Comparative Analysis of the Lignification Process of Two Bamboo Shoots Stored at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatment, and Storage
2.2. Measurement of Shoot Firmness
2.3. Measurement of cellulose and lignin contents
2.4. Enzyme Activity
2.5. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time (RT)-qPCR Analysis
2.6. Statistical Analysis
2.7. Accession Numbers
3. Results
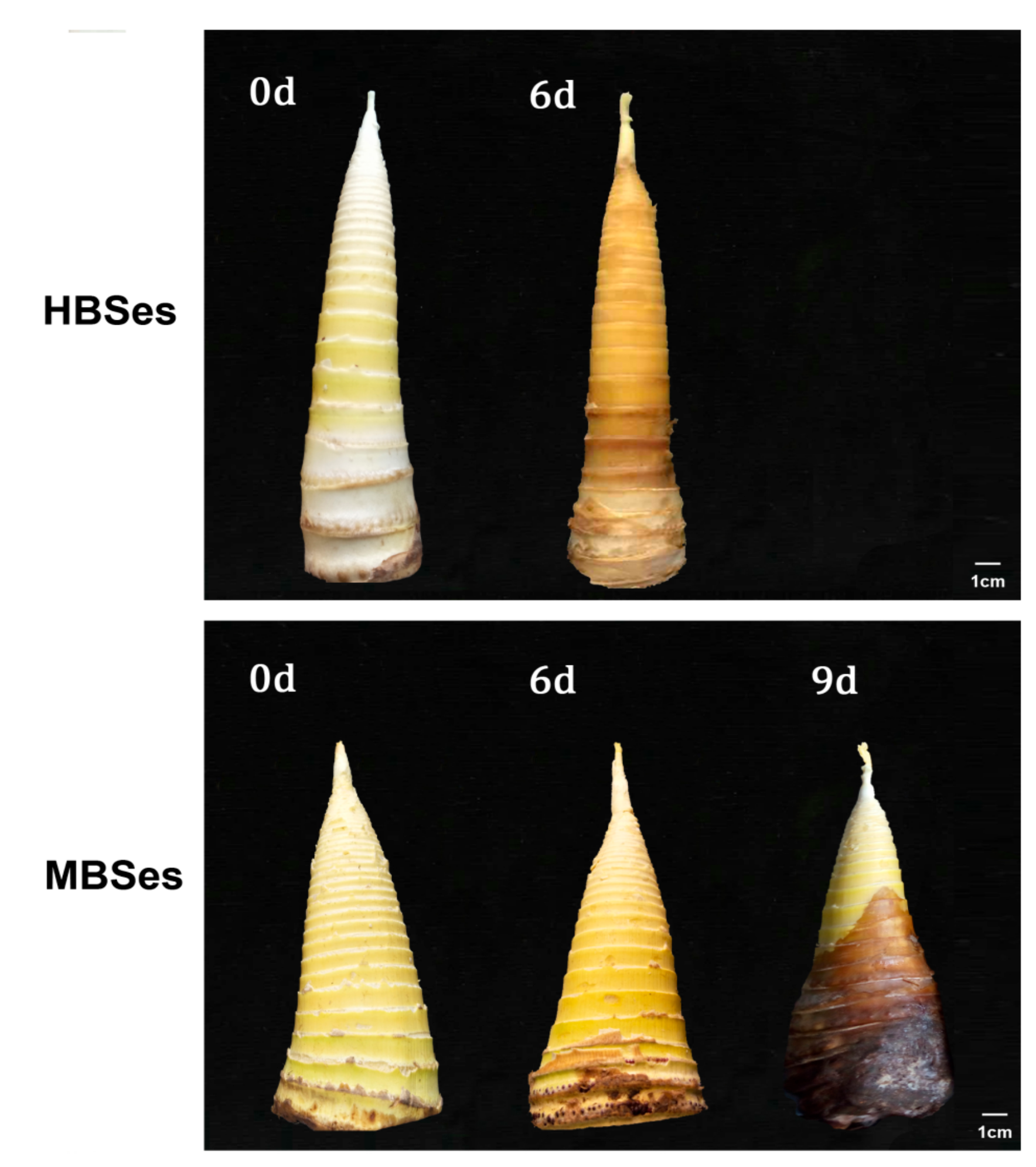
3.1. Bamboo Shoot Surface Quality
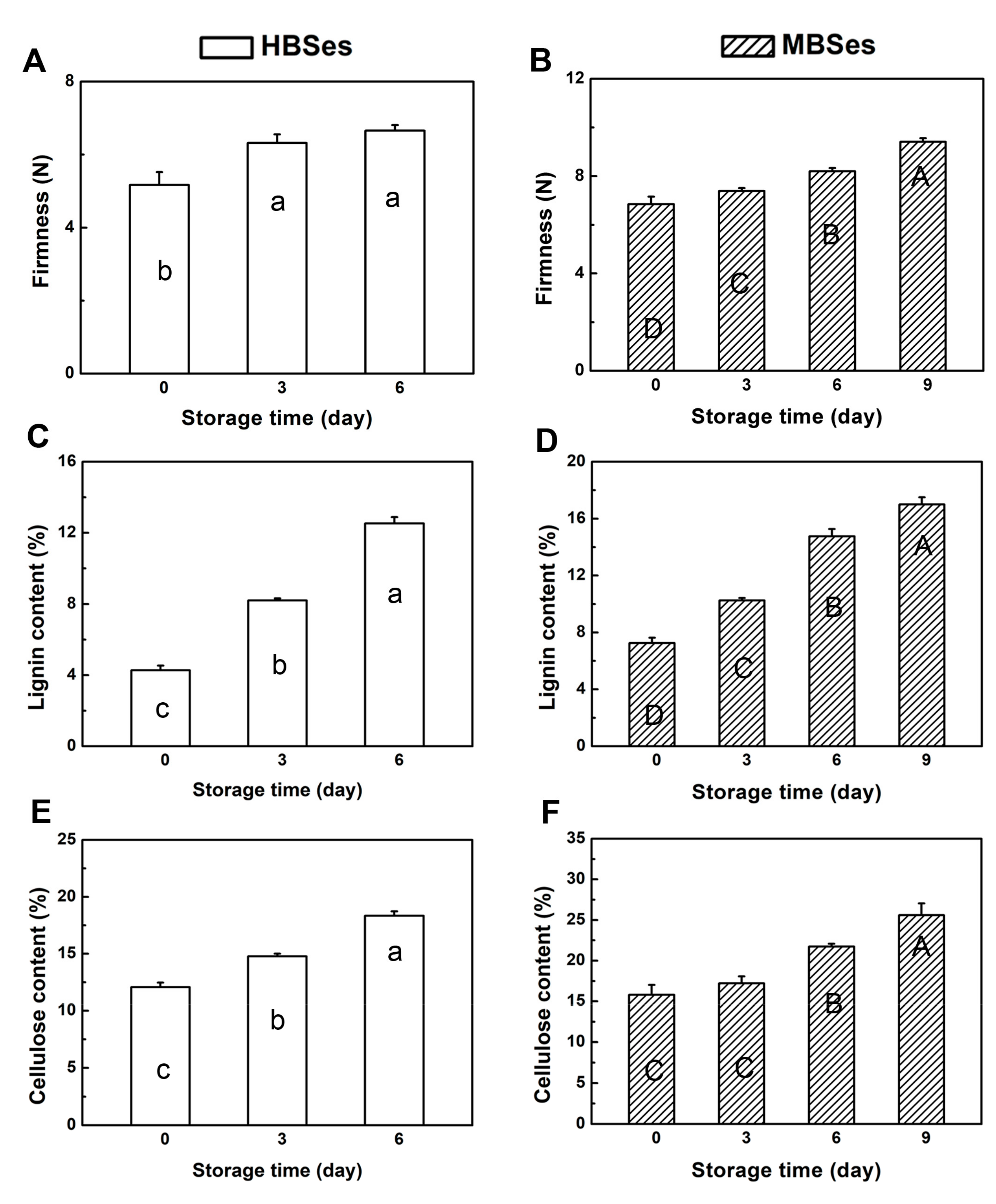
3.2. Lignin and Cellulose Contents
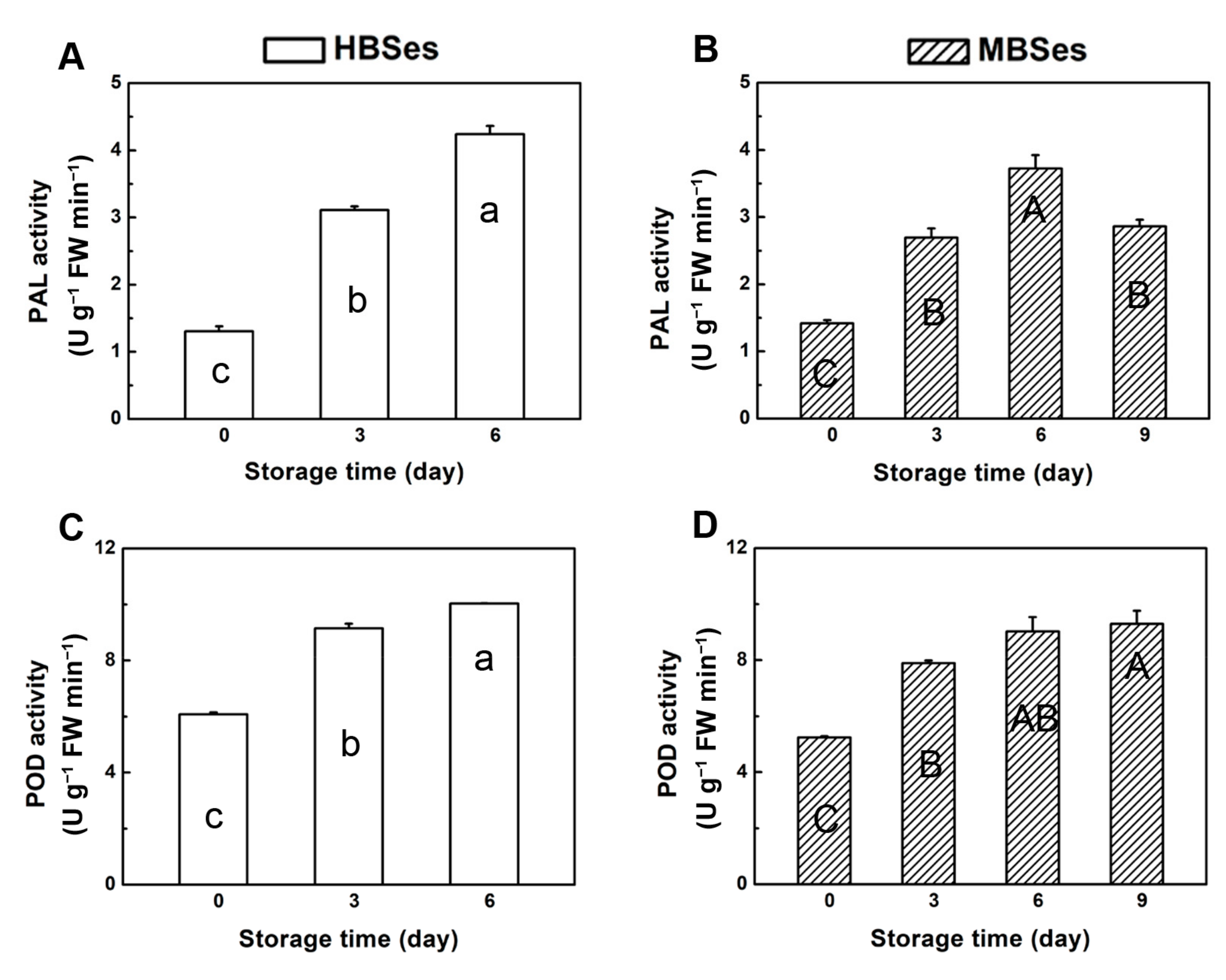
3.3. PAL and POD Activities
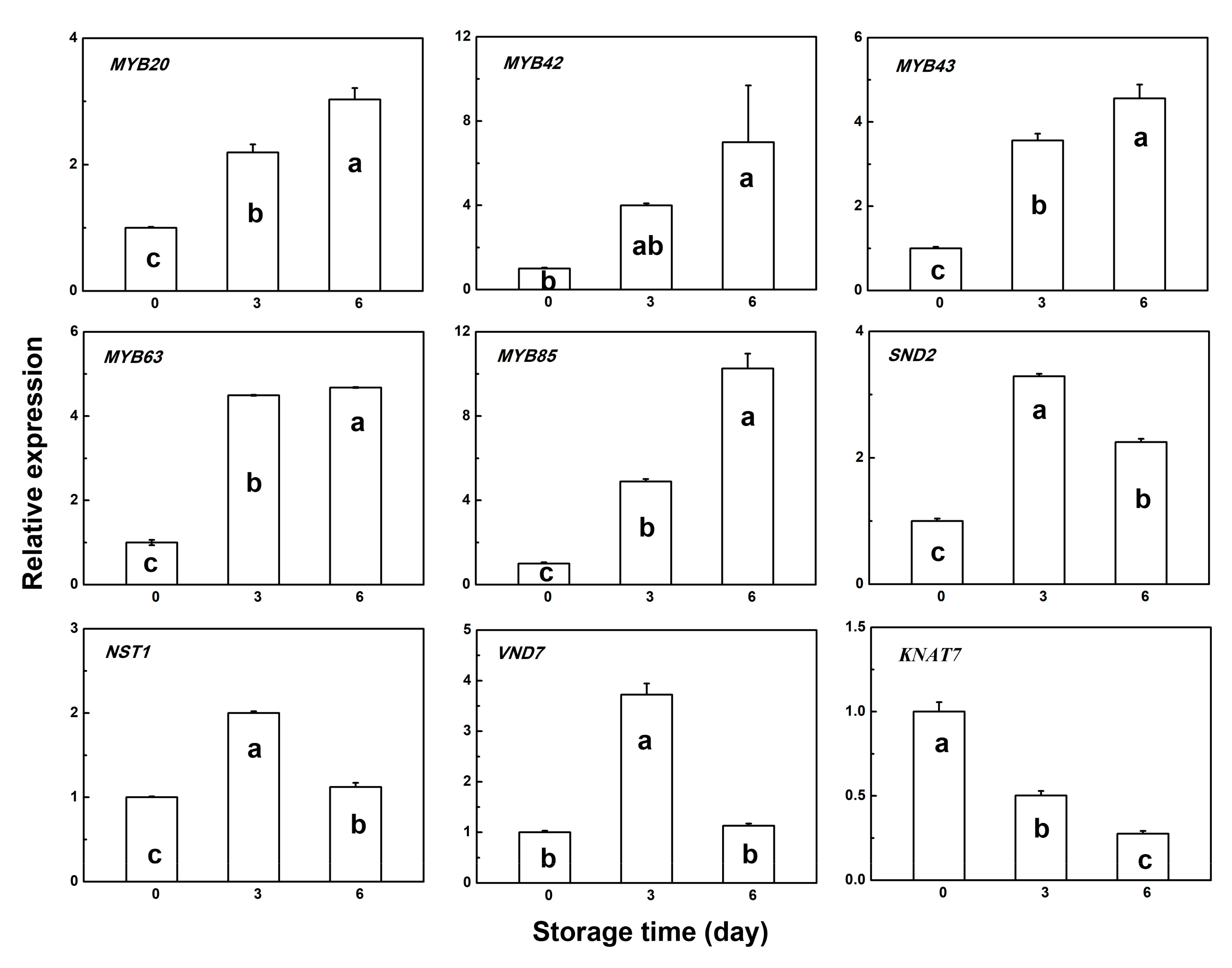
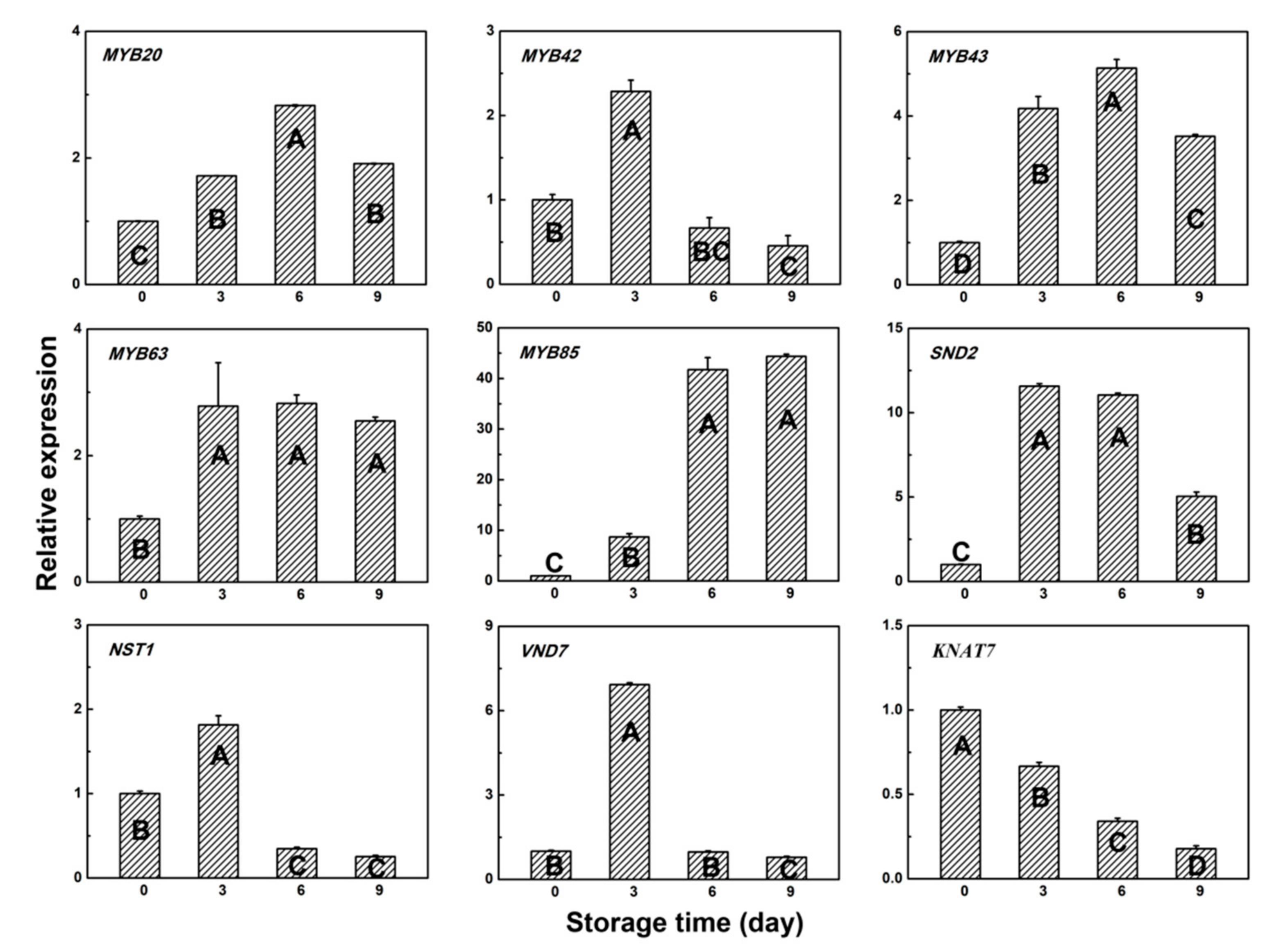
3.4. Gene Expression Pattern of Several Transcription Factors
4. Discussion
4.1. The Effect of Room Temperature Storage on the Lignification Process of Post-Harvest Bamboo Shoots
4.2. Effects of Room Temperature Storage on the Molecular Mechanism of the Lignification Process in Two Varieties of Bamboo Shoots
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleinhenz, V.; Gosbee, M.; Elsmore, S.; Lyall, T.W.; Blackburn, K.; Harrower, K.; Midmore, D.J. Storage methods for extending shelf life of fresh, edible bamboo shoots [Bambusa oldhamii (Munro)]. Postharvest Biol. Technol. 2000, 19, 253–271. [Google Scholar] [CrossRef]
- Satya, S.; Bal, L.M.; Singhal, P.; Naik, S.N. Bamboo shoot processing: Food quality and safety aspect (a review). Trends Food Sci. Tech. 2010, 21, 181–189. [Google Scholar] [CrossRef]
- Lin, J.; He, X.; Hu, Y.; Kuang, T.; Ceulemans, R. Lignifification and lignin heterogeneity for various age classes of bamboo (phyllostachys pubescens) stems. Physiol. Plant. 2002, 114, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Lacombe, E.; Legay, S.; Mihaljevic, S.; Rech, P.; Jauneau, A.; Lapierre, C.; Pollet, B.; Verhaegen, D.; Chaubet-Gigot, N.; et al. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. Plant J. 2005, 43, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Ye, Z. Transcriptional regulation of lignin biosynthesis. Plant Signal Behav. 2009, 4, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Dixon, R. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Xu, X.; Yan, B. Accumulation of lignin and involvement of enzymes in bamboo shoot during storage. Eur. Food Res. Technol. 2008, 226, 635–640. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Plant Biol. 2003, 54, 519–1146. [Google Scholar] [CrossRef]
- Donaldson, L.A. Lignifification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Rogers, L.A.; Campbell, M.M. The genetic control of lignin deposition during plant growth and development. New Phytol. 2004, 164, 17–30. [Google Scholar] [CrossRef]
- Mishra, P.K.; Wimmer, R. Aerosol assisted self-assembly as a route to synthesize solid and hollow spherical lignin colloids and its utilization in layer by layer deposition. Ultrason. Sonochem. 2017, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsick, J.S. One billion years of MYB. Oncogene 1996, 13, 223–235. [Google Scholar] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 2005, 17, 2993–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhong, R.; Ye, Z. Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS ONE 2014, 9, e105726. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; van den Toorn, A.; Sanchez-Perez, G.; Campilho, A.; Willemsen, V.; Snel, B.; Scheres, B. SOMBRERO, BEARSKIN1, and BEARSKIN2 regulate root cap maturation in Arabidopsis. Plant Cell 2010, 22, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Hussey, S.G.; Mizrachi, E.; Creux, N.M.; Myburg, A.A. Navigating the transcriptional roadmap regulating plant secondary cell wall deposition. Front Plant Sci. 2013, 4, 325. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci. 2015, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Fornalé, S.; Shi, X.; Chai, C.; Encina, A.; Irar, S.; Capellades, M.; Fuguet, E.; Torres, J.; Rovira, P.; Puigdomènech, P.; et al. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J. 2010, 64, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, F.; Fornalé, S.; Capellades, M.; Encina, A.; Touriño, S.; Torres, J.; Rovira, P.; Ruel, K.; Puigdomènech, P.; Rigau, J.; et al. ZmMYB42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in Arabidopsis thaliana. Plant Mol. Biol. 2009, 70, 283–296. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Li, C.; Lu, W.; Yang, L.; Jiang, Y.; Luo, K. Functional characterization of the poplar R2R3-MYB transcription factor PtoMYB216 involved in the regulation of lignin biosynthesis during wood formation. PLoS ONE 2013, 8, e76369. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Kim, W.C.; Han, K.H. Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J. 2009, 60, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Liu, C.; Yuan, Y.; Dai, H.; Yan, S.; Xu, J. Regulation of phenolics synthesis and their effects on fruit quality in laiyang pear. Chin. Agric. Sci. 1993, 26, 44–48. (In Chinese) [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Beaulieu, M.; Aprano, G.D.; Lacroix, M. Effect of dose rate of gramma irradia-tion on biochemical quality and browing of mushroom Agaricus bisporus. Radiation Phys. Chem. 2002, 63, 311–315. [Google Scholar] [CrossRef]
- Sala, J.M.; Lafuente, M.T. Antioxidant enzymes activities and rindstaining in ‘Navelate’ oranges as affected by storage relative humidity and ethylene conditioning. Postharvest Biol. Technol. 2004, 31, 277–285. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.C. Relationship between browning and the activities of polynylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Roura, S.I.; Pereyra, L.; del Valle, C.E. Phenylalanine ammonia lyase activity in fresh cut lettuce subjected to the combined action of heat mild shocks and chemical additives. LWT-Food Sci. Technol. 2008, 41, 919–924. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Li, C.; Xuan, L.; He, Y.; Wang, J.; Zhang, H.; Ying, Y.; Wu, A.; Bacic, A.; Zeng, W.; Song, L. Molecular Mechanism of Xylogenesis in Moso Bamboo (Phyllostachys edulis) Shoots during Cold Storage. Polymers 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.L.; Zeng, W.; Wu, A.M.; Picard, K.; Lampugnani, E.R.; Cheetamun, R.; Beahan, C.; Cassin, A.; Lonsdale, A.; Doblin, M.S.; et al. Asparagus spears as a model to study heteroxylan biosynthesis during secondary wall development. PLoS ONE 2015, 10, e0123878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, R.; Demura, T.; Ye, Z. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 2006, 18, 3158–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomal, C.; Bedon, F.; Caron, S.; Mansfield, S.D.; Levasseur, C.; Cooke JE, K.; Blais, S.; Tremblay, L.; Morency, M.; Pavy, N.; et al. Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: A comparative in planta analysis. J. Exp. Bot. 2008, 59, 3925–3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, R.L.; Zhong, R.Q.; Fowler, S.; Lyskowski, D.; Piyasena, H.; Carleton, K.; Spicer, C.; Ye, Z.H. The poplar MYB transcription factors, PtrMYB3 and PtrMYB20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol. 2010, 51, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Wang, H.; Yin, Y.; Xu, Y.; Chen, F.; Dixon, R.A. Syringyl lignin biosynthesis is directly regulated by a secondary cell wall master switch. Proc. Natl. Acad. Sci. USA 2010, 107, 14496–14501. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Mitsuda, N.; Ohtani, M.; Ohme-Takagi, M.; Kato, K.; Demura, T. VASCULAR-RELATED NAC-DOMAIN 7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J. 2011, 66, 579–590. [Google Scholar] [CrossRef]
- Zhang, H.; Ying, Y.; Wang, J.; Zhao, X.; Zeng, W.; Beahan, C.; He, J.; Chen, X.; Bacic, A.; Song, L.; et al. Transcriptome analysis provides insights into xylogenesis formation in Moso bamboo (Phyllostachys edulis) shoot. Sci. Rep. 2018, 8, 3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, E.; Bhargava, A.; Qiang, W.; Friedmann, M.C.; Forneris, N.; Savidge, R.A.; Johnson, L.A.; Mansfield, S.D.; Ellis, B.E.; Douglas, C.J. The class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus. New Phytol. 2012, 194, 102–115. [Google Scholar] [CrossRef]
- Bhargava, A.; Ahad, A.; Wang, S.; Mansfield, S.D.; Haughn, G.W.; Douglas, C.J.; Ellis, B.E. The interacting MYB75 and KNAT7 transcription factors modulate secondary cell wall deposition both in stems and seed coat in Arabidopsis. Planta 2013, 237, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, S.; Taylor-Teeples, M.; Li, W.L.; Schuetz, M.; Brady, S.M.; Douglas, C.J. BEL1-LIKE HOMEODOMAIN6 and KNOTTED ARABIDOPSIS THALIANA7 interact and regulate secondary cell wall formation via repression of REVOLUTA. Plant Cell 2014, 26, 4843–4861. [Google Scholar] [CrossRef] [Green Version]
- He, J.B.; Zhao, X.H.; Du, P.Z.; Zeng, W.; Beahan, C.T.; Wang, Y.Q.; Li, H.L.; Bacic, A.; Wu, A.M. KNAT7 positively regulates xylan biosynthesis by directly activating IRX9 expression in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 514–528. [Google Scholar] [CrossRef]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Actin | TGAGCTTCCTGATGGGCAAG | CCTGATATCCACGTCGCACTT |
| MYB20 | ACCCATCTCACCGTCCCAAA | TCTGCCTCCAGAGAGCTCCA |
| MYB42 | TGGTGAAGTGGCTGCTGGAA | CCAGCAAGCTCGAGTCCCAT |
| MYB43 | GAGTGGCCGGACACCATGTA | CATGCCTCCTGGTCAAACGC |
| MYB63 | AGGAGGACATGCGCCTCATC | TGAAGTTGCCGCGTTTGAGG |
| MYB85 | AGGTCGACCCGCTGGTAAAG | TAGTCGAGCAGCCAGTTCGT |
| SND2 | AGGGTGGCCATGGTGGTAAC | CCCTCCTGTGTGCACCTCAA |
| NST1 | GTCATCCGCGACGTCGATCT | CGGCGTTGTAGATGGCCTTG |
| VND7 | GTACGGGCATGAGGAGCAGT | CGATCACCCTCGACCTGGAC |
| KNAT7 | GCAGGACCTAACTGGTGCGA | TCCTGCCTGACCCTCTCCAT |
| Variety | Value | MYB20 | MYB42 | MYB43 | MYB63 | MYB85 | SND2 | NST1 | VND7 | KNAT7 |
|---|---|---|---|---|---|---|---|---|---|---|
| HBSes | Lignin | 0.97 *** | 0.79 * | 0.93 *** | 0.87 ** | 0.98 *** | 0.52 | 0.09 | 0.02 | −0.95 *** |
| cellulose | 0.95 *** | 0.81 * | 0.89 *** | 0.83 ** | 0.98 *** | 0.46 | 0.04 | −0.03 | −0.91 *** | |
| MBSes | Lignin | 0.64 * | −0.54 | 0.59 * | 0.56 | 0.95 *** | 0.26 | −0.69 * | −0.33 | −0.97 *** |
| cellulose | 0.52 | −0.61 * | 0.43 | 0.46 | 0.88 *** | 0.11 | −0.71 ** | −0.43 | −0.87 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, C.; Zhang, H.; Ying, Y.; Hu, Y.; Song, L. Comparative Analysis of the Lignification Process of Two Bamboo Shoots Stored at Room Temperature. Plants 2020, 9, 1399. https://doi.org/10.3390/plants9101399
Zhang Z, Li C, Zhang H, Ying Y, Hu Y, Song L. Comparative Analysis of the Lignification Process of Two Bamboo Shoots Stored at Room Temperature. Plants. 2020; 9(10):1399. https://doi.org/10.3390/plants9101399
Chicago/Turabian StyleZhang, Zuying, Changtao Li, Hui Zhang, Yeqing Ying, Yuanyuan Hu, and Lili Song. 2020. "Comparative Analysis of the Lignification Process of Two Bamboo Shoots Stored at Room Temperature" Plants 9, no. 10: 1399. https://doi.org/10.3390/plants9101399
APA StyleZhang, Z., Li, C., Zhang, H., Ying, Y., Hu, Y., & Song, L. (2020). Comparative Analysis of the Lignification Process of Two Bamboo Shoots Stored at Room Temperature. Plants, 9(10), 1399. https://doi.org/10.3390/plants9101399




