Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity
Abstract
1. Introduction
2. Results
2.1. Total Polyphenols Are Higher in Roots Than Stems and Increase With Phenological Stage in Different Hop Varieties
2.2. Total Polyphenols in Hop Decrease on VW Infection
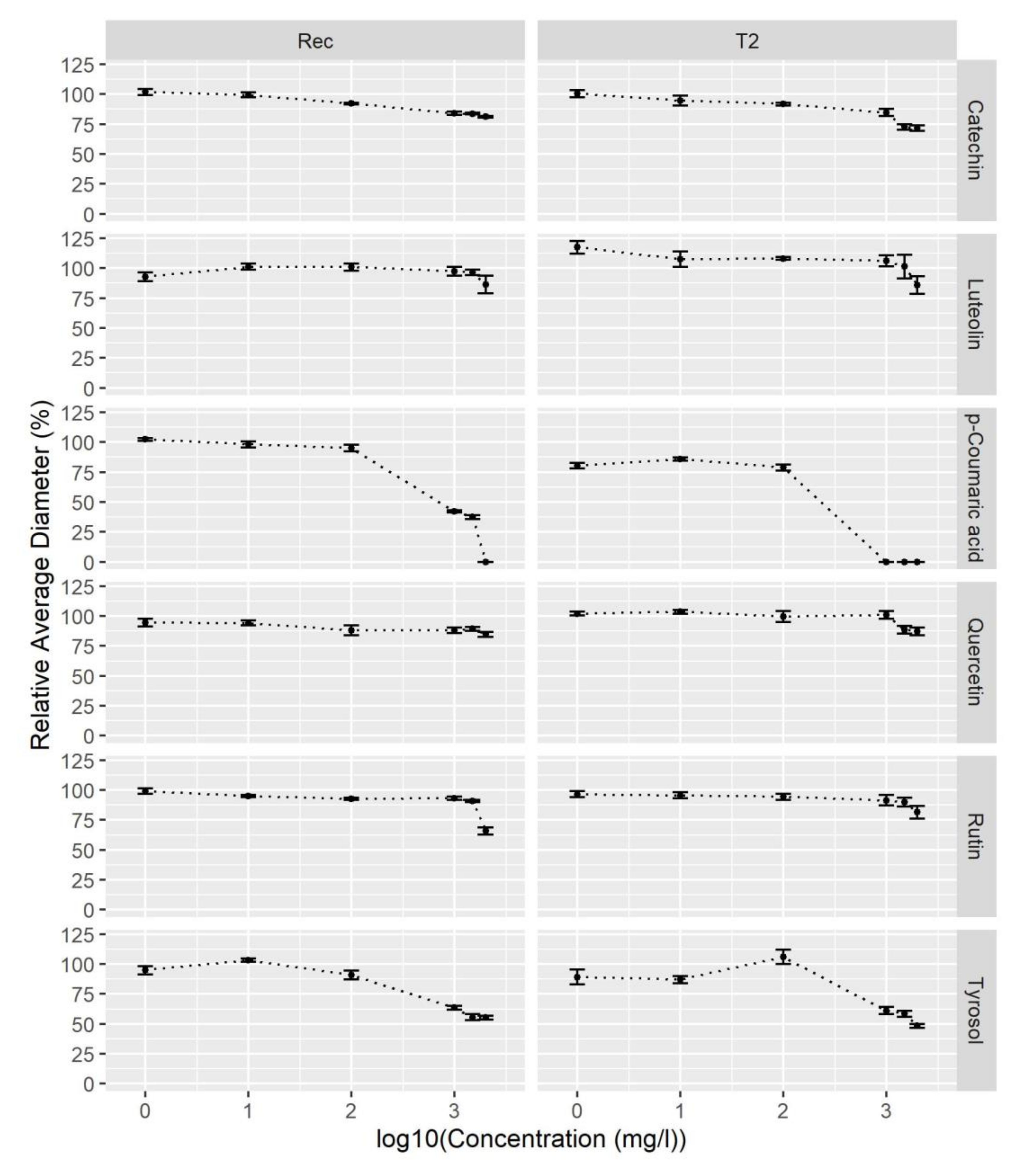
2.3. p-Coumaric Acid and Tyrosol Inhibit V. nonalfalfae Growth In Vitro
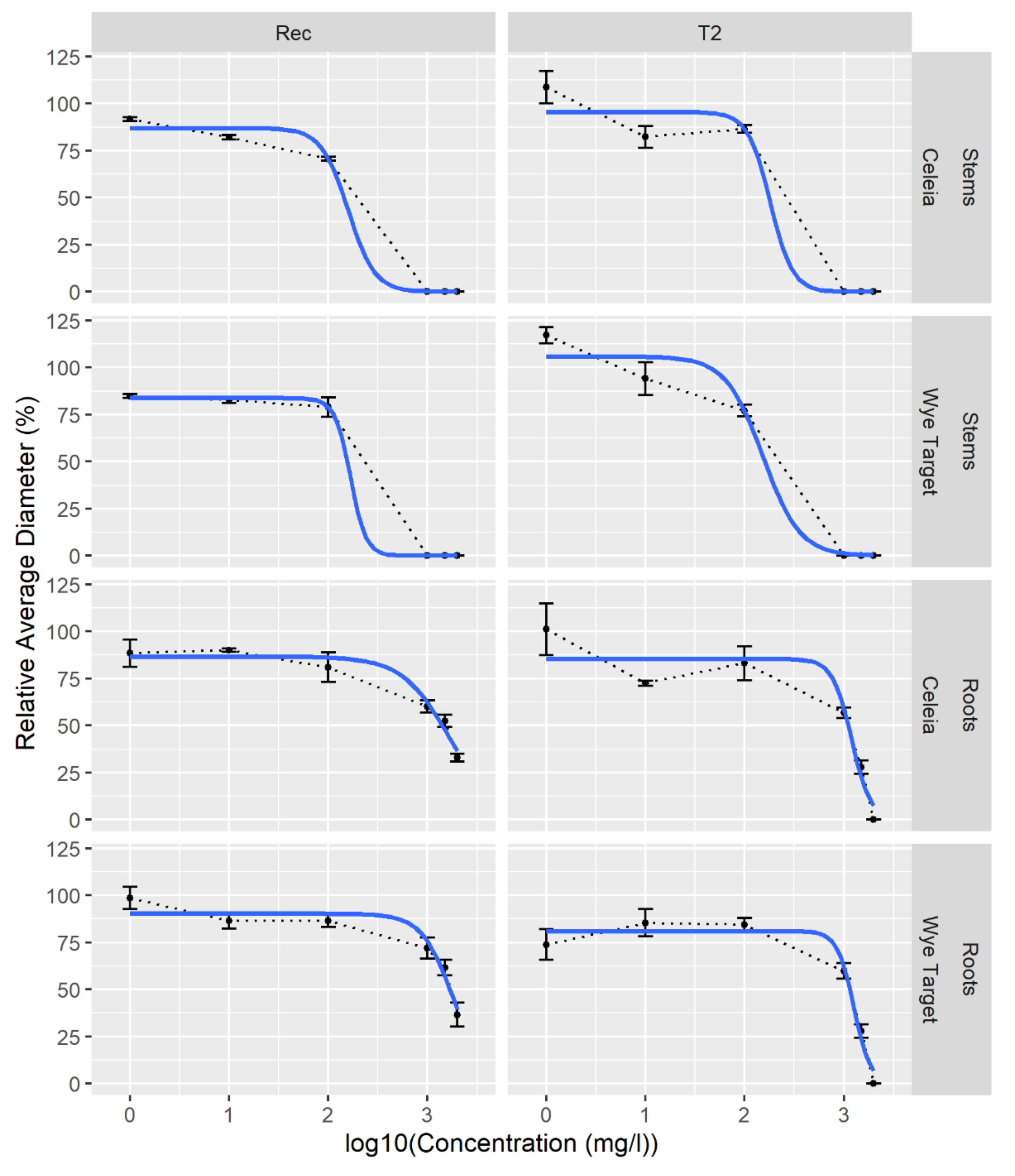
2.4. Total Phenolic Extracts from Hop Show Antifungal Activity against V. nonalfalfae
3. Discussion
3.1. Total Polyphenols in Roots and Stems of Different Hop Varieties
3.2. Phenolic Compounds Are Involved in the Plant Defense against VW Infection, but the Responses Vary among Host Species and with Disease Progression
3.3. V. nonalfalfae Growth In Vitro Is Inhibited by p-Coumaric Acid and Tyrosol
3.4. Antifungal Activity of Total Polyphenol Extracts from Hop
4. Materials and Methods
4.1. Plant Material
4.2. Artificial Inoculation of Hop with Verticillium nonalfalfae
4.3. Extraction of Total Polyphenols from Dry Plant Material
4.4. Spectrophotometric Determination of Hop Total Polyphenols
4.5. Inhibition of Fungal Growth by Commercial Phenolic Compounds
4.6. Determination of Antifungal Activity of Hop Polyphenolic Extracts
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berlanger, I.; Powelson, M.L. Verticillium wilt. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium Species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K. V Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef]
- Harris, R.V. A wilt disease of hops. East. Malling Res. Stn. Annu. Rep. 1927, 1925, 92–93. [Google Scholar]
- Keyworth, W.G. Verticillium wilt of the hop (Humulus lupulus). Ann. Appl. Biol. 1942, 29, 346–357. [Google Scholar] [CrossRef]
- Isaac, I.; Keyworth, W.G. Verticillium wilt of the hop (Humulus lupulus). A study of the pathogenicity of isolates from fluctuating and from progressive outbreaks. Ann. Appl. Biol. 1948, 35, 243–249. [Google Scholar] [CrossRef]
- Sewell, G.W.F.; Wilson, J.F. The nature and distribution of Verticillium albo-atrum strains highly pathogenic to the hop. Plant Pathol. 1984, 33, 39–51. [Google Scholar] [CrossRef]
- Radišek, S.; Jakše, J.; Javornik, B. Genetic variability and virulence among Verticillium albo-atrum isolates from hop. Eur. J. Plant Pathol. 2006, 116, 301–314. [Google Scholar] [CrossRef]
- Radišek, S.; Jakše, J.; Javornik, B. Development of pathotype-specific SCAR markers for detection of Verticillium albo-atrum isolates from hop. Plant Dis. 2004, 88, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.M.; Heale, J.B. Pathogenicity and colonization studies on wild-type and auxotrophic isolates of Verticillium albo-atrum from hop. Plant Pathol. 1985, 34, 119–128. [Google Scholar] [CrossRef]
- Cregeen, S.; Radišek, S.; Mandelc, S.; Turk, B.; Štajner, N.; Jakše, J.; Javornik, B. Different gene expressions of resistant and susceptible hop cultivars in response to infection with a highly aggressive strain of Verticillium albo-atrum. Plant Mol. Biol. Rep. 2015, 33, 689–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunej, U.; Mikulič-Petkovšek, M.; Radišek, S.; Štajner, N. Changes in the phenolic compounds of hop (Humulus lupulus L.) Induced by infection with Verticillium nonalfalfae, the causal agent of hop Verticillium wilt. Plants 2020, 9, 841. [Google Scholar] [CrossRef]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1955, 45, 180–181. [Google Scholar]
- Deketelaere, S.; Tyvaert, L.; França, S.C.; Höfte, M. Desirable Traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef]
- Mott, G.A.; Middleton, M.A.; Desveaux, D.; Guttman, D.S. Peptides and small molecules of the plant-pathogen apoplastic arena. Front. Plant Sci. 2014, 5, 677. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal–plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Yadeta, K.A.J.; Thomma, B.P.H. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, B.; Timmermans, P.; Vos, C.; Cammue, B.P.A.; Kazan, K. What lies beneath: Belowground defense strategies in plants. Trends Plant Sci. 2015, 20, 91–101. [Google Scholar] [CrossRef]
- Talboys, P.W. Association of tylosis and hyperplasia of the xylem with vascular invasion of the hop by Verticillium albo-atrum. Trans. Br. Mycol. Soc. 1958, 41, 249–260. [Google Scholar] [CrossRef]
- Reusche, M.; Thole, K.; Janz, D.; Truskina, J.; Rindfleisch, S.; Drübert, C.; Polle, A.; Lipka, V.; Teichmann, T. Verticillium infection triggers VASCULAR-RELATED NAC DOMAIN7-dependent de novo xylem formation and enhances drought tolerance in Arabidopsis. Plant Cell 2012, 24, 3823–3837. [Google Scholar] [CrossRef]
- Mandelc, S.; Timperman, I.; Radišek, S.; Devreese, B.; Samyn, B.; Javornik, B. Comparative proteomic profiling in compatible and incompatible interactions between hop roots and Verticillium albo-atrum. Plant Physiol Biochem. 2013, 68, 23–31. [Google Scholar] [CrossRef]
- Hu, X.; Puri, K.D.; Gurung, S.; Klosterman, S.J.; Wallis, C.M.; Britton, M.; Durbin-Johnson, B.; Phinney, B.; Salemi, M.; Short, D.P.G.; et al. Proteome and metabolome analyses reveal differential responses in tomato —Verticillium dahliae—interactions. J. Proteom. 2019, 207, 103449. [Google Scholar] [CrossRef]
- Wang, F.X.; Ma, Y.P.; Yang, C.L.; Zhao, P.M.; Yao, Y.; Jian, G.L.; Luo, Y.M.; Xia, G.X. Proteomic analysis of the sea-island cotton roots infected by wilt pathogen Verticillium dahliae. Proteomics 2011, 11, 4296–4309. [Google Scholar] [CrossRef]
- Gayoso, C.; Pomar, F.; Novo-Uzal, E.; Merino, F.; de Ilárduya, O.M. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PALgene expression. BMC Plant Biol. 2010, 10, 232. [Google Scholar] [CrossRef]
- Floerl, S.; Druebert, C.; Majcherczyk, A.; Karlovsky, P.; Kües, U.; Polle, A. Defence reactions in the apoplastic proteome of oilseed rape (Brassica napus var. napus) attenuate Verticillium longisporum growth but not disease symptoms. BMC Plant Biol. 2008, 8, 129. [Google Scholar] [CrossRef]
- Robb, J.; Shittu, H.; Soman, K.V.; Kurosky, A.; Nazar, R.N. Arsenal of elevated defense proteins fails to protect tomato against Verticillium dahliae. Planta 2012, 236, 623–633. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Feussner, K.; Kaever, A.; Landesfeind, M.; Thurow, C.; Karlovsky, P.; Gatz, C.; Polle, A.; Feussner, I. Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum. New Phytol. 2014, 202, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Floerl, S.; Majcherczyk, A.; Possienke, M.; Feussner, K.; Tappe, H. Verticillium longisporum infection affects the leaf apoplastic proteome, metabolome, and cell wall properties in Arabidopsis thaliana. PLoS ONE 2012, 7, e31435. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lu, G.; Guo, H.; Zhang, K.; Li, X.; Cheng, H. The dynamic transcriptome and metabolomics profiling in Verticillium dahliae inoculated Arabidopsis thaliana. Sci. Rep. 2018, 8, 15404. [Google Scholar] [CrossRef]
- Iven, T.; König, S.; Singh, S.; Braus-Stromeyer, S.A.; Bischoff, M.; Tietze, L.F.; Braus, G.H.; Lipka, V.; Feussner, I.; Dröge-Laser, W. Transcriptional activation and production of tryptophan-derived secondary metabolites in Arabidopsis roots contributes to the defense against the fungal vascular pathogen Verticillium longisporum. Mol. Plant 2012, 5, 1389–1402. [Google Scholar] [CrossRef]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Roussos, P.A.; Paplomatas, E.J.; Tjamos, E.C. Phenolic responses of resistant and susceptible olive cultivars induced by defoliating and nondefoliating Verticillium dahliae pathotypes. Plant Dis. 2010, 94, 1156–1162. [Google Scholar] [CrossRef]
- Báidez, A.G.; Gómez, P.; Del Río, J.A.; Ortuño, A. Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb. role of phenolic compounds in plant defense mechanism. J. Agric. Food Chem. 2007, 55, 3373–3377. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, H.; Zhu, X.; Wang, W.; He, X.; Shi, Y.; Yuan, Y.; Du, X.; Cai, Y. Analysis of sea-island cotton and upland cotton in response to Verticillium dahliae infection by RNA sequencing. BMC Genom. 2013, 14, 852. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Tan, G.; Liu, K.; Kang, J.; Xu, K.; Zhang, Y.; Hu, L.; Zhang, J.; Li, C. Transcriptome analysis of the compatible interaction of tomato with Verticillium dahliae using RNA-sequencing. Front. Plant Sci. 2015, 6, 428. [Google Scholar] [CrossRef]
- Progar, V.; Jakše, J.; Štajner, N.; Radišek, S.; Javornik, B.; Berne, S. Comparative transcriptional analysis of hop responses to infection with Verticillium nonalfalfae. Plant Cell Rep. 2017, 36, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Beer-35. In Total Polyphenols (International Method), 8th ed.; The Society: St. Paul, MN, USA, 2011.
- Flajšman, M.; Radišek, S.; Javornik, B. Pathogenicity assay of Verticillium nonalfalfae on hop plants. Bio Protoc. 2017, 7, e2171. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; Daayf, F. Biocontrol treatments confer protection against Verticillium dahliae infection of potato by inducing antimicrobial metabolites. Mol. Plant Microbe Interact. 2011, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- El-Bebany, A.F.; Adam, L.R.; Daayf, F. Differential accumulation of phenolic compounds in potato in response to weakly and highly aggressive isolates of Verticillium dahliae. Can. J. Plant Pathol. 2013, 35, 232–240. [Google Scholar] [CrossRef]
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Bertelli, D.; Brighenti, V.; Marchetti, L.; Reik, A.; Pellati, F. Nuclear magnetic resonance and high-performance liquid chromatography techniques for the characterization of bioactive compounds from Humulus lupulus L. (hop). Anal. Bioanal. Chem. 2018, 410, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An Overview of the antimicrobial properties of hop. In Natural Antimicrobial Agents; Sustainable Development and Biodiversity, 19; Mérillon, J.M., Rivière, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 31–54. [Google Scholar]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.L.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Ind. Crop. Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Čeh, B.; Kač, M.; Košir, I.J.; Abram, V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crop. Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Vidmar, M.; Abram, V.; Čeh, B.; Demšar, L.; Ulrih, N.P. White hop shoot production in Slovenia: Total phenolic, microelement and pesticide residue content in five commercial cultivars. Food Technol. Biotechnol. 2019, 57, 525–534. [Google Scholar] [CrossRef] [PubMed]
- De Keukeleire, J.; Janssens, I.; Heyerick, A.; Ghekiere, G.; Cambie, J.; Roldán-Ruiz, I.; Van Bockstaele, E.; De Keukeleire, D. Relevance of organic farming and effect of climatological conditions on the formation of α-acids, β-acids, desmethylxanthohumol, and xanthohumol in hop (Humulus lupulus L.). J. Agric. Food Chem. 2007, 55, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kavalier, A.R.; Litt, A.; Ma, C.; Pitra, N.J.; Coles, M.C.; Kennelly, E.J.; Matthews, P.D. Phytochemical and morphological characterization of hop (Humulus lupulus L.) cones over five developmental stages using high performance liquid chromatography coupled to time-of-flight mass spectrometry, ultrahigh performance liquid chromatography photodiode array detection, and light microscopy techniques. J. Agric. Food Chem. 2011, 59, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- McAdam, E.L.; Freeman, J.S.; Whittock, S.P.; Buck, E.J.; Jakse, J.; Cerenak, A.; Javornik, B.; Kilian, A.; Wang, C.H.; Andersen, D.; et al. Quantitative trait loci in hop (Humulus lupulus L.) reveal complex genetic architecture underlying variation in sex, yield and cone chemistry. BMC Genom. 2013, 14, 360. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Brighenti, V.; Rodolfi, M.; Mongelli, A.; Dall’Asta, C.; Ganino, T.; Bruni, R.; Pellati, F. Development of a new high-performance liquid chromatography method with diode array and electrospray ionization-mass spectrometry detection for the metabolite fingerprinting of bioactive compounds in Humulus lupulus L. J. Chromatogr. A 2014, 1349, 50–59. [Google Scholar] [CrossRef]
- Talboys, P.W. Resistance to vascular wilt fungi. Proc. Royal Soc. Lond. Ser. B. Biol. Sci. 1972, 181, 319–332. [Google Scholar]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing Series; CABI Pub.: Wallingford, UK, 2002; ISBN 9780851995298. [Google Scholar]
- Kawchuk, L.M.; Hachey, J.; Lynch, D.R.; Kulcsar, F.; van Rooijen, G.; Waterer, D.R.; Robertson, A.; Kokko, E.; Byers, R.; Howard, R.J.; et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 6511–6515. [Google Scholar] [CrossRef]
- De Jonge, R.; van Esse, H.P.; Maruthachalam, K.; Bolton, M.D.; Santhanam, P.; Saber, M.K.; Zhang, Z.; Usami, T.; Lievens, B.; Subbarao, K.V.; et al. Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 5110–5115. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Z.; Seidl, M.F.; Majer, A.; Jakse, J.; Javornik, B.; Thomma, B.P.H.J. Broad taxonomic characterization of Verticillium wilt resistance genes reveals an ancient origin of the tomato Ve1 immune receptor. Mol. Plant Pathol. 2017, 18, 195–209. [Google Scholar] [CrossRef]
- Jakše, J.; Čerenak, A.; Radišek, S.; Satovic, Z.; Luthar, Z.; Javornik, B. Identification of quantitative trait loci for resistance to Verticillium wilt and yield parameters in hop (Humulus lupulus L.). Appl. Genet. 2013. [Google Scholar] [CrossRef]
- Antanaviciute, L.; Surbanovski, N.; Harrison, N.; McLeary, K.J.; Simpson, D.W.; Wilson, F.; Sargent, D.J.; Harrison, R.J. Mapping QTL associated with Verticillium dahliae resistance in the cultivated strawberry (Fragaria × ananassa). Hortic. Res. 2015, 2, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cockerton, H.M.; Li, B.; Vickerstaff, R.J.; Eyre, C.A.; Sargent, D.J.; Armitage, A.D.; Marina-Montes, C.; Garcia-Cruz, A.; Passey, A.J.; Simpson, D.W.; et al. Identifying Verticillium dahliae resistance in strawberry through disease screening of multiple populations and image based phenotyping. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Abdelraheem, A.; Thyssen, G.N.; Fang, D.D.; Jenkins, J.N.; McCarty, J.C.; Wedegaertner, T. Evaluation and genome-wide association study of Verticillium wilt resistance in a MAGIC population derived from intermating of eleven Upland cotton (Gossypium hirsutum) parents. Euphytica 2020, 216, 1–13. [Google Scholar] [CrossRef]
- Leyva-Pérez, M.d.l.O.; Jiménez-Ruiz, J.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Barroso, J.B.; Luque, F.; Mercado-Blanco, J. Tolerance of olive ( Olea europaea ) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytol. 2018, 217, 671–686. [Google Scholar] [CrossRef]
- Trapero, C.; Rallo, L.; López-Escudero, F.J.; Barranco, D.; Díez, C.M. Variability and selection of verticillium wilt resistant genotypes in cultivated olive and in the Olea genus. Plant Pathol. 2015, 64, 890–900. [Google Scholar] [CrossRef]
- Häffner, E.; Karlovsky, P.; Diederichsen, E. Genetic and environmental control of the Verticillium syndrome in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 235. [Google Scholar] [CrossRef]
- Obermeier, C.; Hossain, M.A.; Snowdon, R.; Knüfer, J.; von Tiedemann, A.; Friedt, W. Genetic analysis of phenylpropanoid metabolites associated with resistance against Verticillium longisporum in Brassica napus. Mol. Breed. 2013, 31, 347–361. [Google Scholar] [CrossRef]
- Njoroge, S.M.C.; Vallad, G.E.; Park, S.Y.; Kang, S.; Koike, S.T.; Bolda, M.; Burman, P.; Polonik, W.; Subbarao, K.V. Phenological and phytochemical changes correlate with differential interactions of Verticillium dahliae with broccoli and cauliflower. Phytopathology 2011, 101, 523–534. [Google Scholar] [CrossRef]
- Gharbi, Y.; Barkallah, M.; Bouazizi, E.; Hibar, K.; Gdoura, R.; Triki, M.A. Lignification, phenols accumulation, induction of PR proteins and antioxidant-related enzymes are key factors in the resistance of Olea europaea to Verticillium wilt of olive. Acta Physiol. Plant 2017, 39, 1–15. [Google Scholar] [CrossRef]
- Bruno, G.L.; Sermani, S.; Triozzi, M.; Tommasi, F. Physiological response of two olive cultivars to secondary metabolites of Verticillium dahliae Kleb. Plant Physiol. Biochem. 2020, 151, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Daayf, F.; Nicole, M.; Boher, B.; Pando, A.; Geiger, J.P. Early vascular defense reactions of cotton roots infected with a defoliating mutant strain of Verticillium dahliae. Eur. J. Plant Pathol. 1997, 103, 125–136. [Google Scholar] [CrossRef]
- Marton, K.; Flajšman, M.; Radišek, S.; Košmelj, K.; Jakše, J.; Javornik, B.; Berne, S. Comprehensive analysis of Verticillium nonalfalfae in silico secretome uncovers putative effector proteins expressed during hop invasion. PLoS ONE 2018, 13, e0198971. [Google Scholar] [CrossRef] [PubMed]
- Flajšman, M.; Mandelc, S.; Radišek, S.; Štajner, N.; Jakše, J.; Košmelj, K.; Javornik, B. Identification of novel virulence-associated proteins secreted to xylem by Verticillium nonalfalfae during colonization of hop plants. Mol. Plant Microbe Interact. 2016, 29, 362–373. [Google Scholar] [CrossRef]
- Novo, M.; Silvar, C.; Merino, F.; Martínez-Cortés, T.; Lu, F.; Ralph, J.; Pomar, F. Deciphering the role of the phenylpropanoid metabolism in the tolerance of Capsicum annuum L. to Verticillium dahliae Kleb. Plant Sci. 2017, 258, 12–20. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Karlovsky, P.; Von Tiedemann, A. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 2009, 99, 802–811. [Google Scholar] [CrossRef]
- Svara, A.; Jakse, J.; Radisek, S.; Javornik, B.; Stajner, N. Temporal and spatial assessment of defence responses in resistant and susceptible hop cultivars during infection with Verticillium nonalfalfae. J. Plant Physiol. 2019, 240, 153008. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crop. Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Daayf, F.; El Hadrami, A.; El-Bebany, A.F.; Henriquez, M.A.; Yao, Z.; Derksen, H.; El-Hadrami, I.; Adam, L.R. Phenolic compounds in plant defense and pathogen counter-defense mechanisms. In Recent Advances in Polyphenol Research; Wiley-Blackwell: Oxford, UK, 2012; Volume 3, pp. 191–208. ISBN 9781444337464. [Google Scholar]
- Mace, M.E.; Bell, A.A.; Stipanovic, R.D. Histochemistry and identification of flavanols in Verticillium wilt-resistant and -susceptible cottons. Physiol. Plant Pathol. 1978, 13, 143–149. [Google Scholar] [CrossRef]
- Besbes, F.; Habegger, R.; Schwab, W. Induction of PR-10 genes and metabolites in strawberry plants in response to Verticillium dahliae infection. BMC Plant Biol. 2019, 19, 1–17. [Google Scholar] [CrossRef]
- Veluri, R.; Weir, T.L.; Stermitz, F.R.; Vivanco, J.M. phytotoxic and antimicrobial activities of catechin derivatives. J. Agric. Food Chem. 2004, 52. [Google Scholar] [CrossRef] [PubMed]
- El Hadrami, A.; Rashidul Islam, M.D.; Adam, L.R.; Daayf, F. A cupin domain-containing protein with a quercetinase activity (VdQase) regulates Verticillium dahliae’s pathogenicity and contributes to counteracting host defenses. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, F.; Peragón, J. HPLC analysis of oleuropein, hydroxytyrosol, and tyrosol in stems and roots of Olea europaea L. cv. Picual during ripening. J. Sci. Food Agric. 2010, 90, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Landa, B.B.; Pérez, A.G.; Luaces, P.; Montes-Borrego, M.; Navas-Cortés, J.A.; Sanz, C. Insights Into the effect of Verticillium dahliae defoliating-pathotype infection on the content of phenolic and volatile compounds related to the sensory properties of virgin olive oil. Front. Plant Sci. 2019, 10, 232. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial activity of the phenolic compounds of prunus mume against enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D.; Quideau, S.; Helm, R.F.; Grabber, J.H.; Jung, H.J.G. Pathway of p-Coumaric acid incorporation into maize lignin as revealed by NMR. J. Am. Chem. Soc. 1994, 116, 9448–9456. [Google Scholar] [CrossRef]
- Keskin, Ş.; Şirin, Y.; Çakir, H.E.; Keskin, M. An investigation of Humulus lupulus L.: Phenolic composition, antioxidant capacity and inhibition properties of clinically important enzymes. South Afr. J. Bot. 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Mikyška, A.; Jurková, M. Varietal specificity of polyphenols, free phenolics and antioxidant potential in hops. Kvasny Prumysl 2019, 65, 178–185. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and in vitro effects of cultivars of Humulus lupulus L. Hops on cholinesterase activity and microbial growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial properties of spent hops extracts, flavonoids isolated therefrom, and their derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar] [CrossRef]
- Neve, R.A. Hops; Ray, A., Ed.; Chapman and Hall: London, UK, 1991; ISBN 0412303302. [Google Scholar]
- Radišek, S.; Leskošek, G.; Žveplan, S.; Zmrzlak, M.; Knapič, V. Hmeljeva Uvelost v Slovenskih Hmeljiščih/Hop Wilt in SLOVENE Hop Gardens; Oddelek za varstvo rastlin, Inštitut za Hmeljarstvo in Pivovarstvo Slovenije: Žalec, Slovenia, 2006. [Google Scholar]
- British Hop Association British Hop Varieties. Available online: https://www.britishhops.org.uk/varieties/ (accessed on 30 September 2020).
- Von Rossbauer, G.; Buhr, L.; Hack, H.; Hauptmann, S.; Klose, R.; Meier, U.; Stauss, R.; Weber, E. Phanologische Entwicklungsstadien von Kultur-Hopfen (Humulus lupulus L.). Nachr. Deut. Pflanzenschutzd. 1995, 47, 249–253. [Google Scholar]
- Analytica EBC|Hops and Hop Products|7.14—Total Polyphenols in Hops and Hop Pellets. Available online: https://brewup.eu/ebc-analytica/hops-and-hop-products/total-polyphenols-in-hops-and-hop-pellets/7.14 (accessed on 30 September 2020).
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—a story that begs to be told. Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]



| VW | Variety | WT | StG | KM | Y | H | WC | A | M | WN | Cer | SG | B | F | Cel |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WT | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |||
| 1 | StG | *** | *** | * | ** | ||||||||||
| 1 | KM | *** | *** | * | |||||||||||
| 1 | Y | *** | *** | *** | *** | ** | *** | *** | *** | *** | |||||
| 1 | H | * | |||||||||||||
| 2 | WC | * | ** | * | *** | *** | * | ** | |||||||
| 2 | A | * | |||||||||||||
| 2 | M | *** | ** | * | |||||||||||
| 3 | WN | * | ** | * | *** | * | ** | ||||||||
| 3 | Cer | *** | *** | *** | * | *** | *** | ** | |||||||
| 3 | SG | * | |||||||||||||
| 3 | B | * | |||||||||||||
| 3 | F | *** | |||||||||||||
| 3 | Cel | *** | *** | *** | * | *** | *** | ** | *** |
| VW | Variety | WT | StG | KM | Y | H | WC | A | M | WN | Cer | SG | B | F | Cel |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WT | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| 1 | StG | *** | * | * | *** | ||||||||||
| 1 | KM | ** | *** | ** | *** | * | *** | * | *** | ||||||
| 1 | Y | *** | *** | *** | *** | *** | *** | ** | *** | ** | *** | *** | |||
| 1 | H | *** | * | * | *** | ||||||||||
| 2 | WC | *** | *** | *** | |||||||||||
| 2 | A | ** | *** | ** | ** | *** | |||||||||
| 2 | M | *** | *** | ** | *** | *** | ** | *** | |||||||
| 3 | WN | *** | *** | * | *** | ** | *** | ||||||||
| 3 | Cer | *** | *** | *** | *** | *** | *** | ** | *** | ||||||
| 3 | SG | *** | *** | *** | *** | *** | *** | ||||||||
| 3 | B | *** | ** | *** | * | ** | *** | ||||||||
| 3 | F | *** | * | ** | ** | *** | |||||||||
| 3 | Cel | * | * | * | *** |
| Fungal Strain | Catechin | Luteolin | p-Coumaric Acid | Quercetin | Rutin | Tyrosol |
|---|---|---|---|---|---|---|
| Rec | 18.9 ± 0.8 | 13.7 ± 7.3 | 100 ± 0 | 15.3 ± 2.1 | 34.2 ± 3.1 | 44.8 ± 1.6 |
| T2 | 28.2 ± 2.3 | 14.0 ± 7.3 | 100 ± 0 | 12.9 ± 3.2 | 18.4 ± 5.3 | 51.7 ± 1.7 |
| Extract | Strain | ϕ1, asymptote | IC50 (mg/L) |
|---|---|---|---|
| CE stems | Rec | 87 (CI 79−95) | 148 (CI 121−246) |
| T2 | 96 (CI 88−103) | 183 (CI 137−530) | |
| WT stems | Rec | 84 (CI 76−91) | 212 (CI 141−604) |
| T2 | 106 (CI 98−113) | 130 (CI 113−163) | |
| CE roots | Rec | 84 (CI 77−90) | 1683 (CI 1485−1911) |
| T2 | 86 (CI 80−92) | 1172 (CI 1041−1308) | |
| WT roots | Rec | 89 (CI 83−95) | 1804 (CI 1617−2032) |
| T2 | 82 (CI 76−88) | 1223 (CI 1088−1365) |
| Hop Variety | VW Resistance |
|---|---|
| Wye Target | resistant |
| Styrian Gold | resistant |
| Keyworth Midseason | resistant |
| Yeoman | resistant |
| Herald | resistant |
| Wye Challenger | moderately resistant |
| Atlas | moderately resistant |
| Magnum | moderately resistant |
| Wye Northdown | susceptible |
| Cerera | susceptible |
| Savinjski Golding | susceptible |
| Buket | susceptible |
| Fuggle | susceptible |
| Celeia | susceptible |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berne, S.; Kovačević, N.; Kastelec, D.; Javornik, B.; Radišek, S. Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity. Plants 2020, 9, 1318. https://doi.org/10.3390/plants9101318
Berne S, Kovačević N, Kastelec D, Javornik B, Radišek S. Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity. Plants. 2020; 9(10):1318. https://doi.org/10.3390/plants9101318
Chicago/Turabian StyleBerne, Sabina, Nataša Kovačević, Damijana Kastelec, Branka Javornik, and Sebastjan Radišek. 2020. "Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity" Plants 9, no. 10: 1318. https://doi.org/10.3390/plants9101318
APA StyleBerne, S., Kovačević, N., Kastelec, D., Javornik, B., & Radišek, S. (2020). Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity. Plants, 9(10), 1318. https://doi.org/10.3390/plants9101318







