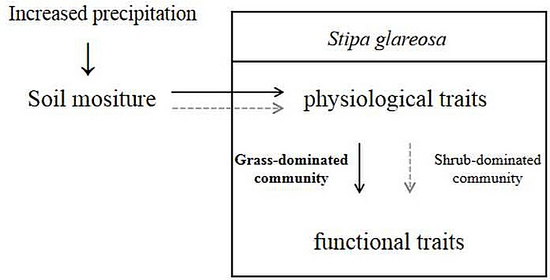
Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe
Abstract
1. Introduction
2. Results
2.1. Effects of Increased Precipitation on Functional Traits
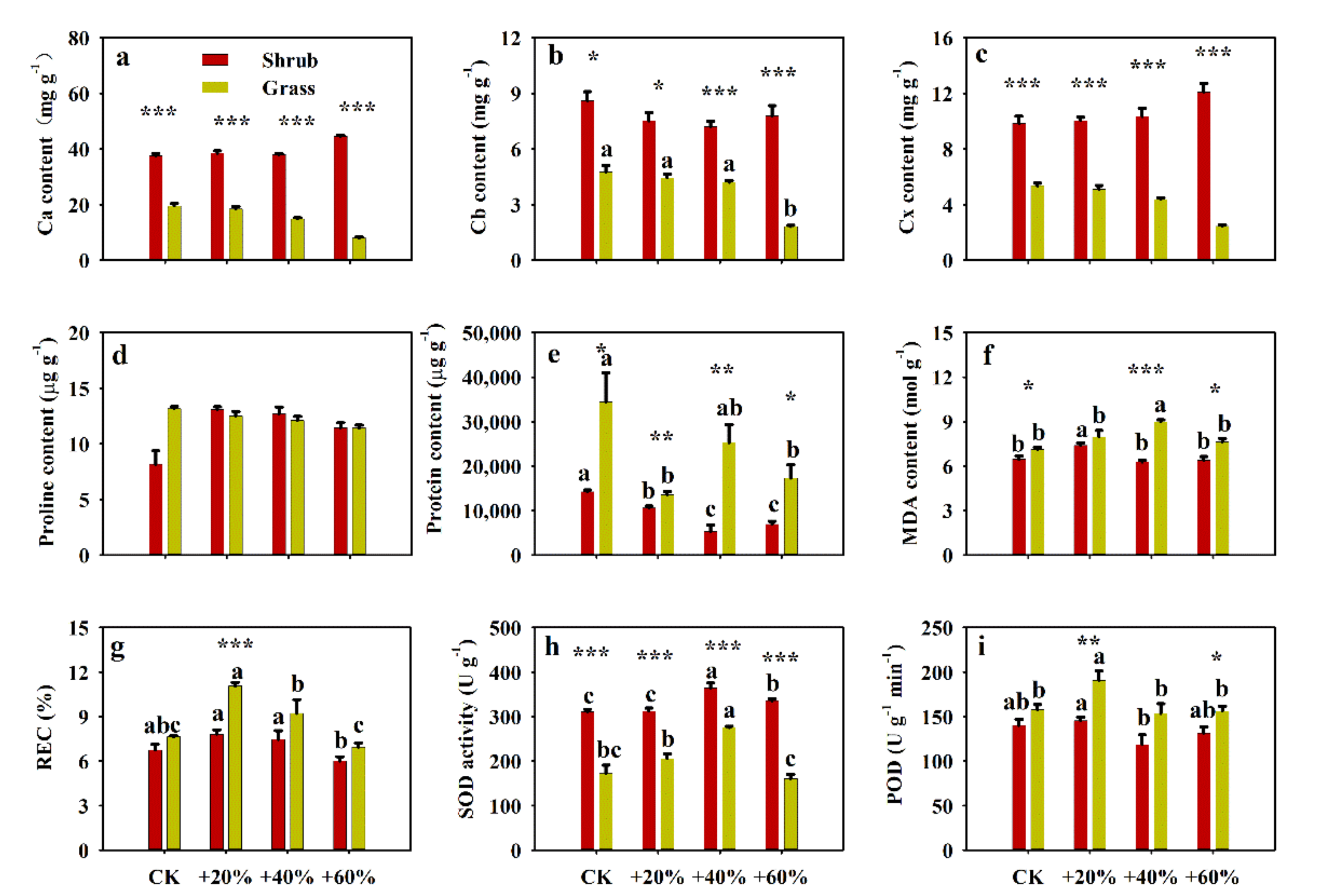
2.2. Effects of Increased Precipitation on Biochemical Traits
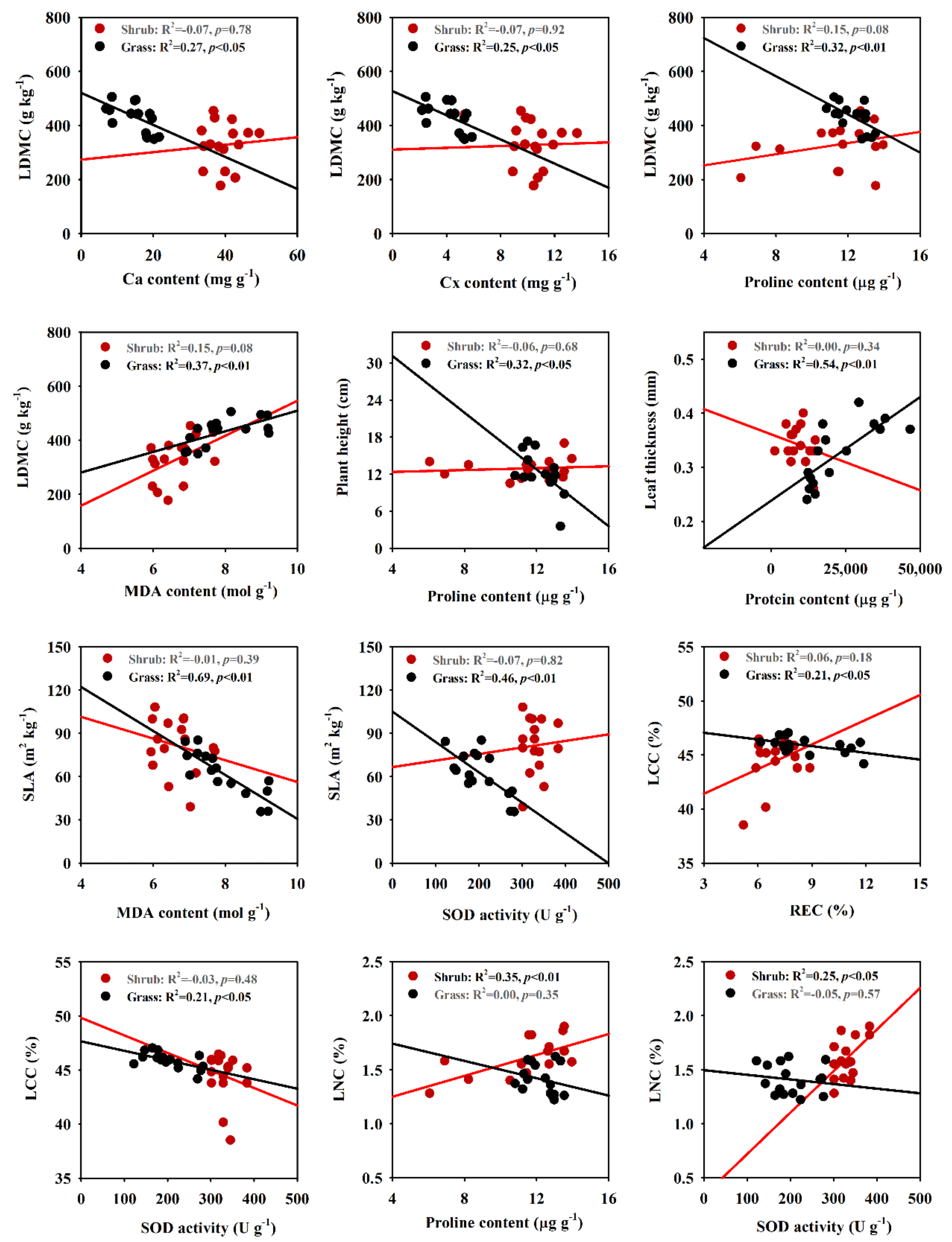
2.3. Correlations between Functional and Biochemical Traits
2.4. Correlations between Soil Water Content and Biochemical Traits
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Experimental Design
4.3. Determination of Traits
4.4. Determination of Soil Water Content
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tramblay, Y.; Badi, W.; Driouech, F.; Adlouni, S.E.; Neppel, L.; Servat, E. Climate change impacts on extreme precipitation in Morocco. Glob. Planet. Chang. 2012, 82–83, 104–114. [Google Scholar] [CrossRef]
- Murray, S.J.; Foster, P.N.; Prentice, I.C. Future global water resources with respect to climate change and water withdrawals as estimated by a dynamic global vegetation model. J. Hydrol. 2012, 448–449, 14–29. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, H.; Ma, J.; Li, G.; Wang, Q.; Rao, Z.; Huang, W.; Huang, X.; Chen, F.H. Trend of increasing Holocene summer precipitation in arid central Asia: Evidence from an organic carbon isotopic record from the LJW10 loess section in Xinjiang, NW China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 509, 24–32. [Google Scholar] [CrossRef]
- Sandel, B.; Low, R. Intraspecific trait variation, functional turnover and trait differences among native and exotic grasses along a precipitation gradient. J. Veg. Sci. 2019, 30, 633–643. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Iii, F.S.C.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Ren, H.; Xu, Z.; Huang, J.; Clark, C.; Chen, S. Nitrogen and water addition reduce leaf longevity of steppe species. Ann. Bot. 2011, 107, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.J.J.M.; Cavenderbares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Summerhayes, B.; Westoby, M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999, 69, 569–588. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Inostroza, L.; Acua, H.; Mendez, J. Multi-physiological-trait selection indices to identify Lotus tenuis genotypes with high dry matter production under drought conditions. Crop Pasture Ence 2017, 66, 90–99. [Google Scholar] [CrossRef]
- Tang, H.L.; Shen, J.B.; Zhang, F.S.; Zed, R. Interactive effects of phosphorus deficiency and exogenous auxin on root morphological and physiological traits in white lupin (Lupinus albus L.). Ence China Life Ences 2013, 56, 313–323. [Google Scholar] [CrossRef]
- Bachle, S.; Nippert, J.B. Physiological and anatomical trait variability of dominant C 4 grasses. Acta Oecologia 2018, 93, 14–20. [Google Scholar] [CrossRef]
- Costa, D.S.; Gerschlauer, F.; Kiese, R.; Fischer, M.; Kleyer, M.; Hemp, A. Plant niche breadths along environmental gradients and their relationship to plant functional traits. Divers. Distrib. 2018, 24, 1869–1882. [Google Scholar] [CrossRef]
- Austin, M.P. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Sherrard, M.E.; Latta, M.R.G. Water stress alters the genetic architecture of functional traits associated with drought adaptation in Avena barbata. Evolution 2009, 63, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Talbi, S.; Romero-Puertas, M.C.; Hernández, A.; Terrón, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2014, 111, 114–126. [Google Scholar] [CrossRef]
- Bin Rahman, A.N.M.R.; Zhang, J. Flood and drought tolerance in rice: Opposite but may coexist. Food Energy Secur. 2016, 5, 76–88. [Google Scholar] [CrossRef]
- Caldwell, C.R.; Britz, S.J. Effect of supplemental ultraviolet radiation on the carotenoid and chlorophyll composition of green house-grown leaf lettuce (Lactuca sativa L.) cultivars. J. Food Compos. Anal. 2006, 19, 637–644. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Monk, L.S.; Fagerstedt, K.V.; Crawford, R.M.M. Superoxide Dismutase as an Anaerobic Polypeptide—A Key Factor in Recovery from Oxygen Deprivation in IRIS Pseudacorus. Plant Physiol. 1987, 85, 1016–1020. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Nevmerzhitskaya, Y.Y.; Mikhaylov, A.L.; Schaimullina, G.K.; Mironov, V.F. Stevioside prevents oxidative stress in wheat seedlings. Dokl. Biol. Sci. 2015, 465, 293–295. [Google Scholar] [CrossRef]
- Pillar, V.D.; Duarte, L.D.S. A framework for metacommunity analysis of phylogenetic structure. Ecol. Lett. 2010, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Liancourt, P.; Boldgiv, B.; Song, D.S.; Spence, L.A.; Helliker, B.R.; Petraitis, P.S.; Casper, B.B. Leaf-trait plasticity and species vulnerability to climate change in a Mongolian steppe. Glob. Chang. Biol. 2015, 21, 3489–3498. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G.; Wang, Y.; Song, X. Sensitive Indicators of Zonal Stipa Species to Changing Temperature and Precipitation in Inner Mongolia Grassland, China. Front. Plant Ence 2016, 7, 73. [Google Scholar] [CrossRef]
- Zuo, X.; Cheng, H.; Zhao, S.; Yue, P.; Liu, X.; Wang, S.; Liu, L.; Xu, C.; Luo, W.; Knops, J.M.H.; et al. Observational and experimental evidence for the effect of altered precipitation on desert and steppe communities. Glob. Ecol. Conserv. 2020, 21, e00864. [Google Scholar] [CrossRef]
- Gibbens, R.P.; Mcneely, R.P.; Havstad, K.M.; Beck, R.F.; Nolen, B. Vegetation changes in the Jornada Basin from 1858 to 1998. J. Arid Environ. 2005, 61, 651–668. [Google Scholar] [CrossRef]
- Armas, C.; Kikvidze, Z.; Pugnaire, F.I. Abiotic conditions, neighbour interactions, and the distribution of Stipa tenacissima in a semiarid mountain range. J. Arid Environ. 2009, 73, 1084–1089. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 2010, 143, 155–162. [Google Scholar] [CrossRef]
- Roche, P.; Díaz-Burlinson, N.; Gachet, S. Congruency analysis of species ranking based on leaf traits: Which traits are the more reliable? Plant Ecol. 2004, 174, 37–48. [Google Scholar] [CrossRef]
- Xia, J.; Wan, S.; Ben, B.L. The Effects of Warming-Shifted Plant Phenology on Ecosystem Carbon Exchange Are Regulated by Precipitation in a Semi-Arid Grassland. PLoS ONE 2012, 7, e32088. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Li, S.; Lü, X.; Wang, X.; Han, X. Changes in specific leaf area of dominant plants in temperate grasslands along a 2500-km transect in northern China. Sci. Rep. 2017, 7, 10780. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.-P.M.; Bartholomeus, R.P.; van Dobben, H.F.; Aerts, R. Leaf habit and woodiness regulate different leaf economy traits at a given nutrient supply. Ecology 2010, 91, 3218–3228. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.G.; Palma, T.; Stanley, D.W. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–217. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, N.; Malik, S.A. Effect of D-mannose on antioxidant defense and oxidative processes in etiolated wheat coleoptiles. Acta Physiol. Plant. 2014, 36, 161–167. [Google Scholar] [CrossRef]
- Aragón, C.F.; Valladares, E.F. Stress-Induced Dynamic Adjustments of Reproduction Differentially Affect Fitness Components of a Semi-Arid Plant. J. Ecol. 2008, 96, 222–229. [Google Scholar] [CrossRef]
- Moro, M.J.; Pugnaire, F.I.; Haase, P.; Puigdefábregas, J. Mechanisms of Interaction Between a Leguminous Shrub and Its Understorey in a Semi-Arid Environment. Ecography 2006, 20, 175–184. [Google Scholar] [CrossRef]
- Geissler, K.; Hahn, C.; Joubert, D.; Blaum, N. Functional responses of the herbaceous plant community explain ecohydrological impacts of savanna shrub encroachment. Perspect. Plant Ecol. Evol. Syst. 2019, 39, 125458. [Google Scholar] [CrossRef]
- Archer, J.S. Woody Plant Establishment and Spatial Heterogeneity in Grasslands. Ecology 2003, 84, 907–919. [Google Scholar]
- Zhang, G.F.; Zhao, W.Z.; Station, L.I.R.B.; Basin, K.L.E.I.R.; Institute, E.E.; Sciences, C.A.O. Species-specific traits determine shrub-annual interactions during a growing season. J. Arid Land 2015, 7, 403–413. [Google Scholar] [CrossRef]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; Kroon, H.D. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, X.; Zuo, X.; Degen, A.A.; Shang, Z.; Luo, Y.; Zhang, Y.; Chen, J. Effect of manipulated precipitation during the growing season on soil respiration in the desert-grasslands in Inner Mongolia, China. Catena 2019, 176, 73–80. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.; Gurvich, D.; et al. New handbook for standardise measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Physiology 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.C.; Desborough, S.L. Rapid estimation of potato tuber total protein content with coomassie brilliant blue G-250. Theor. Appl. Genet. 1978, 52, 135–139. [Google Scholar] [CrossRef]
- Dipierro, S.; De Leonardis, S. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J. Exp. Bot. 1997, 48, 779–783. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Shen, L.; Fan, B.; Liu, K.L.; Yu, M.M.; Zheng, Y.; Ding, Y.; Sheng, J.P. Physiological and Genetic Properties of Tomato Fruits from 2 Cultivars Differing in Chilling Tolerance at Cold Storage. J. Food Sci. 2009, 74, C348–C352. [Google Scholar] [CrossRef]
- Desborough, S.S.L. Superoxide Dismutase, Catalase, and α-Tocopherol Content of Stored Potato Tubers. Plant Physiol. 1990, 94, 1214–1218. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zuo, X.; Yue, P.; Zhao, S.; Guo, X.; Li, X.; Medina-Roldán, E. Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe. Plants 2020, 9, 1463. https://doi.org/10.3390/plants9111463
Hu Y, Zuo X, Yue P, Zhao S, Guo X, Li X, Medina-Roldán E. Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe. Plants. 2020; 9(11):1463. https://doi.org/10.3390/plants9111463
Chicago/Turabian StyleHu, Ya, Xiaoan Zuo, Ping Yue, Shenglong Zhao, Xinxin Guo, Xiangyun Li, and Eduardo Medina-Roldán. 2020. "Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe" Plants 9, no. 11: 1463. https://doi.org/10.3390/plants9111463
APA StyleHu, Y., Zuo, X., Yue, P., Zhao, S., Guo, X., Li, X., & Medina-Roldán, E. (2020). Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe. Plants, 9(11), 1463. https://doi.org/10.3390/plants9111463