Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
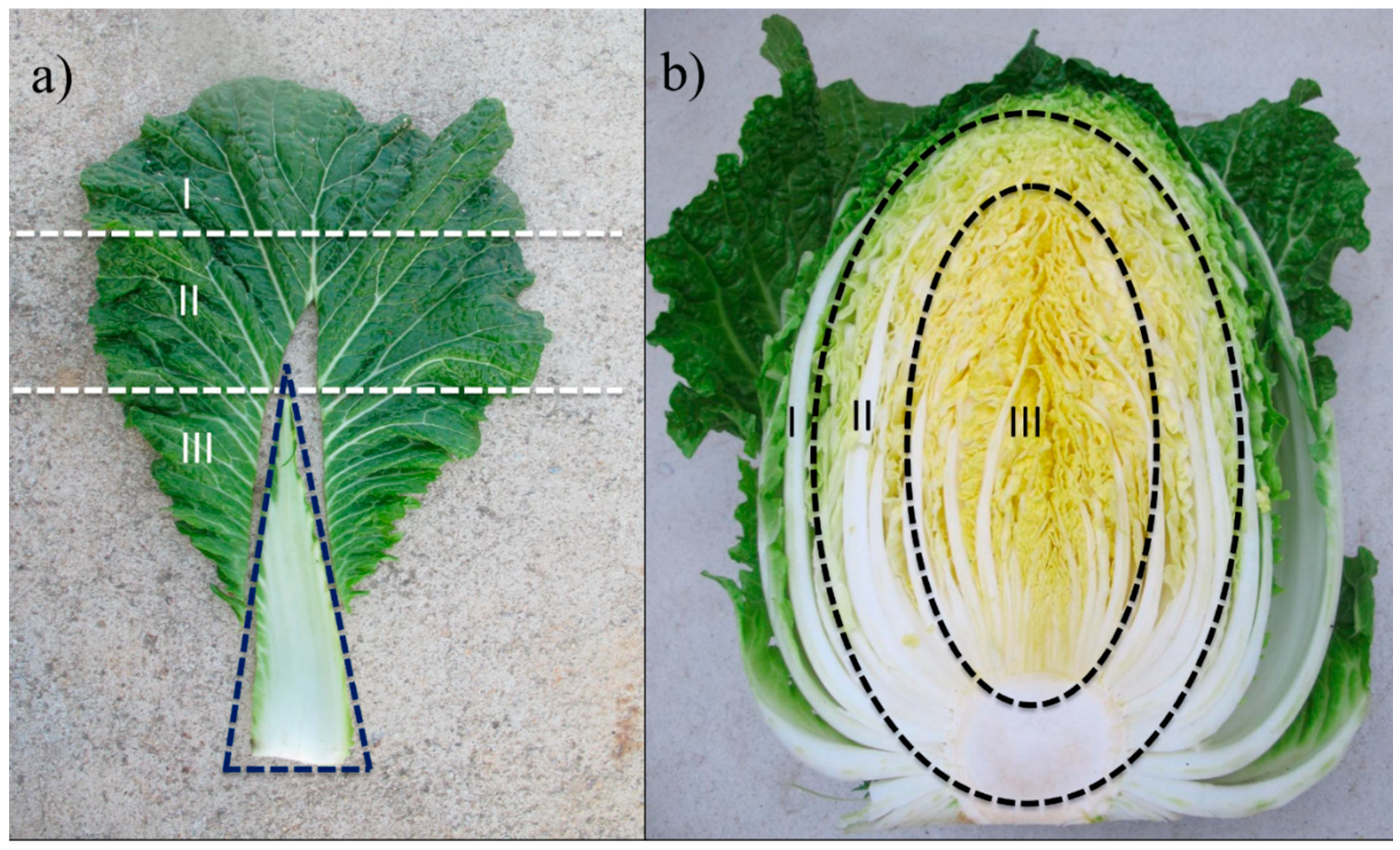
2.2. Plant Materials
2.3. Sample Pretreatment, Extraction, and Analysis of GSLs
2.4. Statistical Analysis
3. Results and Discussion
3.1. Variation in GSL Content between Germplasm Collections
3.2. Intra- and Inter-Leaf Distribution of GSLs in Kimchi Cabbage
3.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2019, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.; Zalcmann, A.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates-A review. LWT Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Hong, E.; Kim, G.-H. Anticancer and antimicrobial activities of β-phenylethyl isothiocyanate in Brassica rapa L. Food Sci. Technol. Res. 2008, 14, 377–382. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-Y.; Kim, H.-J.; Kim, D.-K.; Jo, H.-J. Anticancer activity and quantitative analysis of glucosinolates from green and red leaf mustard. Korean J. Food Nutr. 2011, 24, 362–366. [Google Scholar] [CrossRef]
- Catanzaro, E.; Fimognari, C. Antileukemic activity of sulforaphane. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 301–317. [Google Scholar]
- Blažević, I.; Radonić, A.; Skočibušić, M.; Denicola, G.R.; Montaut, S.; Iori, R.; Rollin, P.; Mastelić, J.; Zekić, M.; Maravić, A. Glucosinolate profiling and antimicrobial screening of Aurinia leucadea (Brassicaceae). Chem. Biodivers. 2011, 8, 2310–2321. [Google Scholar] [CrossRef]
- Tierens, K.; Thomma, B.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.; Broekaert, W. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef]
- Saladino, F.; Bordin, K.; Luciano, F.B.; Franzón, M.F.; Mañes, J.; Meca, G. Antimicrobial activity of the glucosinolates. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 249–274. [Google Scholar]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Sørensen, J.C.; Frandsen, H.B.; Jensen, S.K.; Kristensen, N.B.; Sørensen, S.; Sørensen, H. Bioavailability and in vivo metabolism of intact glucosinolates. J. Funct. Foods 2016, 24, 450–460. [Google Scholar] [CrossRef]
- Mithen, R. Glucosinolates—Biochemistry, genetics and biological activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Singh, A. Glucosinolates in plant defense. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 237–246. [Google Scholar]
- Lazzeri, L.; Baruzzi, G.; Malaguti, L.; Antoniacci, L. Replacing methyl bromide in annual strawberry production with glucosinolate-containing green manure crops. Pest Manag. Sci. 2003, 59, 983–990. [Google Scholar] [CrossRef]
- Bangarwa, S.K.; Norsworthy, J.K. Glucosinolate and isothiocyanate production for weed control in plasticulture production system. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 201–235. [Google Scholar]
- Aires, A.; Dias, C.S.; Carvalho, R.; Oliveira, M.H.; Monteiro, A.A.; Simões, M.V.; Rosa, E.A.; Bennett, R.N.; Saavedra, M.J. Correlations between disease severity, glucosinolate profiles and total phenolics and Xanthomonas campestris pv. campestris inoculation of different Brassicaceae. Sci. Hortic. 2011, 129, 503–510. [Google Scholar] [CrossRef]
- Rodman, J.E.; Soltis, P.S.; Soltis, D.E.; Sytsma, K.J.; Karol, K.G. Parallel evolution of glucosinolate biosynthesis inferred from congruent nuclear and plastid gene phylogenies. Am. J. Bot. 1998, 85, 997–1006. [Google Scholar] [CrossRef]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in food. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 87–132. [Google Scholar]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Lee, M.K.; Chun, J.H.; Byeon, D.H.; Chung, S.O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.P.; Kim, S.J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Khan, M.; Ulrichs, C.; Mewis, I. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 229–236. [Google Scholar] [CrossRef]
- Rosa, E.A.S.; Rodrigues, P.M.F. The effect of light and temperature on glucosinolate concentration in the leaves and roots of cabbage seedlings. J. Sci. Food Agric. 1998, 78, 208–212. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate profiles in broccoli: Variation in levels and implications in breeding for cancer chemoprotection. J. Amer. Soc. Hort. Sci. 2002, 127, 807–813. [Google Scholar] [CrossRef]
- Rosen, C.J.; Fritz, V.A.; Gardner, G.M.; Hecht, S.S.; Carmella, S.G.; Kenney, P.M. Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. HortScience 2005, 40, 1493–1498. [Google Scholar] [CrossRef]
- Ulmer, B.J.; Dosdall, L.M. Glucosinolate profile and oviposition behavior in relation to the susceptibilities of Brassicaceae to the cabbage seedpod weevil. Entomol. Exp. Appl. 2006, 121, 203–213. [Google Scholar] [CrossRef]
- Park, M.-H.; Arasu, M.V.; Park, N.-Y.; Choi, Y.-J.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, J.B.; Kim, S.-J. Variation of glucoraphanin and glucobrassicin: Anticancer components in Brassica during processing. Food Sci. Technol. Camp. 2013, 33, 624–631. [Google Scholar] [CrossRef]
- Windsor, A.J.; Reichelt, M.; Figuth, A.; Svatoš, A.; Kroymann, J.; Kliebenstein, D.J.; Gershenzon, J.; Mitchell-Olds, T. Geographic and evolutionary diversification of glucosinolates among near relatives of Arabidopsis thaliana (Brassicaceae). Phytochemistry 2005, 66, 1321–1333. [Google Scholar] [CrossRef]
- Park, S.; Valan Arasu, M.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Lee, S.W.; Al-Dhabi, N.A.; Kim, S.J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef]
- Tian, Q.; Rosselot, R.A.; Schwartz, S.J. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Biochem. 2005, 343, 93–99. [Google Scholar] [CrossRef]
- Hennig, K.; Verkerk, R.; Bonnema, G.; Dekker, M. Pitfalls in the desulphation of glucosinolates in a high-throughput assay. Food Chem. 2012, 134, 2355–2361. [Google Scholar] [CrossRef]
- Matthäus, B.; Fiebig, H. Simultaneous determination of isothiocyanates, indoles, and oxazolidinethiones in myrosinase digests of rapeseeds and rapeseed meal by HPLC. J. Agric. Food Chem. 1996, 44, 3894–3899. [Google Scholar] [CrossRef]
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Bodnaryk, R.P.; Palaniswamy, P. Glucosinolate levels in cotyledons of mustard, Brassica juncea L. and rape, B. Napus L. do not determine feeding rates of flea beetle, Phyllotreta cruciferae (goeze). J. Chem. Ecol. 1990, 16, 2735–2746. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Deighton, N.; Birch, A.E.; Patrian, B.; Baur, R.; Städler, E. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry 2001, 57, 693–700. [Google Scholar] [CrossRef]
- Velasco, L.; Becker, H.C. Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet. Resour. Crop Evol. 2000, 47, 231–238. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Yang, B.; Quiros, C.F. Survey of glucosinolate variation in leaves of Brassica rapa crops. Genet. Resour. Crop Evol. 2010, 57, 1079–1089. [Google Scholar] [CrossRef]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordas, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef]
- Baek, S.A.; Jung, Y.H.; Lim, S.H.; Park, S.U.; Kim, J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. subsp. pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef]
- Ishida, M.; Kakizaki, T.; Ohara, T.; Morimitsu, Y. Development of a simple and rapid extraction method of glucosinolates from radish roots. Breed. Sci. 2011, 61, 208–211. [Google Scholar] [CrossRef]
- Kim, J.K.; Chu, S.M.; Kim, S.J.; Lee, D.J.; Lee, S.Y.; Lim, S.H.; Ha, S.H.; Kweon, S.J.; Cho, H.S. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem. 2010, 119, 423–428. [Google Scholar] [CrossRef]
- Hong, E.; Kim, S.J.; Kim, G.H. Identification and quantitative determination of glucosinolates in seeds and edible parts of Korean Chinese cabbage. Food Chem. 2011, 128, 1115–1120. [Google Scholar] [CrossRef]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two year under different climatic conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Variyar, P.S.; Chatterjee, S.; Sharma, A. Effect of post harvest radiation processing and storage on the volatile oil composition and glucosinolate profile of cabbage. Food Chem. 2014, 151, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk-Plonska, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.B. Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by postharvest temperature and radiation treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]
- Birch, A.N.E.; Wynne Griffiths, D.; Hopkins, R.J.; Macfarlane Smith, W.H.; McKinlay, R.G. Glucosinolate responses of swede, kale, forage and oilseed rape to root damage by turnip root fly (Delia floralis) larvae. J. Sci. Food Agric. 1992, 60, 1–9. [Google Scholar] [CrossRef]
- Smith, T.K.; Lund, E.K.; Clarke, R.G.; Bennett, R.N.; Johnson, I.T. Effects of Brussels sprout juice on the cell cycle and adhesion of human colorectal carcinoma cells (HT29) in vitro. J. Agric. Food Chem. 2005, 53, 3895–3901. [Google Scholar] [CrossRef]
- Wiesner, M.; Zrenner, R.; Krumbein, A.; Glatt, H.; Schreiner, M. Genotypic variation of the glucosinolate profile in pak choi (Brassica rapa ssp. chinensis). J. Agric. Food Chem. 2013, 61, 1943–1953. [Google Scholar] [CrossRef]
- Giallourou, N.; Oruna-Concha, M.J.; Harbourne, N. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale). Food Chem. 2016, 212, 411–419. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Rdven, R.; Hansen, E.; Mlmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of different cooking methods on color, phytochemical concentration, and antioxidant capacity of raw and frozen Brassica vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Maganj, J.; Zhang, Y.A.N.; Rowland, I.R.; Wagstaff, C. Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ishii, G. Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativa Mill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci. Plant Nutr. 2006, 52, 394–400. [Google Scholar] [CrossRef]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD-MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Villatoro-Pulido, M.; Priego-Capote, F.; Álvarez-Sánchez, B.; Saha, S.; Philo, M.; Obregón-Cano, S.; De Haro-Bailón, A.; Font, R.; Del Río-Celestino, M. An approach to the phytochemical profiling of rocket [Eruca sativa (Mill.) Thell]. J. Sci. Food Agric. 2013, 93, 3809–3819. [Google Scholar] [CrossRef] [PubMed]
- Taranto, F.; Francese, G.; Di Dato, F.; D’Alessandro, A.; Greco, B.; Onofaro Sanajà, V.; Pentangelo, A.; Mennella, G.; Tripodi, P. Leaf metabolic, genetic, and morphophysiological profiles of cultivated and wild rocket salad (Eruca and Diplotaxis Spp.). J. Agric. Food Chem. 2016, 64, 5824–5836. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, S.; Lim, Y.P.; Kim, S.J.; Park, J.T.; An, G. Metabolite profiles of glucosinolates in cabbage varieties (Brassica oleracea var. capitata) by season, color, and tissue position. Hortic. Environ. Biotechnol. 2014, 55, 237–247. [Google Scholar] [CrossRef]
- Shelton, A.L. Within-plant variation in glucosinolate concentrations of Raphanus sativus across multiple scales. J. Chem. Ecol. 2005, 31, 1711–1732. [Google Scholar] [CrossRef]
- Shroff, R.; Vergara, F.; Muck, A.; Svatos, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef] [PubMed]
- Reintanz, B.; Lehnen, M.; Reichelt, M.; Gershenzon, J.; Kowalczyk, M.; Sandberg, G.; Godde, M.; Uhl, R.; Palme, K. Bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 2001, 13, 351–367. [Google Scholar] [CrossRef]
- Hamilton, J.; Zangerl, A.R.; DeLucia, E.H.; Berenbaum, M.R. The carbon-nutrient balance hypothesis: Its rise and fall. Ecol. Lett. 2001, 4, 86–95. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]


| S/No | Accession No. | Scientific Name * | Crop Name | Given Name | Origin | Classification |
|---|---|---|---|---|---|---|
| 1 | IT260816 | Brassica rapa L. | Kimchi cabbage | CHINA-YAAS-2010-103 | China | Breeding line |
| 2 | IT 260822 | Brassica rapa L. | Kimchi cabbage | CHINA-YAAS-2010-109 | China | Breeding line |
| 3 | IT 100390 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100390 | Taiwan | - |
| 4 | IT 260819 | Brassica rapa L. | Kimchi cabbage | CHINA-YAAS-2010-106 | China | Breeding line |
| 5 | IT 100414 | Brassica rapa L. | Turnip | AVRDC-KJH-1985-100414 | Taiwan | - |
| 6 | IT 260820 | Brassica rapa L. | Kimchi cabbage | CHINA-YAAS-2010-107 | China | Breeding line |
| 7 | IT 100416 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100416 | Taiwan | - |
| 8 | IT 100413 | Brassica rapa L. | Turnip | AVRDC-KJH-1985-100413 | Taiwan | - |
| 9 | IT 100388 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100388 | Taiwan | - |
| 10 | IT 100408 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100408 | Taiwan | - |
| 11 | IT 260824 | Brassica rapa L. | Kimchi cabbage | CHINA-YAAS-2010-111 | China | Breeding line |
| 12 | IT 228167 | Brassica rapa L. | Kimchi cabbage | 36197 | Taiwan | - |
| 13 | IT 100404 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100404 | Taiwan | - |
| 14 | IT 100352 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100352 | Taiwan | - |
| 15 | IT 293231 | Brassica rapa L. | Kimchi cabbage | WIR68 | Ethiopia | Cultivar |
| 16 | IT 100412 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100412 | Taiwan | - |
| 17 | IT 100411 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100411 | Taiwan | - |
| 18 | IT 100371 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100371 | Taiwan | - |
| 19 | IT 135409 | Brassica rapa L. | Kimchi cabbage | Shingatsuna | Japan | Landrace |
| 20 | IT 32750 | Brassica rapa L. | Kimchi cabbage | Ching Pao 26 | China | Cultivar |
| 21 | IT 100406 | Brassica rapa L. | Mibuna | AVRDC-KJH-1985-100406 | Taiwan | |
| 22 | IT 100353 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100353 | Taiwan | |
| 23 | IT 100372 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100372 | Taiwan | |
| 24 | Commercial | Brassica rapa L. | Kimchi cabbage | Hangamssam 1 | South Korea | Cultivar |
| 25 | IT 100366 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100366 | Taiwan | |
| 26 | IT 100393 | Brassica rapa L. | Leaf mustard | AVRDC-KJH-1985-100393 | Taiwan | |
| 27 | IT 100395 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100395 | Taiwan | |
| 28 | IT 163625 | Brassica rapa L. | Kimchi cabbage | Yeongdeog Sandongchae-2 | South Korea | Landrace |
| 29 | Commercial | Brassica rapa L. | Kimchi cabbage | Weoldongdaewang | South Korea | Cultivar |
| 30 | IT 199678 | Brassica rapa L. | Kimchi cabbage | WIR33507 | China | Landrace |
| 31 | IT 199706 | Brassica rapa L. | Kimchi cabbage | WIR30643 | China | Landrace |
| 32 | IT 32733 | Brassica rapa L. | Kimchi cabbage | Song Dao Xin 2 | China | Cultivar |
| 33 | IT 32738 | Brassica rapa L. | Kimchi cabbage | Weonsi-1984-Kimchicabbage32738 | South Korea | - |
| 34 | IT 219574 | Brassica rapa L. | Kimchi cabbage | Kang re jieqiuxiayangbaoxinbai 50 tian | China | Cultivar |
| 35 | IT 262102 | Brassica rapa L. | Kimchi cabbage | Namyeon1-ho | North korea | Cultivar |
| 36 | IT 100383 | Brassica rapa L. | Kimchi cabbage | AVRDC-KJH-1985-100383 | Taiwan | - |
| 37 | IT 120112 | Brassica rapa L. | Kimchi cabbage | Shuang Ching 156 | China | Cultivar |
| 38 | IT 163707 | Brassica rapa L. | Kimchi cabbage | JangsuSandongchae | South Korea | Landrace |
| 39 | IT 166984 | Brassica rapa L. | Kimchi cabbage | Tianjin qing | China | Landrace |
| 40 | IT 163708 | Brassica rapa L. | Kimchi cabbage | Muju Sandongchae1 | South Korea | Landrace |
| 41 | Commercial | Brassica rapa L. | Kimchi cabbage | Balgang 3-ho | South Korea | Cultivar |
| 42 | Commercial | Brassica rapa L. | Kimchi cabbage | Alchandul | South Korea | Cultivar |
| 43 | IT 215003 | Brassica rapa L. | Kimchi cabbage | Jeonnam Haenam-2000-36 | South Korea | Landrace |
| 44 | IT 199670 | Brassica rapa L. | Kimchi cabbage | Dak-se | South Korea | Landrace |
| 45 | IT 206799 | Brassica oleracea L. | Cabbage | NPL-KIG-1997-278 | South Korea | - |
| 46 | IT 216342 | Brassica rapa L. | Kimchi cabbage | Baoshou 3 | China | Cultivar |
| 47 | IT 100409 | Brassica juncea L. Czern. | Leaf mustard | AVRDC-KJH-1985-100409 | Taiwan | - |
| 48 | Commercial | Brassica rapa L. | Kimchi cabbage | Hangamssam2 | South Korea | Cultivar |
| Glucosinolates | RT (min) | MRM Transition | CID (ev) | Dwell Time (sec) | Calibration Curve Parameters |
|---|---|---|---|---|---|
| Progoitrin (PRO) | 1.41 | 387.77 > 194.85 | 20 | 0.033 | Y = 3.59902X – 20.5808 (r2 = 0.999) |
| Gluconapin (GNA) | 3.02 | 371.74 > 258.74 | 20 | 0.033 | Y = 3.50074X + 3.51886 (r2 = 0.996) |
| Glucobrassicanapin (GBN) | 4.42 | 385.71 > 258.87 | 25 | 0.033 | Y = 2.68899X – 2.8434 (r2 = 0.994) |
| Glucotropaeolin (TRO) | 4.84 | 407.72 > 258.87 | 20 | 0.033 | Y = 6.27084X – 4.49552 (r2 = 0.999) |
| Glucoerucin (ERU) | 4.97 | 419.69 > 258.74 | 25 | 0.033 | Y = 2.41077X + 16.6315 (r2 = 0.999) |
| Glucobrassicin (GBC) | 5.61 | 446.69 > 204.94 | 20 | 0.033 | Y = 1.76969X – 11.3033 (r2 = 0.999) |
| Glucoberteroin (BER) | 6.29 | 433.72 > 275.06 | 20 | 0.033 | Y = 2.92616X – 3.54071 (r2 = 0.993) |
| Gluconasturtiin (NAS) | 6.33 | 421.69 > 274.87 | 25 | 0.033 | Y = 1.98894X + 1.81048 (r2 = 0.994) |
| S/No | Gluconapin | Glucobrassicanapin | Progoitrin | Glucotropaeolin | Glucoerucin | Gluconasturtiin | Glucoberteroin | Glucobrassicin | Sum |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59.88 ± 9.48A | 14,961.04 ± 64R | 1191.24 ± 46.86U | 21.08 ± 0.36P | 59.85 ± 4.55L | 3713.98 ± 118.91Q | 450.56 ± 8.05P | 1257.38 ± 46.19M | 21,715.00 ± 186.56Q |
| 2 | 13,634.53 ± 32.45S | 19,279.35 ± 711.33V | 1548.95 ± 12.76X | 20.96 ± 1.63OP | 39.21 ± 2.26J | 6307.25 ± 365.08W | 664.07 ± 34.97R | 939.88 ± 77.28KL | 42,434.21 ± 1042.22X |
| 3 | 24.07 ± 2.22A | 19,737.72 ± 527.7W | 1207.7 ± 23.17UV | 18.96 ± 1.38OP | 20.73 ± 0.51H | 4701.79 ± 91.65T | 247.37 ± 7.70K-M | 2352.8 ± 65.59Q | 28,311.15 ± 639.34V |
| 4 | 54.11 ± 7.74A | 18,361.17 ± 307.39U | 1979.79 ± 43.03Y | 9.13 ± 0.46JK | 116.8 ± 2.76Q | 6353.11 ± 137.87W | 1221.33 ± 13.01T | 909.34 ± 15.88KL | 29,004.78 ± 212.28V |
| 5 | 11.9 ± 0.61A | 5877.32 ± 85.72HI | 13.78 ± 0.19AB | 31.77 ± 1.12R | 10.3 ± 0.62E-G | 1070.32 ± 20.69HIJ | 17.19 ± 1.18AB | 375.17 ± 24.16C-E | 7407.74 ± 124.18FG |
| 6 | 3476.23 ± 182.91lM | 13,029.46 ± 135.44P | 147.35 ± 5.72I | 6.21 ± 0.71F-I | 27.7 ± 2.97I | 5471.57 ± 147.53V | 310.33 ± 5.7NO | 633.73 ± 27.11G-I | 23,102.58 ± 347ST |
| 7 | 4735.79 ± 147.57P | 17,678.49 ± 118.58T | 30.92 ± 2.36ABC | 8.14 ± 1.13IJ | 3.48 ± 0.31A-E | 1808.78 ± 49.56L | 52.03 ± 2.5A-F | 967.2 ± 9.61KL | 25,284.81 ± 204.38U |
| 8 | 15,276.5 ± 3.34T | 6141.34 ± 63.04HI | 109.45 ± 6.7G | 8.91 ± 0.17JK | 3.55 ± 0.22A-E | 635.54 ± 15.93DEF | 16.21 ± 0.23AB | 413.84 ± 4.04D-F | 22,605.35 ± 44.95RS |
| 9 | 3728.9 ± 164.19M | 13,101.21 ± 394.68P | 560.33 ± 11.65O | 15.61 ± 1.58N | 4.74 ± 0.23A-E | 3677.9 ± 184.07Q | 61.98 ± 1.17B-G | 1623.02 ± 116.35N | 22,773.70 ± 830.86R-T |
| 10 | 2946.79 ± 232.69K | 9778.47 ± 286.18M | 32.95 ± 1.13A-D | 21.01 ± 0.89OP | 2.76 ± 0.2A-D | 918.89 ± 33.45GHI | 13.13 ± 1.09AB | 278.44 ± 8.22A-D | 13,992.44 ± 545.46L |
| 11 | 3299.16 ± 59L | 9673.08 ± 198.58LM | 296.33 ± 10.91L | 2.3 ± 0.1A-C | 7.61 ± 0.17B-F | 4023.25 ± 75.16R | 160.08 ± 3.25I | 821.22 ± 19.58I-K | 18,283.02 ± 281.06O |
| 12 | 3535.26 ± 199.95lM | 5062.22 ± 88.73GH | 12.64 ± 1.72AB | 7.87 ± 0.42H-J | 725.93 ± 10.93U | 3134.41 ± 121.18O | 2574.83 ± 88.13Y | 842.07 ± 20.2I-K | 15,895.23 ± 287.36M |
| 13 | 4191.84 ± 46.19NO | 9971.01 ± 269.09M | 331.44 ± 19.07L | 14.61 ± 1.27MN | 3.25 ± 0.35A-E | 643.2 ± 12.04DEF | 78.21 ± 1.74C-H | 500.03 ± 11.73E-G | 15,733.6 ± 283.92M |
| 14 | 3336.34 ± 165.05L | 13,541.59 ± 96.59Q | 474.05 ± 17.5N | 9.05 ± 1.17JK | 5.92 ± 0.2A-E | 2722.06 ± 114.26N | 85.32 ± 1.8D-H | 547.25 ± 7.77E-G | 20,721.58 ± 158.21P |
| 15 | 3467.66 ± 112.06LM | 6626.38 ± 172.30J | 227.4 ± 4.68K | 4.03 ± 0.51B-F | 76.69 ± 0.99MN | 4261.8 ± 210.19S | 279.83 ± 7.15L-N | 603.83 ± 27.82F-H | 15,547.64 ± 447.31M |
| 16 | 4010.97 ± 74.12N | 3497.18 ± 12.57EF | 165.63 ± 1.76I | 8.18 ± 0.28IJ | 85.21 ± 2.39O | 1773.78 ± 68.97L | 166.24 ± 2.24IJ | 1088.08 ± 34.99LM | 10,795.26 ± 175.08J |
| 17 | 11.93 ± 2.53A | 2924.5 ± 44.36DE | 111.5 ± 4.38GH | 29.87 ± 1.33R | 85.48 ± 6.99O | 858.03 ± 28.63F-H | 236.7 ± 11.87KL | 546.02 ± 33.92E-G | 4804.03 ± 115.95D |
| 18 | 2070.69 ± 49J | 9270.47 ± 118.15L | 918.9 ± 12.68T | 20.1 ± 0.32OP | 125.37 ± 0.74R | 4700.27 ± 183.2T | 1616.48 ± 36.22V | 3206.66 ± 111.92S | 21,928.94 ± 399.73QR |
| 19 | 21.75 ± 4.52A | 15,704.45 ± 392.29S | 417.99 ± 1.94M | 11.73 ± 0.51L | 204.85 ± 6.86T | 4700.35 ± 145.32T | 457.36 ± 8.04P | 1953.32 ± 63.03O | 23,471.8 ± 375.26T |
| 20 | 1190.43 ± 38.54FG | 6263.87 ± 114.31HIJ | 1474.34 ± 9.07W | 39.75 ± 0.45S | 106.73 ± 2.46P | 3441.64 ± 180.17P | 806.95 ± 12.97S | 12134.4 ± 474.88U | 25,458.11 ± 810.62U |
| 21 | 2973.98 ± 57.69k | 10,636.42 ± 86.88N | 96.36 ± 4.52FG | 18.51 ± 0.65O | 3.26 ± 0.41A-E | 2264.32 ± 39.6M | 49.01 ± 2.14A-F | 647.84 ± 17.57G-J | 16,689.69 ± 163.39N |
| 22 | 5144.32 ± 213.54q | 1101.59 ± 71.18B | 0.52 ± 0.05A | 12.04 ± 0.66L | 0.76 ± 0.06AB | 46.6 ± 5.15A | 0.19 ± 0.01A | 186.51 ± 12A-C | 6492.51 ± 297.72E |
| 23 | 1602.12 ± 31.52hi | 10,590.99 ± 161.77N | 873.97 ± 13.86S | 29.87 ± 0.55R | 78.11 ± 5.31N | 5047.2 ± 100.45U | 1494.98 ± 35.08U | 2955.72 ± 34.85R | 22,672.94 ± 351.75R-T |
| 24 | 2866.29 ± 596.67K | 9938.31 ± 179.31M | 626.3 ± 13.28P | 15.83 ± 0.68N | 188.01 ± 6.86S | 2814.85 ± 71.76N | 2426.77 ± 72.17W | 1256.82 ± 29.37M | 20,133.19 ± 869.75P |
| 25 | 1266.69 ± 23.11FG | 11,840.19 ± 410.97O | 795.24 ± 21.51R | 57.1 ± 3.27U | 48.10 ± 1.20K | 5079.75 ± 283.32U | 663.97 ± 26.40R | 2310.74 ± 95.76PQ | 22,061.78 ± 819.36QR |
| 26 | 10,185.75 ± 257.48R | 23,026.64 ± 620.66X | 240.42 ± 10.62K | 5.41 ± 0.57D-H | 0.47 ± 0.03AB | 3414.3 ± 90.05P | 70.51 ± 2.29B-H | 510.19 ± 9.12E-G | 37,453.7 ± 927.2W |
| 27 | 4356.59 ± 95.73O | 2446.97 ± 29.44C | 15.49 ± 1.39AB | 1.08 ± 0.17A | 9.21 ± 1.25C-H | 306.57 ± 10.02BC | 21.45 ± 0.6A-C | 174.01 ± 3.42A-C | 7331.37 ± 125.6EF |
| 28 | 1395.66 ± 27.59GH | 8532.77 ± 182.94K | 941.11 ± 43.58T | 13.42 ± 0.41lMN | 15.45 ± 2.45GH | 1008.44 ± 20.59HIJ | 215.08 ± 8.93JK | 369.49 ± 21.95C-E | 12,491.42 ± 280.55K |
| 29 | 489.47 ± 9.08DE | 6278.88 ± 54.44H-J | 1240.81 ± 21.58V | 11.2 ± 0.81KL | 5.24 ± 0.65A-E | 2180.63 ± 94.1M | 112.39 ± 0.96G-I | 3089.58 ± 115.3RS | 13,408.2 ± 184.56L |
| 30 | 255.83 ± 11.73A-D | 3853.9 ± 24.7F | 619.18 ± 23.47P | 10.9 ± 1.15KL | 71.37 ± 1.69M | 1179.72 ± 17.94J | 802.45 ± 24.23S | 2944.82 ± 114.14R | 9738.15 ± 97.86I |
| 31 | 257.55 ± 8.54A-D | 2780.93 ± 77.85CD | 561.96 ± 10.64O | 47.77 ± 5.06T | 56.59 ± 2.96L | 1514.37 ± 95.45K | 658.95 ± 42.59R | 2141.2 ± 140.05OP | 8019.32 ± 348.92F-H |
| 32 | 544.53 ± 6.47E | 3671.15 ± 20.04F | 170.87 ± 12.02I | 13.03 ± 0.67LM | 0.29 ± 0.00A | 858.07 ± 43.68F-H | 17.74 ± 1.92AB | 1995 ± 80.33O | 7270.68 ± 126.13EF |
| 33 | 1074.06 ± 29.14F | 4647.27 ± 136.25GH | 207.8 ± 40.27JK | 6.08 ± 0.44E-I | 2.44 ± 0.55A-C | 1466.53 ± 33.59K | 44.76 ± 0.61A-F | 774.18 ± 36.47H-K | 8223.13 ± 263.56GH |
| 34 | 466.79 ± 1.68C-E | 3113.24 ± 68.28DE | 169.68 ± 7.9I | 7.85 ± 0.36HIJ | 15.06 ± 2.73GH | 1026.78 ± 23.48H-J | 111.86 ± 4.39G-I | 2335.53 ± 123.13PQ | 7246.78 ± 214.09EF |
| 35 | 51.05 ± 1.94A | 305.54 ± 12.68A | 39.96 ± 6.33BCD | 7.73 ± 0.53HIJ | 2.13 ± 0.27A-C | 891.29 ± 31.8GH | 36.19 ± 0.89A-E | 3054.82 ± 161.62RS | 4388.7 ± 197.89C |
| 36 | 194.61 ± 8.14A-C | 1402.18 ± 28.1B | 95.62 ± 7.09FG | 4.68 ± 0.45C-G | 9.22 ± 1.13C-G | 477.73 ± 14.12C-E | 87.78 ± 1.76D-H | 1759.17 ± 53.61N | 4030.99 ± 61.51CD |
| 37 | 126.23 ± 1.92AB | 1419.5 ± 21.17B | 177.16 ± 3.65IJ | 6.94 ± 0.99GHIJ | 3.51 ± 0.39A-E | 697.43 ± 29.58E-G | 94.04 ± 2.96E-H | 1280.23 ± 46.13M | 3805.05 ± 83.11C |
| 38 | 592.17 ± 12.37E | 6582.48 ± 116.76IJ | 753.42 ± 15.53Q | 3.59 ± 0.46A-E | 14.2 ± 1.19FG | 1241.58 ± 60.26J | 352.78 ± 3.66O | 263.48 ± 10.36A-D | 9803.7 ± 116.59I |
| 39 | 381.38 ± 9.82B-E | 2785.14 ± 49.21CD | 300.07 ± 6.27L | 3.06 ± 0.25A-D | 77.62 ± 3.87N | 1136.92 ± 10.75IJ | 526.19 ± 20.32Q | 3186.07 ± 86.65S | 8396.45 ± 101.55H |
| 40 | 1791.19 ± 29.09I | 11,617.64 ± 110.72O | 391.31 ± 23.16M | 4.02 ± 0.33B-F | 3.01 ± 0.22A-D | 3232.81 ± 41.13OP | 103.98 ± 5.05F-H | 944.95 ± 13.14KL | 18,088.92 ± 172.28O |
| 41 | 70.72 ± 3.11A | 1309.85 ± 21.19B | 144.92 ± 14.72HI | 2.47 ± 0.49A-C | 5.19 ± 0.53A-E | 443.64 ± 11.57CD | 294.7 ± 7.18MN | 139.05 ± 6.11AB | 2410.54 ± 48.13B |
| 42 | 15.71 ± 1.75A | 210.42 ± 2.84A | 69.41 ± 14.23D-F | 6.01 ± 0.29E-I | 6.5 ± 0.99A-E | 161.18 ± 2.06AB | 31.09 ± 1.34A-D | 120.81 ± 7.66A | 621.15 ± 10.07A |
| 43 | 33.68 ± 2.34A | 216.5 ± 2.46A | 58.56 ± 5.03C-E | 1.97 ± 0.17AB | 9.97 ± 0.79D-G | 145.54 ± 6.6AB | 123.9 ± 6.94HI | 207.89 ± 11.57A-D | 798.00 ± 14.86A |
| 44 | 27.63 ± 4.55A | 282.4 ± 5.17A | 26.03 ± 3.18ABC | 1.04 ± 0.26A | 4.36 ± 0.42A-E | 181.09 ± 5.68AB | 67.08 ± 3.94B-H | 326.18 ± 20.08A-E | 915.81 ± 20.17A |
| 45 | 105.54 ± 2.09AB | 0.04 ± 0.01A | 42.05 ± 0.57B-D | 1.16 ± 0.27A | ND | 302.73 ± 6.55BC | 0.04 ± 0.01A | 856.47 ± 49.15J-L | 1308.02 ± 43.14A |
| 46 | 21.56 ± 0.84A | 67.31 ± 1.05A | 88.33 ± 8.34E-G | 8.13 ± 0.39IJ | 1.53 ± 0.21AB | 291.44 ± 12.69BC | 20.23 ± 2.05A-C | 350.9 ± 25.66B-E | 849.43 ± 30.04A |
| 47 | 35.79 ± 1.8A | 1.68 ± 0.06A | 0.32 ± 0.08A | 11.25 ± 0.48KL | ND | 681.72 ± 11.04E-G | 0.05 ± 0.01A | 121.37 ± 8.89A | 852.18 ± 21.09A |
| 48 | 233.33 ± 5.72A-D | 946.67 ± 19.13B | 801.03 ± 16.99R | 24.5 ± 0.91Q | ND | 1610.44 ± 50.06KL | 2510.9 ± 94.18X | 3547.13 ± 191.53T | 9673.98 ± 326.32I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhee, J.-H.; Choi, S.; Lee, J.-E.; Hur, O.-S.; Ro, N.-Y.; Hwang, A.-J.; Ko, H.-C.; Chung, Y.-J.; Noh, J.-J.; Assefa, A.D. Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants 2020, 9, 1421. https://doi.org/10.3390/plants9111421
Rhee J-H, Choi S, Lee J-E, Hur O-S, Ro N-Y, Hwang A-J, Ko H-C, Chung Y-J, Noh J-J, Assefa AD. Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants. 2020; 9(11):1421. https://doi.org/10.3390/plants9111421
Chicago/Turabian StyleRhee, Ju-Hee, Susanna Choi, Jae-Eun Lee, On-Sook Hur, Na-Young Ro, Ae-Jin Hwang, Ho-Cheol Ko, Yun-Jo Chung, Jae-Jong Noh, and Awraris Derbie Assefa. 2020. "Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves" Plants 9, no. 11: 1421. https://doi.org/10.3390/plants9111421
APA StyleRhee, J.-H., Choi, S., Lee, J.-E., Hur, O.-S., Ro, N.-Y., Hwang, A.-J., Ko, H.-C., Chung, Y.-J., Noh, J.-J., & Assefa, A. D. (2020). Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants, 9(11), 1421. https://doi.org/10.3390/plants9111421





