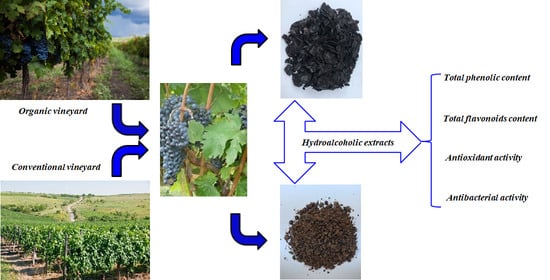
Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization of the Extracts
2.2. Antibacterial Activity of the Grapes Extracts
2.3. Statistical Analysis of Data
2.3.1. Correlations of the Phytochemical Parameters and the Antibacterial Activity of the Bacterial Strains
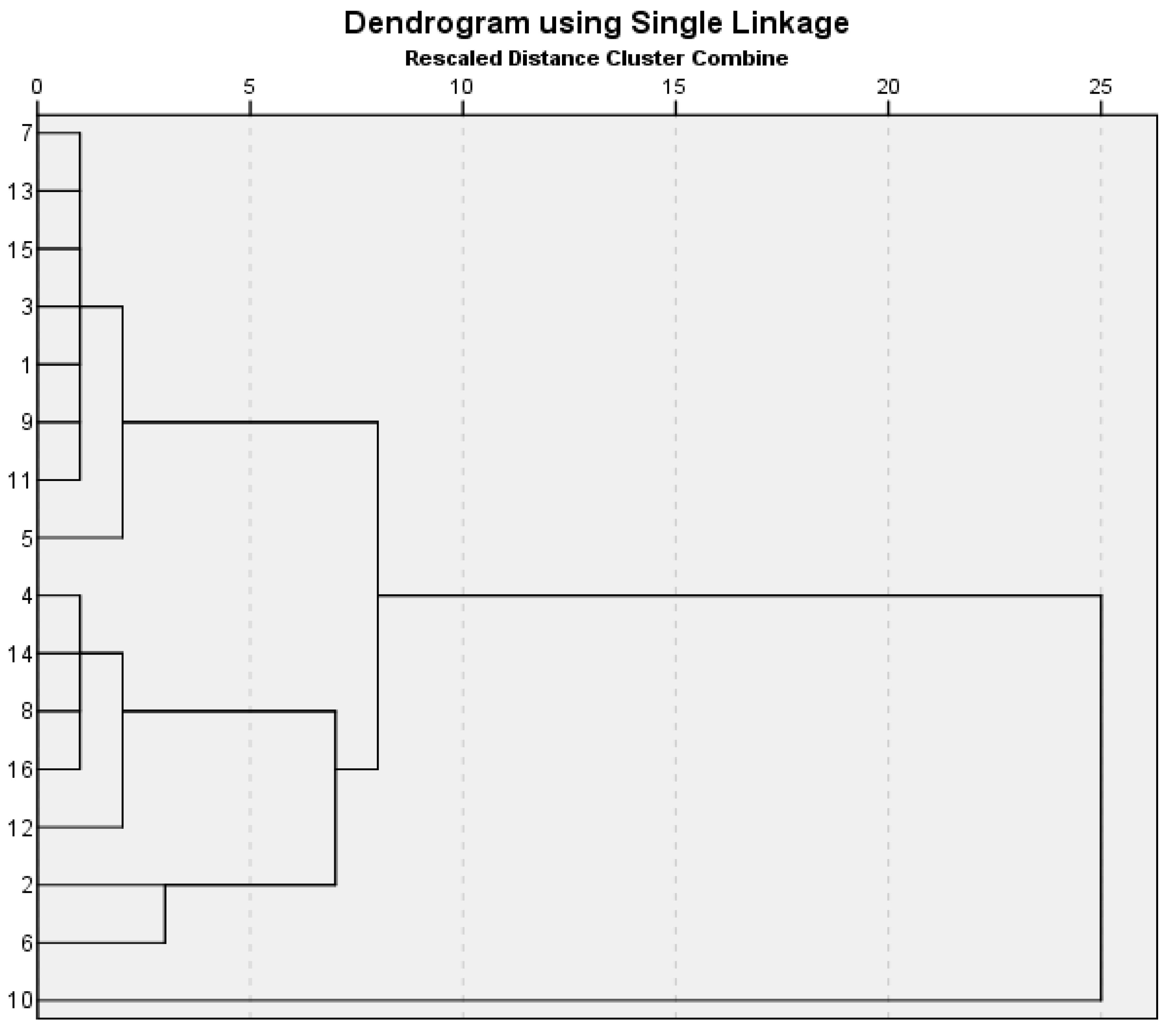
2.3.2. Statistical Package for the Social Sciences SPSS Classification: Hierarchical Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Vineyard Description
4.2. Preparation of Grape Extracts
4.3. Phytochemical Characterization of Grape Extracts
4.4. Test Microorganisms
4.5. Determination of the Antibacterial Activity
4.6. Determination of Minimum Inhibitory Concentration (MIC)
4.7. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat—Database. Available online: https://ec.europa.eu/eurostat/web/agriculture/data (accessed on 18 September 2020).
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crop. Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.J.E.; Karoutounian, S.A. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Coderoni, S.; Perito, M.A. Sustainable consumption in the circular economy. An analysis of consumers’ purchase intentions for waste-to-value food. J. Clean. Prod. 2020, 252, 119870. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; Dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.; Rosalen, P.L.; de Alencar, S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; José Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibacterial, Antiviral, and Antifungal Properties of Wine and Winery Byproducts in Relation to Their Flavonoid Content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Garcia-Lomillo, J.; Gonzalez-SanJose, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Business Wire. Available online: https://www.businesswire.com/news/home/20200228005268/en/Grape-Seed-Oil-Market-2020-2024-Increasing-Application (accessed on 13 May 2020).
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2017, 143, 922–935. [Google Scholar] [CrossRef]
- Queiroz, M.; Oppolzer, D.; Gouvinhas, I.; Silva, A.M.; Barros, A.I.R.N.A.; Domínguez-Perles, R. New grape stems’ isolated phenolic compounds modulate reactive oxygen species, glutathione, and lipid peroxidation in vitro: Combined formulations with vitamins C and E. Fitoterapia 2017, 120, 146–157. [Google Scholar] [CrossRef]
- Das, R.; Bhattacharjee, C. Chapter 43—Grapes. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 695–708. [Google Scholar]
- Peixoto, C.M.; Ines, M.; Alves, M.J.; Calhelha, R.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, C.M.; Bumbac, M.; Olteanu, R.L.; Alecu (Holban), G.C.; Boboaca-Mihaescu, D.N.; Necula, C.; Radulescu, C. Influence Of Extraction Method On Chemical Composition From Red Grapes Skin Extract. J. Sci. Arts 2019, 1, 201–208. [Google Scholar]
- Radulescu, C.; Olteanu, R.L.; Stihi, C.; Florescu, M.; Stirbescu, R.M.; Stanescu, S.G.; Nicolescu, C.M.; Bumbac, M. Chemometrics based-vibrational spectroscopy for Juglandis semen extracts investigation. J. Chemom. 2020, 34, e3234. [Google Scholar] [CrossRef]
- Alecu (Holban), G.C.; Olteanu, R.L.; Radulescu, C.; Stirbescu, R.M.; Necula, C.; Boboaca-Mihaescu, D.N. Characterization of red grapes skin extracts using vibrational spectroscopy and chemometrics. J. Sci. Arts 2020, 2, 475–490. [Google Scholar]
- Radulescu, C.; Olteanu, R.L.; Stihi, C.; Florescu, M.; Lazurca, D.; Dulama, I.D.; Stirbescu, R.M.; Teodorescu, S. Chemometric assessment of spectroscopic techniques and antioxidant activity for Hippophae rhamnoides L. extracts obtained by different isolation methods. Anal. Lett. 2019, 52, 2393–2415. [Google Scholar] [CrossRef]
- Buruleanu, L.; Radulescu, C.; Georgescu, A.A.; Nicolescu, C.M.; Olteanu, R.L.; Dulama, I.D.; Stanescu, G.S. Chemometric Assessment of the Interactions between the Metal Contents, Antioxidant Activity, Total Phenolics, and Flavonoids in Mushrooms. Anal. Lett. 2019, 52, 1195–1214. [Google Scholar] [CrossRef]
- David, M.; Serban, A.; Radulescu, C.; Danet, A.F.; Florescu, M. Bioelectrochemical evaluation of plant extracts with antioxidant capacity using gold nanozyme-based sensor. Bioelectrochemistry 2019, 129, 124–134. [Google Scholar] [CrossRef]
- Draelos, Z.D. Quality skincare on a budget, summer sun protection. Dermatol. Times 2019, 40, 14–15. [Google Scholar]
- Reed, R. The definition of “cosmeceutical”. J. Soc. Cosmet. Chem. 1962, 13, 103–106. [Google Scholar]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.M.; Montans, F.J. The mechanical behavior of skin: Structures and models for the finite element analysis. Comput. Struct. 2017, 190, 75–107. [Google Scholar] [CrossRef]
- Azimi, H.; Fallah-Tafti, M.; Khalshur, A.A.; Abdollahi, M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia 2012, 83, 1306–1317. [Google Scholar] [CrossRef]
- Kligman, A. The Future of Cosmeceuticals: An Interview with Albert Kligman, MD, PhD. Dermatol. Surg. 2005, 31 (Suppl. 1), 890–891. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Calvarheiro, M.; Ribeiro, H.M.; Simoes, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Anunciato, T.P.; Rocha-Filho, P. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products. Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simoes, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological breakthroughs in the development of topical phytocompounds-based formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef]
- Zucchinelli, M.; D’Ammaro, D.; Giubilato, E.; Zabeo, A.; Criscione, P.; Pizzol, L.; Cohen, Y.; Tarolli, P.; Lamastra, L.; Marinello, F. Use of multiple indicators to compare sustainability performance of organic vs. conventional vineyard management. Sci. Tot. Environ. 2020, 711, 135081. [Google Scholar] [CrossRef]
- Milićević, T.; Aničić Urošević, M.; Relić, D.; Jovanović, G.; Nikolić, D.; Vergel, K.; Popović, A. Environmental pollution influence to soil–plant–air system in organic vineyard: Bioavailability, environmental, and health risk assessment. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Uzman, D.; Leyer, I.; Reineke, A.; Entling, M.H. Differential effects of semi-natural habitats and organic management on spiders in viticultural landscapes. Agric. Ecosyst. Environ. 2020, 287, 106695. [Google Scholar] [CrossRef]
- Xu, W.; Bo, L.; Wang, C.; Kong, X. Organic management of grape affects yeast succession and wine sensory quality during spontaneous fermentation. LWT Food Sci. Technol. 2020, 120, 108894. [Google Scholar] [CrossRef]
- Zahedipour, P.; Asghari, M.; Abdollahi, B.; Alizadeh, M.; Danesh, Y.R. A comparative study on quality attributes and physiological responses of organic and conventionally grown table grapes during cold storage. Sci. Hortic. 2019, 247, 86–95. [Google Scholar] [CrossRef]
- Cravero, M.C. Organic and biodynamic wines quality and characteristics: A review. Food Chemistry 2020, 295, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chemistry. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Ivanišová, E.; Terentjeva, M.; Kántor, A.; Frančáková, H.; Kačániová, M. Phytochemical and Antioxidant Profile of Different Varieties of Grape from the Small Carpathians Wine Region of Slovakia. Erwerbs Obstbau 2019, 61, 53–59. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Dobrodeeva, L.; Druzhinina, A.; Ovchinnikov, D.; Parshina, A.; Shulgina, E. Biological activity of a polyphenolic complex of Arctic brown algae. J. Appl. Phycol. 2019, 31, 3341–3348. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, D.S.; Jung, Y.J.; Park, J.H.; Choi, J.I.; Yim, M.J.; Jeon, J.M.; Kim, H.W.; Son, K.T.; Je, J.Y.; et al. The mechanism of antibacterial activity of phlorofucofuroeckol-A against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef]
- Cherian, C.; Jannet Vennila, J.; Sharan, L. Marine bromophenols as an effective inhibitor of virulent proteins (peptidyl arginine deiminase, gingipain R and hemagglutinin A) in Porphyromas gingivalis. Arch. Oral Biol. 2019, 100, 119–128. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal Activity of Phlorotannins against Dermatophytes and Yeasts: Approaches to the Mechanism of Action and Influence on Candida albicans Virulence Factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Kim, S.J.; Lee, W.S. Influenza virus neuraminidase inhibitory activity of phlorotannins from the edible brown alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Morán-Santibañez, K.; Peña-Hernáncez, M.A.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Skouta, R.; Vasquez, A.H.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Virucidal and Synergistic Activity of Polyphenol-Rich Extracts of Seaweeds against Measles Virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.; Liu, G.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Iun, J.J.; Jung, M.; Jeong, I.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Zeid, A.H.A.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Kim, S.-K. Seaweeds: Valuable Ingredients for the Pharmaceutical Industries; Springer: Cham, Switzerland, 2018; ISBN 9783319690759. [Google Scholar]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Kasanah, N.; Amelia, W.; Mukminin, A.; Isnansetyo, T.; Isnansetyo, A. Antibacterial activity of Indonesian red algae Gracilaria edulis against bacterial fish pathogens and characterization of active fractions. Nat. Prod. Res. 2019, 33, 3303–3307. [Google Scholar] [CrossRef] [PubMed]
- Anjali, K.P.; Sangeetha, B.M.; Devi, G.; Raghunathan, R.; Dutta, S. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine-a comparative study. J. Photochem. Photobiol. B Biol. 2019, 200, 111622. [Google Scholar] [CrossRef] [PubMed]
- de Felício, R.; de Albuquerque, S.; Young, M.C.M.; Yokoya, N.S.; Debonsi, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Shen, W.; Rui, W.; Ma, X.; Cen, Y. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar] [CrossRef]
- Rajauria, G.; Abu-ghannam, N. Isolation and Partial Characterization of Bioactive Fucoxanthin from Himanthalia elongata Brown Seaweed: A TLC-Based Approach. Int. J. Anal. Chem. 2013, 2013, 802573. [Google Scholar] [CrossRef]
- Karpinski, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Y.; Xu, C.; Lu, J.; Wang, Y. The growing season impacts the accumulation and composition of flavonoids in grape skins in two-crop-a-year viticulture. J. Food Sci. Technol. 2017, 54, 2861–2870. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature of grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grape and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Haselgrove, L.; Botting, D.; van Heeswijck, R.; Hoj, P.B.; Dry, P.R.; Ford, C.; Iland, P.G. Canopy microclimate and berry composition: The effect of bunch exposure in the phenolic composition of Vitis vinifera L. cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar] [CrossRef]
- Bunea, C.I.; Pop, N.; Babes, A.C.; Mantea, C.; Dulf, F.V.; Bunea, A. Carotenoids, total polyphenols, and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem. Cent. J. 2012, 6, 66. [Google Scholar] [CrossRef]
- Winter, C.K.; Davis, S.F. Organic Foods. J. Food Sci. 2006, 71, 117–124. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Chirila, F.; Ober, C.; Socaciu, C. Antibacterial action of an aqueous grape seed polyphenolic extract. Afr. J. Biotechnol. 2011, 10, 6276–6280. [Google Scholar]
- Nirmala, G.; Narendhirakannan, R.T. In vitro antioxidant and antimicrobial activities of grapes (Vitis vinifera L) seed and skin extracts—Muscat variety. Int. J. Pharm. Sci. 2011, 3, 242–249. [Google Scholar]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Baydar, N.G.; Sagdic, O.; Ozkan, G.; Cetin, S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. Technol. 2006, 41, 799–804. [Google Scholar] [CrossRef]
- Yigit, D.; Yigit, N.; Mavi, A.; Yildirim, A.; Güleryüz, M. Antioxidant and antimicrobial activities of methanol and water extracts of fruits, leaves and seeds of Vitis vinifera L. cv. Karaerik. Asian J. Chem. 2009, 21, 183–194. [Google Scholar]
- Over, K.F.; Hettiarachchy, N.; Johnson, M.G.; Davis, B. Effect of organic acids and plant extracts on Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium in broth culture model and chicken meat systems. J. Food Sci. 2009, 74, M515–M521. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J. Food Prot. 2004, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, K.; Yang, J.; Sakharkar, M.K. Gallic Acid Potentiates the Antimicrobial Activity of Tulathromycin against Two Key Bovine Respiratory Disease (BRD) Causing-Pathogens. Front Pharmacol. 2018, 9. article 1486. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Siddiqi, R.; Ahmad, S.; Rasool, S.; Sayeed, S.A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007, 72, M341–M345. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.L.; Kahkonen, M.; Heinone, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimia, R. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobials agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, C.; Johnson, D.; Pinto, M.D.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.R.; Wang, H.Y.; Wang, S.T.; Sun, S.L.; Zhou, J.; Luan, Y.Y. Assessment of the antibacterial activity and the antidiarrheal function of flavonoids from bayberry fruit. J. Agric. Food Chem. 2011, 59, 5312–5317. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Abramovič, H.; Terpinc, P.; Generalić, I.; Skroza, D.; Klancnik, A.; Katalinic, V.; Možina, S.S. Antioxidant and antimicrobial activity of extracts obtained from rosemary (Rosmarinus officinalis) and vine (Vitis vinifera) leaves. Croat. J. Food Sci. Technol. 2012, 4, 1–8. [Google Scholar]
- El Darra, N.; Tannous, J.; Bou Mounsef, P.; Palge, J.; Vaghi, J.; Voroliev, E.; Louka, N.; Maroun, R.S. A Comparative Study on Antiradical and Antimicrobial Properties of Red Grapes Extracts Obtained from Different Vitis vinifera Varieties. Food Nutr. Sci. 2012, 3, 1420–1432. [Google Scholar] [CrossRef]
- Soto, M.L.; Falque, E.; Dominguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]









| Grape Variety | Vineyard Type | Total Phenolic Content [mg GAE/g] | Total Flavonoids Content [mg Quercetin/g] | Antioxidant Activity [mg Ascorbic Acid/g] | |||
|---|---|---|---|---|---|---|---|
| Grape Berry Tissue | |||||||
| Skin | Seeds | Skin | Seeds | Skin | Seeds | ||
| Merlot | Organic | 55.69 ± 3.18 ab | 146.80 ± 6.53 b | 43.94 ± 3.84 | 172.19 ± 9.67 | 21.22 ± 1.39 a | 355.77 ± 9.57 |
| Conventional | 15.82 ± 0.50 ab | 47.38 ± 0.90 b | 51.89 ± 3.44 | 120.69 ± 8.53 | 24.22 ± 1.92 a | 143.2 ± 7.04 | |
| Feteasca Neagra | Organic | 71.98 ± 4.04 ab | 150.92 ± 4.87 b | 87.72 ± 5.95 | 158.36 ± 11.10 | 23.99 ± 2.16 a | 286.58 ± 10.47 |
| Conventional | 22.17 ± 0.58 ab | 64.48 ± 1.36 b | 47.02 ± 2.87 | 122.14 ± 7.18 | 23.82 ± 2.62 a | 157.07 ± 9.31 | |
| Pinot Noir | Organic | 47.04 ± 1.87 ab | 169.53 ± 7.32 b | 26.28 ± 1.46 | 388.25 ± 10.72 | 15.98 ± 1.53 a | 312.84 ± 12.81 |
| Conventional | 20.64 ± 1.53 ab | 77.05 ± 2.76 b | 15.79 ± 1.51 | 135.13 ± 5.68 | 19.36 ± 1.99 a | 209.59 ± 11.38 | |
| Muscat Hamburg | Organic | 20.41 ± 1.26 ab | 52.78 ± 1.90 b | 49.23 ± 3.07 | 123.58 ± 8.66 | 26.55 ± 2.35 a | 135.77 ± 8.14 |
| Conventional | 19.94 ± 1.73 ab | 73.53 ± 1.37 b | 43.41 ± 3.63 | 131.76 ± 6.70 | 26.32 ± 2.09 a | 164.5 ± 6.45 | |
| Association | Eta Squared |
|---|---|
| Antioxidant activity * Management system | 0.051 |
| Flavonoids * Management system | 0.075 |
| Phenolics * Management system | 0.241 |
| Antioxidant activity * Anatomic part | 0.752 |
| Flavonoids * Anatomic part | 0.502 |
| Phenolics * Anatomic part | 0.445 |
| Equation of Regression | R Square |
|---|---|
| Total phenolic content (TPC) = 70.529−8.688 * variety −49.065 * vineyard management +61.299 * anatomic part | 0.72 |
| Total flavonoid content (TFC) = 14.027−1.927 * variety −52.094 * vineyard management +118.972 * anatomic Part | 0.56 |
| Antioxidant activity = −25.377−17.632 * variety −59.569 * vineyard management +189.74 * anatomic Part | 0.82 |
| Association | Eta Squared | Association | Eta Squared |
|---|---|---|---|
| CCB1 * management system | 0.122 | CCB1 * anatomic part | 0.030 |
| CCB3 * management system | 0.000 | CCB3 * anatomic part | 0.010 |
| CCB4 * management system | 0.019 | CCB4 * anatomic part | 0.171 |
| CCB5 * management system | 0.003 | CCB5 * anatomic part | 0.030 |
| CCB6 * management system | 0.001 | CCB6 * anatomic part | 0.001 |
| CCB7 * management system | 0.015 | CCB7 * anatomic part | 0.029 |
| CCB10 * management system | 0.005 | CCB10 * anatomic part | 0.164 |
| Type | Compounds | Antimicrobial Activity | Reference |
|---|---|---|---|
| Polyphenols | Phlorotannins | Alteration of the cell membrane and cell destruction of S. aureus, S. pneumonia and P. aeruginosa | [40] |
| Phlorotannins | Alteration of the cell membrane, cytoplasm’s leakage and cell destruction of V. parahaemolyticus | [41] | |
| Phlorofucofuroeckol | Cell membrane damage and suppression of genes related to methicillin resistance in S. aureus | [42] | |
| Bromophenols | Downregulation of pathogenic genes of P. gingivalis | [43] | |
| Dieckol | Alteration of cell integrity and metabolism of T. rubrum | [44] | |
| Phlorotannins | Alterations of the cell wall composition, increased mitochondrial respiration. Inhibition of the formation of the germ tube of C. albicans | [45] | |
| Phlorotannins | Inhibition of the enzyme neuraminidase of the Influenza A virus | [46] | |
| Polyphenolic rich extracts | Inhibition of the viral particle | [47] | |
| Polysaccharides | Depolymerized fucoidans | Interaction with protein of the cell membrane and cellular rupture of E. coli and S. aureus | [48] |
| Fucoidan | Inhibition of dental plaque bacteria and foodborne pathogens. | [49] | |
| Laminarin rich extracts | Inhibition of S. aureus, L. monocytogenes, E. coli and S. typhimurium. | [50] | |
| Water soluble polysaccharide extracts | Inhibition of F. oxysporium Inhibition of C. albicans and M. phaseli | [51] | |
| Sulfated polysaccharides | Obstruction of herpes simplex virus type 1 and 2 attachment to the cells | [52] | |
| Interference with fusion between HIV infected cells. Inhibition of the viral enzyme reverse transcriptase | |||
| Inhibition of dengue virus by interaction with the glycoprotein of the viral envelop | |||
| Proteins & peptides | Lectins | Inhibition of several Gram-negative bacteria by interaction with compounds of the cell wall | [53] |
| Lectins | Inhibition of T. rubrum and C. lindemuthianum | ||
| Lectins | Antiviral effects against HIV, Hepatitis C virus and SARS-CoV by preventing the entry in the host cells | [54] | |
| Fatty acids | Bioactive fraction | Perforation of the cell wall of S. aureus and K. neumoniae, cytoplasmic leakage and cell death | [55] |
| Bioactive fraction | Rupture of cell membrane of Vibrio spp and A. hydrophila | [56] | |
| Bioactive fraction | Fatty acids could be involved in the inhibition S. aureus, E. coli and P. vulgaris | [57] | |
| Bioactive fraction | Inhibition of C. cladosporioides and C. sphaerospermum by disrupting the cell membrane | [58] | |
| Sulfoquinovosyldia-cylglycerol | Antiviral effects against HSV type 2 by disturbing the initial stages of the viral life cycle | [59] | |
| Pigments | Fucoxanthin | Inhibition of L. monocytogenes | [60] |
| Fucoxanthin | Inhibition of several pathogenic bacteria by increasing cell membrane permeability, leakage of cytoplasm and inhibition of nucleic acid | [61] |
| No. | Specie/Code | Source |
|---|---|---|
| 1 | Lactococcus sp./CCB1 | Wheat |
| 2 | Bacillus sp. T3/CCB3 | Nuts |
| 3 | Bacillus sp./CCB4 | Seeds |
| 4 | Lactobacillus sp./CCB5 | Meat products |
| 5 | Streptococcus sp./CCB6 | Dairy products |
| 6 | Leuconostoc sp./CCB7 | Vegetables |
| 7 | Micrococcus sp./CCB10 | Air (vineyard) |
| 8 | Bacillus sp./CCB11 | Wheat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants 2020, 9, 1470. https://doi.org/10.3390/plants9111470
Radulescu C, Buruleanu LC, Nicolescu CM, Olteanu RL, Bumbac M, Holban GC, Simal-Gandara J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants. 2020; 9(11):1470. https://doi.org/10.3390/plants9111470
Chicago/Turabian StyleRadulescu, Cristiana, Lavinia Claudia Buruleanu, Cristina Mihaela Nicolescu, Radu Lucian Olteanu, Marius Bumbac, Georgeta Carmen Holban, and Jesus Simal-Gandara. 2020. "Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards" Plants 9, no. 11: 1470. https://doi.org/10.3390/plants9111470
APA StyleRadulescu, C., Buruleanu, L. C., Nicolescu, C. M., Olteanu, R. L., Bumbac, M., Holban, G. C., & Simal-Gandara, J. (2020). Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants, 9(11), 1470. https://doi.org/10.3390/plants9111470