S.; Court, J. Evidence-Based Policymaking: What Is It? How Does It Work? What Relevance for Developing Countries; Overseas Development Institute: London, UK, 2005. [Google Scholar]
- Beltrame, D.; Borelli, T.; Oliveira, C.; Coradin, L.; Hunter, D. Biodiversity for Food and Nutrition: Promoting Food Security and Nutrition through Institutional Markets in Brazil. In Public Food Procurement for Sustainable Food Systems and Healthy Diets; Swensson, L., Tartanac, F., Hunter, D., Schneider, S., Eds.; Food and Agricultural Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Baldi, G.; Martini, E.; Catharina, M.; Muslimatun, S.; Fahmida, U.; Jahari, A.B.; Hardinsyah; Frega, R.; Geniez, P.; Grede, N.; et al. Cost of the Diet (CoD) tool: First results from Indonesia and applications for policy discussion on food and nutrition security. Food Nutr. Bull. 2013, 34 (Suppl. S2), S35–S42. [Google Scholar] [CrossRef]
- Gee, E.; Lee, H. Start Me up! Food Biodiversity and Youth-Led Innovations. In Biodiversity, Food and Nutrition. A New Agenda for Sustainable Food Systems; Hunter, D., Borelli, T., Gee, E., Eds.; Routledge: Abingdon, UK, 2020; pp. 255–274. [Google Scholar]









| Country | Species Name | Local Name | Edible Use | Main Nutritional Benefits | Habitat | Threat Status (IUCN, Community to Other) | Threats/Suggestion for Conservation | Photo |
|---|---|---|---|---|---|---|---|---|
| Brazil | Astrocaryum aculeatum | Tucumã | Fruit pulp | Rich in vitamin A as well as lauric, myristic and oleic acid [99] | Amazon rainforest | No IUCN assessment | Habitat loss—deforestation/ Preserve natural habitats |  Credit: J. Camillo |
| Euterpe edulis | Jussara | Fruit pulp consumed as puree, palm heart (discouraged) | The fruit is rich in antioxidants [99] | Dense shady forest (Atlantic forest) | No IUCN assessment, listed as Vulnerable in the Red Book of Brazilian Flora [100] | Habitat loss—deforestation, overharvesting of palm heart/ Preserve natural habitats |  Credit: A. Popovkin | |
| Butia eriospatha | Butiá | Fruit pulp, seed | Good source of fiber, potassium, and vitamin C (equivalent to levels found in oranges) [99] | Highland mixed shady forests (Araucaria forest), around 800–900 m elevation | IUCN—Vulnerable | Habitat loss—deforestation/ Preserve natural habitats |  Credit: G. Lopes | |
| Dipteryx alata | Baru nut | Fruit/Nut | High in fiber; the nut is high in quality protein [99] | Tropical savannah (Cerrado) | IUCN—Vulnerable | Habitat loss—deforestation/Preserve natural habitats |  Credit: J. Camillo | |
| Hancornia speciosa | Mangaba | Fruit | Excellent source of vitamin C, folates and a good source of carotenoids and vitamin E [99] | Scrublands (Caatinga) and barren lands in central Brazil | No IUCN assessment | Habitat loss—deforestation/Preserve natural habitats |  Credit: J. Camillo | |
| Ecuador | Vasconcellea microcarpa (Carica microcarpa) | Col de monte | Leaves | N/A | Forest | IUCN—Least concern | Deforestation/Nutrition education needed |  Credit: X. Scheldeman |
| Pouteria multiflora | Logma | Fruit | N/A | Forest | No IUCN assessment | Deforestation/Nutrition education needed |  Credit: IKIAM | |
| Hypolepis hostilis | Garabato yuyo | Leafy green vegetable (fern) | N/A | Forest | No IUCN assessment | Loss of traditional food culture /Use as complementary food for infants |  Credit: IKIAM | |
| Plukenetia volubilis | Sachainchi | Nut | Good source of lipids, proteins, and essential amino acids (e.g., cysteine, tyrosine, threonine, and tryptophan), vitamin E and polyphenols [101] | Home garden | No IUCN assessment | Loss of traditional food culture /Use as complementary food for infants |  Credit: IKIAM | |
| Fiji/ Samoa | Caulerpa racemosa | Nama, Limu | Sea vegetable | Contains proteins, fiber, minerals, vitamins, polyunsaturated fatty acids, and bioactive anti-oxidants [102] | Near reefs, in shallow waters | No IUCN assessment | Unsustainable harvesting, storm surges, cyclones |  Credit: N.Hobgood |
| Kenya | Cleome gynandra | Spider plant, Ofsaga, saga, liSaga, lisaka | Leaves used as vegetables [103] | High in β-carotene, folic acid, vitamin C, calcium and a good source of vitamin E, iron [104] | Roadsides, field margins, semi-domesticated | No IUCN assessment | No organized collecting missions |  Credit: BFN Kenya |
| Amaranthus tortuosus | Amaranth, Ekichabo, Dodo | Leaves used as vegetables and seed crushed for flour | Good source of proteins, fibers, calcium, iron, riboflavin, niacin and vitamin C and an excellent source of lysine [104] | Roadsides, field margins, semi-domesticated | No IUCN assessment | No organized collecting missions |  Credit: BFN Kenya | |
| Chorchorus olitorius | Jute mallow, murere | Leaves used as vegetables | High levels of β -carotene, vitamin C, folic acid, calcium and iron [104] | Roadsides, field margins, semi-domesticated | No IUCN assessment | No organized collecting missions |  Credit: C. Kerr | |
| Morocco | Nasturtium officinale | Watercress, Gernounch | Leafy vegetable | Rich in vitamin K, vitamin A, vitamin C, vitamin B6, manganese, calcium, and folate [105] | Springs, river edges, irrigation canals | IUCN—Least Concern | Paving of irrigation canals may decrease community access, changing diet and preference and leading to decreased use |  Credit: M. Lavin |
| Malva sylvestris | Mallow, Tibi, Khobiza, Bakola houra | Leafy vegetable | Strong antioxidant properties, rich in phenols, flavonoids, carotenoids, and tocopherols, alpha-linolenic acid and minerals [106] | Fields, field margins, along irrigation canals and roads | IUCN—Least Concern | Changing diet and preferences may lead to decreased use |  Credit: B. Powell | |
| Sideroxylon spinosum | Argan | Edible oil | Good source of linoleic and oleic fatty acids. Rich source of tocopherol (vitamin E) [107] | Dry forests from the Atlantic coast to 800 m elevation | IUCN criteria at national level—Vulnerable [108] | Social-ecological systems change driven by commodification and globalization |  Credit: B. Powell | |
| Niue | Tacca leontopetaloides | Kai Niue | Root starch | Good source of carbohydrate, also contains vitamin C, fat, and protein [109] | Uncultivated land | IUCN Least Concern | General lack of information |  Credit: B. Dupont |
| Papua New Guinea | Pandanus brosimos | Karuka | Fruit (boiled) & extracted nut | Good source of protein and oil especially for highland communities [71] | Forest, high altitudes | No IUCN assessment | No known threat, but general lack of information |  Credit: Green Dean |
| Turkey | Scolymus hispanicus | Golden thistle, Şevketi bostan | Roots and young leaves | Rich in dietary fiber, magnesium and calcium [105,110] | Disturbed habitats and fallow fields | No IUCN assessment | Overharvesting/domestication programs initiated |  Credit: BFN Turkey |
| Eremurus spectabilis | Foxtail lily, Çiriş otu | Shoots, buds and young leaves | Rich in antioxidants and minerals [111]. High in vitamin C [112] | Dry and stony grazed hillsides | No IUCN assessment | Overharvesting/domestication programs initiated |  Credit: K.D. Zinnert | |
| West Sumatra, Indonesia | Elateriospermum tapos | Tapuih | Seeds consumed raw or fermented | Rich in protein and unsaturated fatty acids [113] | Forest/agroforest | No IUCN assessment | Perceived as rare by local communities/Preserve forest and multi-strata agroforests |  Credit: L. Pawera |
| Mangifera foetida | Ambacam, Bacang | Fruits consumed raw or cooked | Rich in vitamins A and C [114] | Forest, agroforest, homegardens | IUCN -Least Concern | Perceived as rare by local communities/Preserve forests and multi-strata agroforests |  Credit: L. Pawera | |
| Diplazium esculentum | Pakis, Pahu | Young shoots as a vegetable, cooked | Rich in vitamin B9 (folate) [114] | Forests, wetlands | IUCN—Least concern | Relatively common/Preserve forests and wetlands |  Credit: L. Pawera | |
| Ipomoea aquatica | Kangkung air, Kangkuang liar | Leaves and stems as a vegetable, cooked | Rich in Iron and provitamin A [114] | Rivers, ponds, rice fields | No IUCN assessment | Threatened by overuse of herbicides/Reduce the use of herbicides and keep clean water bodies |  Credit: L. Pawera |
| Awareness | Focus | Financial Support | External Pressures |
|---|---|---|---|
| No adequate data | Mismatch with national priorities | No international financial or donor support | International trade favor R&D on conventional crops |
| Lack of priority in education and information systems | Limited capacity (institutional, research) to work with WFPs | Weak economies for investing in R&D for WFPs | International R&D priorities influence national priorities |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borelli, T.; Hunter, D.; Powell, B.; Ulian, T.; Mattana, E.; Termote, C.; Pawera, L.; Beltrame, D.; Penafiel, D.; Tan, A.; et al. Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition. Plants 2020, 9, 1299. https://doi.org/10.3390/plants9101299
Borelli T, Hunter D, Powell B, Ulian T, Mattana E, Termote C, Pawera L, Beltrame D, Penafiel D, Tan A, et al. Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition. Plants. 2020; 9(10):1299. https://doi.org/10.3390/plants9101299
Chicago/Turabian StyleBorelli, Teresa, Danny Hunter, Bronwen Powell, Tiziana Ulian, Efisio Mattana, Céline Termote, Lukas Pawera, Daniela Beltrame, Daniela Penafiel, Ayfer Tan, and et al. 2020. "Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition" Plants 9, no. 10: 1299. https://doi.org/10.3390/plants9101299
APA StyleBorelli, T., Hunter, D., Powell, B., Ulian, T., Mattana, E., Termote, C., Pawera, L., Beltrame, D., Penafiel, D., Tan, A., Taylor, M., & Engels, J. (2020). Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition. Plants, 9(10), 1299. https://doi.org/10.3390/plants9101299








