Functional Principles of Morphological and Anatomical Structures in Pinecones
Abstract
:1. Introduction
2. Results and Discussions
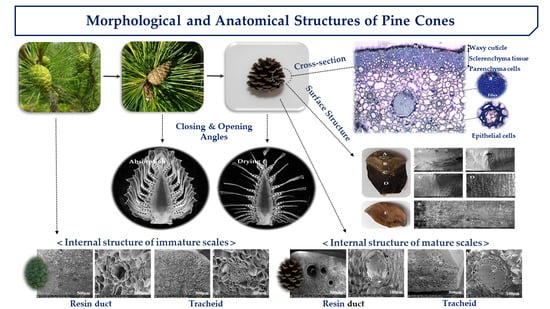
2.1. Estimation of Angle Observation from 3D Images
2.2. Thickness Changes of Internal Structure
2.3. Cross-Section Structure of Cone Scale
2.4. Internal and Surface Structure of Cone Scale
2.5. Pore Distribution of Cone Scales
3. Materials and Methods
3.1. Plant Material
3.2. Microcomputed Tomography Analysis
3.3. Dissecting-Microscope Appearance
3.4. Optical Microscope Analysis
3.5. FE-SEM (Field Emission Scanning Electron Microscope) Analysis
3.6. Mercury Intrusion Porosimetry Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tafforeau, M.; Verdus, M.C.; Norris, V.; Ripoll, C.; Thellier, M. Memory processes in the response of plants to environmental signals. Plant Signal. Behav. 2006, 1, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant surfaces: Structures and functions for biomimetic innovations. Nano Micro Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, N.D.; Dolan, L. Morphological evolution in land plants: New designs with old genes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 508–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Dai, E.; Han, X.; Xie, S.; Chao, E.; Chen, Z. Fast nastic motion of plants and bioinspired structures. J. R. Soc. Interface 2015, 12, 20150598. [Google Scholar] [CrossRef] [Green Version]
- Dumais, J.; Forterre, Y. “Vegetable dynamicks”: The role of water in plant movements. Annu. Rev. Fluid Mech. 2012, 44, 453–478. [Google Scholar] [CrossRef] [Green Version]
- Volkov, A.G.; Foster, J.C.; Ashby, T.A.; Walker, R.K.; Johnson, J.A.; Markin, V.S. Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ. 2010, 33, 163–173. [Google Scholar] [CrossRef]
- Bae, H.; Lee, E. Biological and ecological classification of biomimicry from a biology push standpoint. Ecosphere 2019, 10, e02959. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Yeom, E.; Seo, S.-J.; Kim, K.; Kim, H.; Kim, J.-H.; Lee, S.J. Journey of water in pine cones. Sci. Rep. 2015, 5, 9963. [Google Scholar] [CrossRef] [Green Version]
- Monika, A.; Krzysztof, S. Effects of microwave irradiation by means of a horn antenna in the process of seed extraction on Scots pine (Pinus sylvestris L.) cone moisture content and seed germination energy and capacity. Eur. J. For. Res. 2016, 135, 633–642. [Google Scholar]
- Aniszewska, M. The change in weight and surface temperature of a pine cone (Pinus sylvestris L.) as a result of microwave irradiation. For. Res. Paper 2016, 77, 56. [Google Scholar] [CrossRef]
- Lin, S.; Xie, Y.M.; Li, Q.; Huang, X.; Zhou, S. On the shape transformation of cone scales. Soft Matt. 2016, 12, 9797–9802. [Google Scholar] [CrossRef] [PubMed]
- Duigou, A.L.; Castro, M. Evaluation of force generation mechanisms in natural, passive hydraulic actuators. Sci. Rep. 2016, 6, 18105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Chang, S.-S.; Roper, M.; Kim, H.; Lee, S.J. A biologically-inspired symmetric bidirectional switch. PLoS ONE 2017, 12, e0169856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anttila, A.-K.; Pirttilä, A.M.; Häggman, H.; Harju, A.; Venäläinen, M.; Haapala, A.; Holmbom, B.; Julkunen-Tiitto, R. Condensed conifer tannins as antifungal agents in liquid culture. Holzforschung 2013, 67, 825. [Google Scholar] [CrossRef]
- Reyssat, E.; Mahadevan, L. Hygromorphs: From pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Bello, A.; Manyala, N.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Renewable pine cone biomass derived carbon materials for supercapacitor application. RSC Adv. 2016, 6, 1800–1809. [Google Scholar] [CrossRef] [Green Version]
- Walling, N.P.; Philamore, H.; Landenberger, K.B.; Matsuno, F. Towards a Temperature-Responsive Pinecone-Inspired Actuator Using Silicone Encapsulated Hydrogels; Springer International Publishing: Cham, Switzerland, 2019; pp. 369–373. [Google Scholar]
- Dawson, C.; Vincent, J.F.V.; Rocca, A.-M. How pine cones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Youssef, Z.; Jacquemin, F.; Gloaguen, D.; Guillén, R. Hygro-elastic internal stresses in porous composite materials: A multi-scale analysis. J. Reinf. Plast. Compos. 2007, 26, 1865–1880. [Google Scholar] [CrossRef] [Green Version]
- Baley, C. Analysis of the flax fibres tensile behaviour and analysis of the tensile stiffness increase. Compos. Part A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Song, K.; Lee, S.J. Pine cone scale-inspired motile origami. NPG Asia Mater. 2017, 9, e389. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, Y.; He, X.; Ceulemans, R. Systematic survey of resin canals in Pinaceae. Belg. J. Bot. 2002, 135, 3–14. [Google Scholar]
- Aniszewska, M.; Gendek, A.; Śliwińska, J. Variability of silver fir (Abies alba Mill.) cones—Variability structure of scale surface area. For. Res. Paper 2017, 78, 5. [Google Scholar] [CrossRef] [Green Version]
- Stawiarski, A.; Chwał, M.; Barski, M.; Muc, A. The influence of the manufacturing constraints on the optimal design of laminated conical shells. Compos. Struct. 2020, 235, 111820. [Google Scholar] [CrossRef]






| Rigida | Drying Angle (°) | Absorption Angle (°) | ||||||
| Scale no. | Left | Right | Left | Right | ||||
| Bract | Scale | Bract | Scale | Bract | Scale | Bract | Scale | |
| 1 | 42.6 | 23.6 | 45.3 | 45.5 | 30.0 | 12.4 | 30.5 | 16.7 |
| 2 | 58.6 | 55.4 | 46.7 | 47.3 | 33.3 | 5.1 | 36.6 | 7.4 |
| 3 | 68.6 | 78.8 | 65.9 | 63.2 | 43.8 | 3.0 | 48.3 | 3.8 |
| 4 | 77.8 | 82.3 | 74.6 | 77.6 | 50.4 | 1.7 | 52.5 | 1.5 |
| 5 | 86.7 | 89.6 | 69.0 | 86.0 | 45.6 | 2.7 | 43.1 | 4.8 |
| 6 | 87.5 | 102.6 | 80.0 | 88.3 | 46.2 | 7.7 | 54.1 | 5.3 |
| 7 | 96.8 | 119.1 | 90.3 | 113.5 | 59.5 | 9.1 | 63.0 | 8.9 |
| 8 | 103.5 | 112.2 | 97.9 | 117.4 | 75.6 | 18.9 | 80.5 | 19.0 |
| 9 | 133.1 | 111.6 | 115.5 | 120.7 | 60.0 | 25.6 | 83.4 | 28.9 |
| Gomsol | Drying Angle (°) | Absorption Angle (°) | ||||||
| Scale no. | Left | Right | Left | Right | ||||
| Bract | Scale | Bract | Scale | Bract | Scale | Bract | Scale | |
| 1 | 18.63 | 39.8 | 23.9 | 21.7 | 12.6 | 4.4 | 26.5 | 4.8 |
| 2 | 7.2 | 45.5 | 28.1 | 12.9 | 41.6 | 54.8 | 48.2 | 29.6 |
| 3 | 3.3 | 56.1 | 45.5 | 6.5 | 56.5 | 49.1 | 52.3 | 53.4 |
| 4 | 4.4 | 48.8 | 57.1 | 2.3 | 60.4 | 56.3 | 67.6 | 83.2 |
| 5 | 15.5 | 47.2 | 41.5 | 9.61 | 69.4 | 84.8 | 77.2 | 136.7 |
| 6 | 43.5 | 65.6 | 50.3 | 21.8 | - | - | - | - |
| 7 | 70.3 | 95.7 | 56.9 | 44.5 | - | - | - | - |
| Position | Rigida | Gomsol | ||||
|---|---|---|---|---|---|---|
| Dryness (µm) | Absorption (µm) | Change (%) | Dryness (µm) | Absorption (µm) | Change (%) | |
| 1 | 367.9 ± 35.4 | 374.0 ± 24.5 | 1.7 | 325.3 ± 96.4 | 384.3 ± 19.3 | 18.2 |
| 2 | 264.8 ± 40.9 | 287.9 ± 46.3 | 8.7 | 168.1 ± 79.3 | 176.1 ± 107.2 | 4.7 |
| 3 | 199.5 ± 36.4 | 231.7 ± 54.0 | 16.1 | 101.5 ± 44.0 | 132.3 ± 46.5 | 30.3 |
| 4 | 117.4 ± 22.1 | 157.4 ± 53.3 | 34.0 | 112.3 ± 9.3 | 149.1 ± 35.7 | 32.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.; Kim, J. Functional Principles of Morphological and Anatomical Structures in Pinecones. Plants 2020, 9, 1343. https://doi.org/10.3390/plants9101343
Bae H, Kim J. Functional Principles of Morphological and Anatomical Structures in Pinecones. Plants. 2020; 9(10):1343. https://doi.org/10.3390/plants9101343
Chicago/Turabian StyleBae, Haejin, and Jinhee Kim. 2020. "Functional Principles of Morphological and Anatomical Structures in Pinecones" Plants 9, no. 10: 1343. https://doi.org/10.3390/plants9101343
APA StyleBae, H., & Kim, J. (2020). Functional Principles of Morphological and Anatomical Structures in Pinecones. Plants, 9(10), 1343. https://doi.org/10.3390/plants9101343