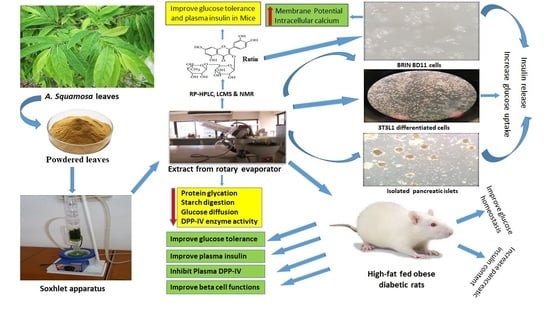
Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Plant Extracts
2.2. In Vitro Insulin-Releasing Studies
2.3. Membrane Potential and Intracellular Calcium ([Ca2+]i) Concentration
2.4. Glucose Uptake Assay
2.5. Glycation of Insulin
2.6. DPP-IV Enzyme Activity In Vitro
2.7. Starch Digestion
2.8. Glucose Diffusion In Vitro
2.9. Animals
2.10. Oral Glucose Tolerance
2.11. DPP-IV Enzyme Activity In Vivo
2.12. Glucose Homeostasis after 9-Days Treatment with Hot Water Extract of A. squamosa Leaves in High-Fat Fed Rats
2.13. Islet Morphology after 9-Days Treatment with Hot Water Extract of A. squamosa Leaves in High-Fat Fed Rats
2.14. Purification of Crude Extracts
2.15. Structural Characterization of Purified Extracts
2.16. Confirmation of Extract Purity and Identity
2.17. Statistical Analysis
3. Results
3.1. Effects of A. squamosa Leaves on Insulin Release from BRIN-BD11 Cells
3.2. Effects of A. squamosa Leaves on Insulin Release from Isolated Mouse Islets
3.3. Effects of A. squamosa Leaves on Protein Glycation In Vitro
3.4. Effects of A. squamosa Leaves on Insulin Release in the Presence of Known Modulators of Insulin Secretion and Absence of Extracellular Calcium from BRIN BD11 Cells
3.5. Effects of A. squamosa Leaves on Membrane Depolarization and Intracellular Calcium from BRIN BD11 Cells
3.6. Effects of A. squamosa Leaves on Glucose Uptake and Insulin Action
3.7. Effects of A. squamosa Leaves on Starch Digestion In Vitro
3.8. Effects of A. squamosa Leaves on Glucose Diffusion In Vitro
3.9. Effects of A. squamosa Leaves on DPP-IV Enzyme Activity In Vitro
3.10. Effects of A. squamosa Leaves on Oral Glucose Tolerance and Insulin Responses
3.11. Effects of A. squamosa Leaves on DPP-IV Enzyme Activity In Vivo
3.12. Effects of A. squamosa Leaves on Food Intake, Energy Intake, Fluid Intake, Body Weight, Non-Fasting Blood Glucose, Plasma Insulin and DPP-IV Concentration in High-Fat Fed Rats
3.13. Effects of A. squamosa Leaves on Glucose Tolerance and, Associated Plasma Insulin and DPP-IV Levels in High-Fat Fed Rats
3.14. Effects of A. squamosa Leaves on Pancreatic Insulin and Islet Morphology in High-Fat Fed Rats
3.15. Purification and Structural Characterization of Purified Extract
3.16. Effects of Peak Samples of A. squamosa Leaves on Insulin Release In Vitro
3.17. Effects of Isolated Compound Rutin on Insulin Release In Vitro
3.18. Effects of Isolated Compound Rutin on Beta Cell Membrane Depolarization and Intracellular Calcium Ion Concentration
3.19. Effects of Isolated Compound Rutin on Oral Glucose Tolerance and Insulin Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American diabetes association |
| AUC | Area under the curve |
| cAMP | Cyclic adenosine monophosphate |
| CE | Cellulose ester |
| DAPI | 4, 6-diamidino-2-phenylindole |
| DPP-IV | Dipeptidyl peptidase-IV |
| EI-MS | Electron ionization mass spectroscopy |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT-4 | Glucose transporter type 4 |
| GLY-PRO-AMC | Gly-Pro-7-Amino-4-Methyl-Coumarin |
| HDL | High-density lipoproteins |
| HWAS | Hot water extract of Annona squamosa |
| IBMX | Isobutylmethylxanthine |
| KATP | ATP-sensitive potassium channel |
| KCL | Potassium chloride |
| LC-MS | Liquid chromatography mass spectrometry |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| NaBH3CN | Sodium cyanoborohydride |
| NaCl | Sodium chloride |
| 2-NBDG | 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose |
| NMR | Nuclear Magnetic Resonance |
| RP-HPLC | Reverse phase high performance liquid chromatography |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| TFA | Trifluoroacetic acid |
| UARC | University Ayurvedic Research Centre |
References
- ADA, American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2008, 32, S62–S67. [Google Scholar] [CrossRef]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Wallia, A.; Molitch, M.E. Insulin Therapy for Type 2 Diabetes Mellitus. JAMA 2014, 311, 2315–2325. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2014, 38, 140–149. [Google Scholar] [CrossRef]
- Kato, E. Bioactive compounds in plant materials for the prevention of diabetesand obesity. Biosci. Biotechnol. Biochem. 2019, 83, 975–985. [Google Scholar] [CrossRef]
- Sarmiento, B.E.; Menezes, L.F.S.; Schwartz, E.F. Insulin Release Mechanism Modulated by Toxins Isolated from Animal Venoms: From Basic Research to Drug Development Prospects. Molecules 2019, 24, 1846. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Phys. 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Hannan, J.; Flatt, P.R.; Abdel-Wahab, Y.H. Anti-hyperglycaemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, H.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goray, V.N.P. Pharmacognostical studies on the leaf of Annona squamosa Linn. Pharmacogn. J. 2009, 1, 88–93. [Google Scholar]
- Varadharaj, V.; Kumba, U.; Krishnamurthy, V. Physicochemical, phytochemical screening and profiling of secondary metabolites of Annona squamosa leaf extract. World J. Pharm. Res. 2012, 1, 1143–1164. [Google Scholar]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Murthy, P.; Chandra, R.; Tandon, V.; Watal, G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J. Ethnopharmacol. 2005, 99, 75–81. [Google Scholar] [CrossRef]
- Kaleem, M.; Asif, M.; Ahmed, Q.U.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singap. Med. J. 2006, 47, 670–675. [Google Scholar]
- Gupta, R.K.; Kesari, A.N.; Diwakar, S.; Tyagi, A.; Tandon, V.; Chandra, R.; Watal, G. In vivo evaluation of anti-oxidant and anti-lipidimic potential of Annona squamosa aqueous extract in Type 2 diabetic models. J. Ethnopharmacol. 2008, 118, 21–25. [Google Scholar] [CrossRef]
- Panda, A.K.; Das, M.C.; Panda, P.K.; Mekap, S.K.; Pani, S.R. In-vivo anti-hyperglycemic and anti-hyperlipidemic activity of Annona squamosa (Linn.) leaves, collected from southern Odisha. World J. Pharm. Res. 2013, 2, 3347–3359. [Google Scholar]
- Sharma, A.; Chand, T.; Khardiya, M.; Yadav, K.C.; Mangal, R.; Sharma, A. Antidiabetic and antihyperlipidemic activity of Annona squamosa fruit peel in streptozotocin induced diabetic rats. IJTPR 2013, 5, 15–21. [Google Scholar]
- Sangala, R.; Burra, S.; Gopu, J.; Kodati, D.R.; Dubasi, A. Evaluation of antidiabetic activity of Annona squamosa linn seed in alloxan—Induced diabetic rats. IJPPR 2011, 2, 100–106. [Google Scholar]
- Sheikuduman, M.S.; Christina, A.J.M.; Chidambaranathan, N.; Ravi, V.; Karunakaran, G. Hepatoprotective activity of Annona squamosa Linn. on experimental animal model. Int. J. Appl. Res. Nat. Prod. 2008, 1, 1–8. [Google Scholar]
- Sharma, M.C.; Sharma, S.; Kohli, D.V. In vitro studies of the use of some medicinal herbals leaves against antidepressant, analgesic activity, and anti-inflammatory activity. Dig. J. Nanomater. Biostruct. 2010, 5, 131–134. [Google Scholar]
- Hannan, J.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S.; Huzifa, A.; Mishuk, A.R. Nigella sativa stimulates insulin secretion from isolated rat islets and inhibits the digestion and absorption of (CH2O)n in the gut. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Vasu, S.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H. Esculentin-2CHa-Related Peptides Modulate Islet Cell Function and Improve Glucose Tolerance in Mice with Diet-Induced Obesity and Insulin Resistance. PLoS ONE 2015, 10, e0141549. [Google Scholar] [CrossRef]
- Rorsman, P. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef]
- Ojo, O.O.; Abdel-Wahab, Y.; Flatt, P.R.; Mechkarska, M.; Conlon, J. Tigerinin-1R: A potent, non-toxic insulin-releasing peptide isolated from the skin of the Asian frog, Hoplobatrachus rugulosus. Diabetes Obes. Metab. 2011, 13, 1114–1122. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y. Antihyperglycaemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. Br. J. Nutr. 2011, 107, 1316–1323. [Google Scholar] [CrossRef]
- Kasabri, V.; Flatt, P.R.; Abdel-Wahab, Y. Terminalia bellirica stimulates the secretion and action of insulin and inhibits starch digestion and protein glycation in vitro. Br. J. Nutr. 2009, 103, 212–217. [Google Scholar] [CrossRef]
- O’Harte, F.P.; Højrup, P.; Barnett, C.R.; Flatt, P.R. Identification of the site of glycation of human insulin. Peptides 1996, 17, 1323–1330. [Google Scholar] [CrossRef]
- Duffy, N.A.; Green, B.D.; Irwin, N.; Gault, V.A.; McKillop, A.M.; O’Harte, F.P.; Flatt, P.R. Effects of antidiabetic drugs on dipeptidyl peptidase IV activity: Nateglinide is an inhibitor of DPP IV and augments the antidiabetic activity of glucagon-like peptide-1. Eur. J. Pharmacol. 2007, 568, 278–286. [Google Scholar] [CrossRef]
- Thomson, H.; Ojo, O.O.; Flatt, P.; Abdelwahab, Y. Antidiabetic actions of aqueous bark extract of Swertia chirayita on insulin secretion, cellular glucose uptake and protein glycation. J. Exp. Integr. Med. 2014, 4, 268. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- McCloskey, A.; Miskelly, M.; Moore, C.; Nesbit, M.; Christie, K.; Owolabi, A.; Flatt, P.; McKillop, A.M.; McMullen, C. CRISPR/Cas9 gene editing demonstrates metabolic importance of GPR55 in the modulation of GIP release and pancreatic beta cell function. Peptides 2020, 125, 170251. [Google Scholar] [CrossRef]
- Srinivasan, D.; Ojo, O.O.; Owolabi, B.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H. The frog skin host-defense peptide CPF-SE1 improves glucose tolerance, insulin sensitivity and islet function and decreases plasma lipids in high-fat fed mice. Eur. J. Pharmacol. 2015, 764, 38–47. [Google Scholar] [CrossRef]
- Grace, M.H.; Warlick, C.W.; Neff, S.A.; Lila, M.A. Efficient preparative isolation and identification of walnut bioactive components using high-speed counter-current chromatography and LC-ESI-IT-TOF-MS. Food Chem. 2014, 158, 229–238. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K. Annonaceae: Breaking the Wall of Inflammation. Front. Pharmacol. 2017, 8, 752. [Google Scholar] [CrossRef]
- Tomlinson, P.R.; Wilson, J.W.; Stewart, A.G. Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: Relationship to elevated cAMP levels. Biochem. Pharmacol. 1995, 49, 1809–1819. [Google Scholar] [CrossRef]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin Signaling and the Regulation of Glucose Transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Kawabata, J.; Mizuhata, K.; Sato, E.; Nishioka, T.; Aoyama, Y.; Kasai, T. 6-Hydroxyflavonoids as α-Glucosidase Inhibitors from Marjoram (Origanum majorana) Leaves. Biosci. Biotechnol. Biochem. 2003, 67, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Lee, L.-C.; Hu, K.-Y.; Tsai, W.-J.; Huang, C.; Tsay, H.-J.; Liu, H.-K. The application of post-translational modification oriented serum proteomics to assess experimental diabetes with complications. PLoS ONE 2018, 13, e0206509. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, Y.H.; Marenah, L.; Flatt, P.R.; Michael, C. Insulin releasing properties of the temporin family of antimicrobial peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, N.; Thimmaiah, N.V.; Jagadeesh, N.V.; Nandeep, R.; Swetha, S.; Kalidindi, B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J. Food Drug Anal. 2015, 23, 795–802. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; Van Dijck, L.; Töns, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.P.B.; De Koning, E.J.P. Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am. J. Physiol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef]
- Mizokami, A.; Yasutake, Y.; Gao, J.; Matsuda, M.; Takahashi, I.; Takeuchi, H.; Hirata, M. Osteocalcin Induces Release of Glucagon-Like Peptide-1 and Thereby Stimulates Insulin Secretion in Mice. PLoS ONE 2013, 8, e57375. [Google Scholar] [CrossRef]
- Desai, N.S.; Barhate, C.R.; Biyani, S.O.; Kulkarni, S.R.; Nagarsenker, M.S. Quantitative analysis of flavonoids in Annona squamosa leaf extracts and its pellet formulation by validated high-performance thin-layer chromatographic technique. JPC J. Planar Chromatogr. Mod. TLC 2011, 24, 306–311. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; De Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid.-Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.-W.; Li, W.-N.; Fu, X.-Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic Acid and Rutin Play a Major Role in the In Vivo Anti-Diabetic Activity of Morus alba Leaf Extract on Type II Diabetic Rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Munhoz, A.C.M.; Fröde, T.S. Isolated Compounds from Natural Products with Potential Antidiabetic Activity—A Systematic Review. Curr. Diabetes Rev. 2017, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teucrium polium on beta-cell destruction in a model of streptozotocin-induced diabetes. Planta Med. 2009, 75, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef]
- Yadav, S.; Yadava, S.; Yadav, K.D. Alpha-l-rhamnosidase selective for rutin to isoquercitrin transformation from Penicillium griseoroseum MTCC-9224. Bioorg. Chem. 2017, 70, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, B.-L.; Xie, T.; Li, G.-C.; Tuo, Y.; Xiang, Y.-T. Biotransformation of rutin to isoquercitrin using recombinant α-l-rhamnosidase from Bifidobacterium breve. Biotechnol. Lett. 2015, 37, 1257–1264. [Google Scholar] [CrossRef] [PubMed]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. https://doi.org/10.3390/plants9101348
Ansari P, Flatt PR, Harriott P, Abdel-Wahab YHA. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants. 2020; 9(10):1348. https://doi.org/10.3390/plants9101348
Chicago/Turabian StyleAnsari, Prawej, Peter R. Flatt, Patrick Harriott, and Yasser H.A. Abdel-Wahab. 2020. "Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals" Plants 9, no. 10: 1348. https://doi.org/10.3390/plants9101348
APA StyleAnsari, P., Flatt, P. R., Harriott, P., & Abdel-Wahab, Y. H. A. (2020). Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants, 9(10), 1348. https://doi.org/10.3390/plants9101348