Histological Changes Associated with the Graft Union Development in Tomato
Abstract
:1. Introduction
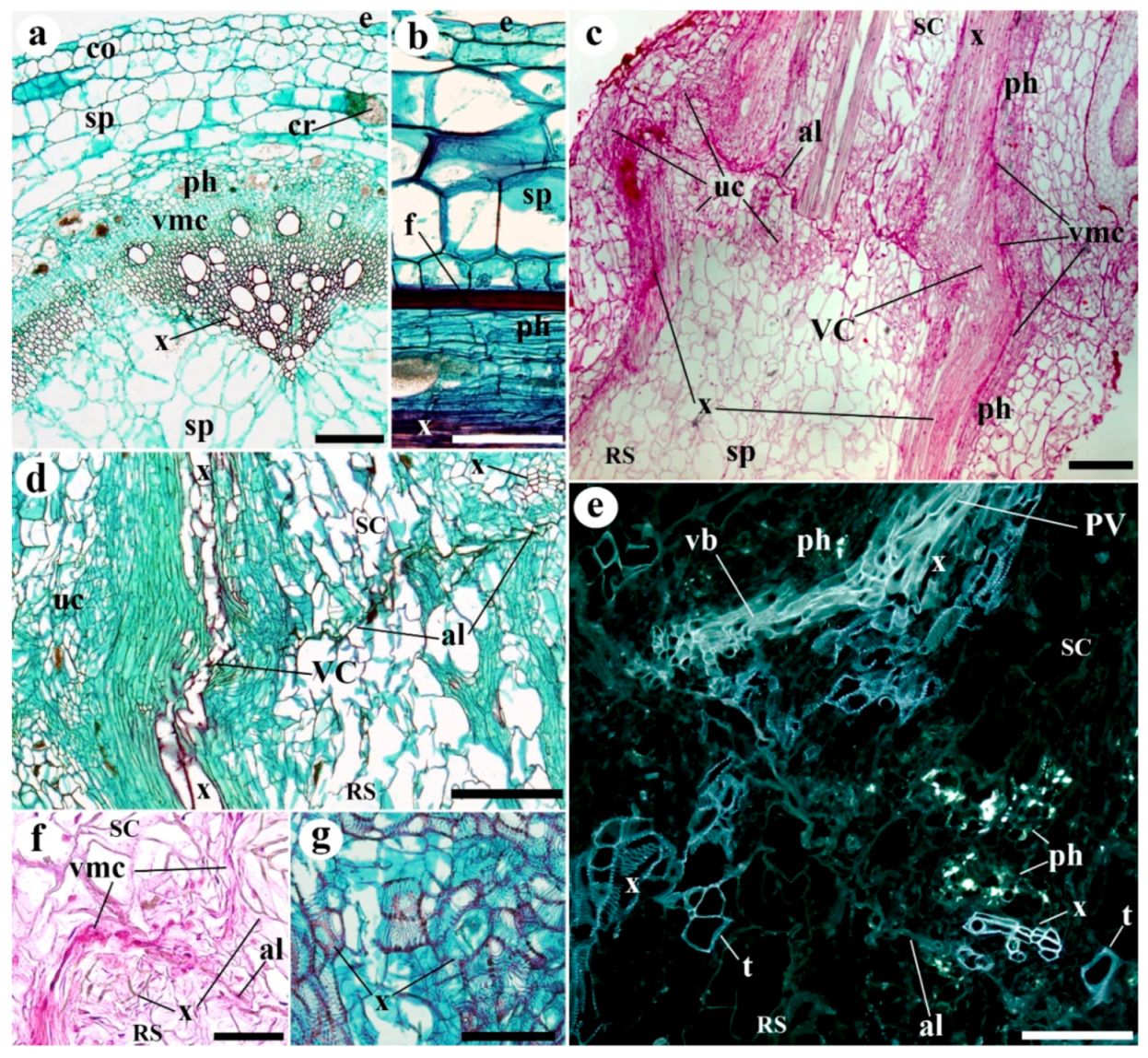
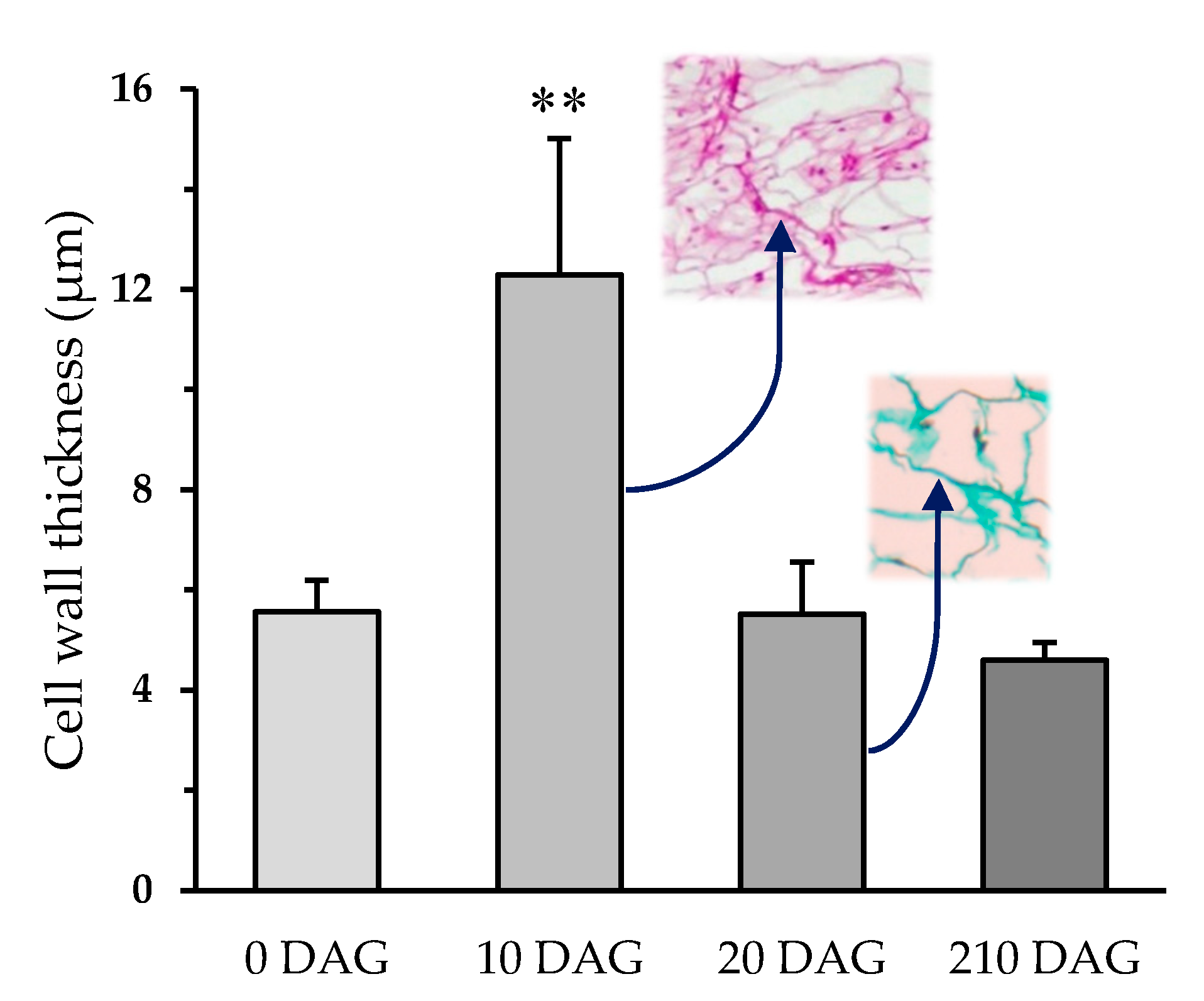
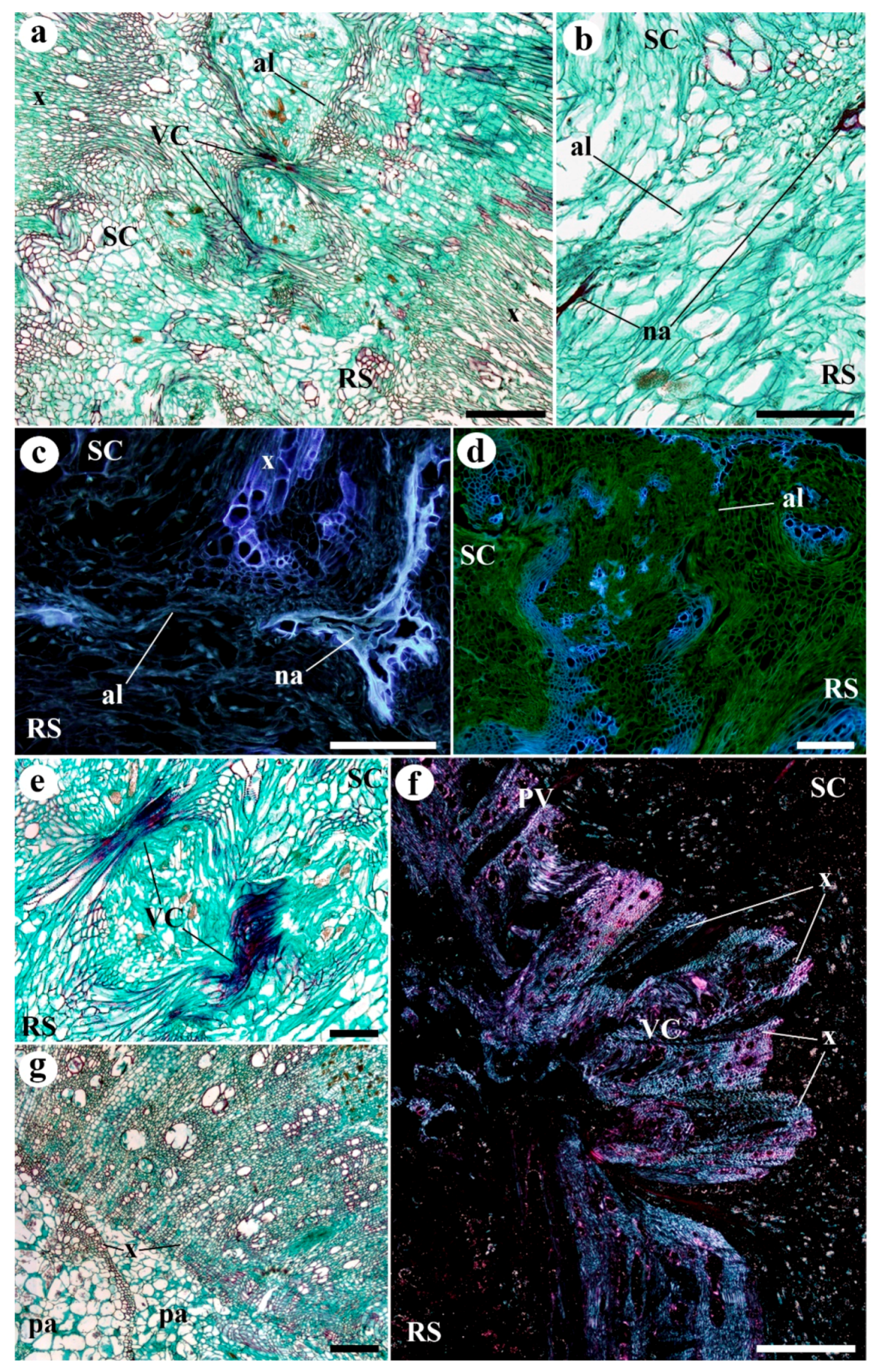
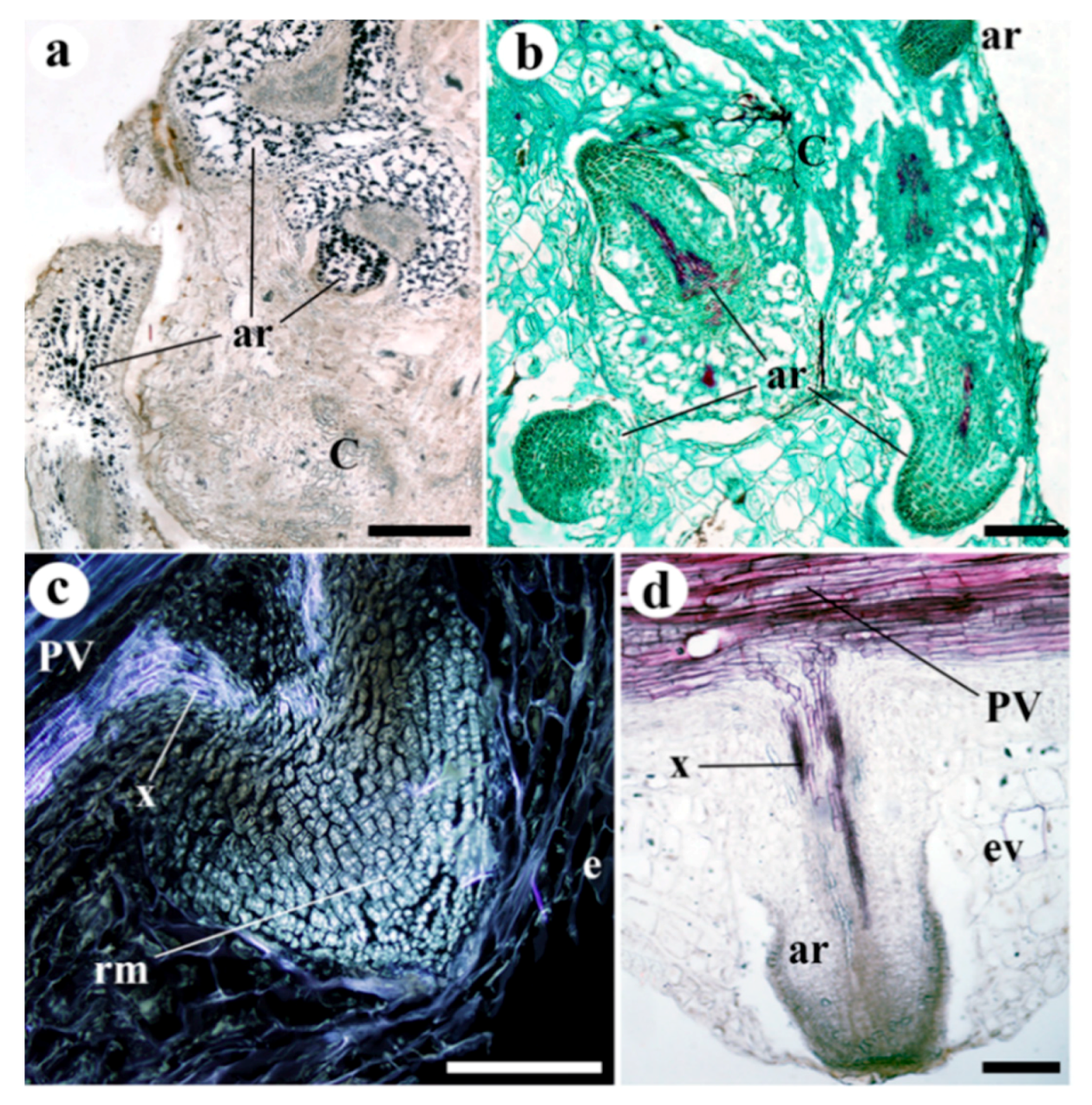
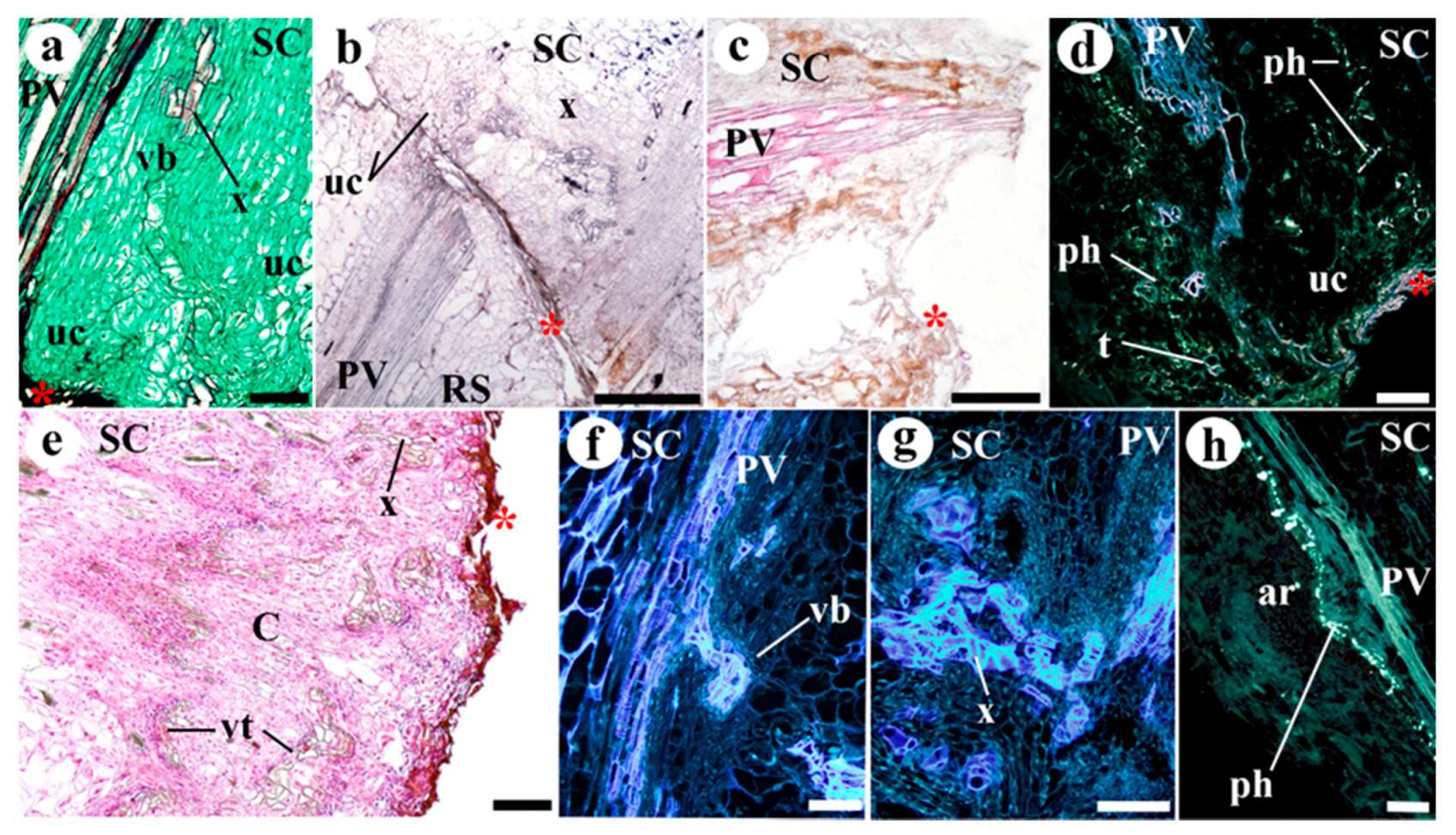
2. Results
3. Discussion
4. Material and Methods
4.1. Plants and Growth Conditions
4.2. Grafting Method and Healing Conditions
4.3. Post-Healing Cultivation
4.4. Cell Wall Thickness
4.5. Callus Thickness
4.6. Histological Techniques
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A History of Grafting. In Horticultural Reviews; Janick, J., Ed.; Wiley-Blackwell: West Lafayette, IN, USA, 2009; Volume 35, pp. 437–493. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann & Kester’s Plant Propagation Principles and Practices, 9th ed.; Pearson: Harlow, UK, 2018. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato grafting: A global perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Tirupathamma, T.L.; Ramana, C.V.; Naidu, L.N.; Sasikala, K. Vegetable grafting: A multiple crop improvement methodology. Curr. J. Appl. Sci. Technol. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Notaguchi, M. The use of grafting to study systemic signaling in plants. Plant Cell Physiol. 2017, 58, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- Garner, R.J.; Bradley, S. The Grafter’s Handbook, 6th ed.; Mitchell Beazly: London, UK, 2013. [Google Scholar]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer: New York, NY, USA, 2018. [Google Scholar]
- Schweingruber, F.H.; Börner, A. The Plant Stem: A Microscopic Aspect; Springer: New York, NY, USA, 2018. [Google Scholar]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef] [Green Version]
- The 100 Tomato Genome Sequencing Consortium. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014, 80, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Bauchet, G.; Causse, M. Genetic Diversity in Tomato (Solanum lycopersicum) and Its Wild Relatives. In Genetic Diversity of Plants; Caliskan, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 133–162. [Google Scholar]
- Knapp, S.; Peralta, I.E. The Tomato (Solanum lycopersicum L., Solanaceae) and Its Botanical Relatives. In The Tomato Genome; Causse, M., Giovannoni, J., Bouzayen, M., Zouine, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–21. [Google Scholar]
- Jeffree, C.E.; Yeoman, M.M. Development of intercellular connections between opposing cells in a graft union. New Phytol. 1983, 93, 491–509. [Google Scholar] [CrossRef]
- Miller, H.; Barnett, J.R. The structure and composition of bead-like projections on Sitka spruce callus cells formed during grafting and in culture. Ann. Bot. 1993, 72, 441–448. [Google Scholar] [CrossRef]
- Yeoman, M. Cellular Recognition Systems in Grafting. In Cellular Interactions; Springer: New York, NY, USA, 1993; pp. 441–448. [Google Scholar]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Sala, K.; Karcz, J.; Rypie, A.; Kurczy, E.U. Unmethyl-esterified homogalacturonan and extensins seal Arabidopsis graft union. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-ono, K.; Ono, M. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Yang, R.; Li, X.; Zhao, W.; Zhao, F.; Wang, S. The processes of graft union formation in tomato. Hortic. Environ. Biotechnol. 2015, 56, 569–574. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-union development: A delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef] [Green Version]
- Asahina, M.; Azuma, K.; Pitaksaringkarn, W.; Yamazaki, T.; Mitsuda, N.; Ohme-Takagi, M.; Yamaguchi, S.; Kamiya, Y.; Okada, K.; Nishimura, T.; et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16128–16132. [Google Scholar] [CrossRef] [Green Version]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immanen, J.; Nieminen, K.; Smolander, O.-P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sachs, T. The role of the root in the induction of xylem differentiation in peas. Ann. Bot. 1968, 32, 391–399. [Google Scholar] [CrossRef]
- Sachs, T. The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Aloni, R. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 1980, 150, 255–263. [Google Scholar] [CrossRef]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef] [Green Version]
- Biedroń, M.; Banasiak, A. Auxin-mediated regulation of vascular patterning in Arabidopsis thaliana leaves. Plant Cell Rep. 2018, 37, 1215–1229. [Google Scholar] [CrossRef] [Green Version]
- Křeček, P.; Skůpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zažímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.; Balla, J.; Luschnig, C.; Wisniewska, J.; Reinöhl, V.; Friml, J.; Benková, E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006, 20, 2902–2911. [Google Scholar] [CrossRef] [Green Version]
- Mazur, E.; Benková, E.; Friml, J. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 2016, 6, 33754. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.W.; Yeoman, M.M.; Brown, R. An analysis of the development of the graft union in Lycopersicon esculentum. Ann. Bot. 1974, 38, 639–646. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Ann. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Sala, K.; Malarz, K.; Barlow, P.W.; Kurczyńska, E.U. Distribution of some pectic and arabinogalactan protein epitopes during Solanum lycopersicum (L.) adventitious root development. BMC Plant Biol. 2017, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.; Darrouy, N.; Iturrieta, R. Franqueamiento: A new vegetative propagation technique for loquat. Acta Hortic. 2007, 750, 325–330. [Google Scholar] [CrossRef]
- Meyer, L.J.; Kennelly, M.M.; Pliakoni, E.D.; Rivard, C.L. Leaf removal reduces scion adventitious root formation and plant growth of grafted tomato. Sci. Hortic. 2017, 214, 147–157. [Google Scholar] [CrossRef]
- Biggs, A.R.; Miles, N.W. Suberin deposition as a measure of wound response in peach bark. J. Am. Soc. Hortic. Sci. 1985, 20, 903–905. [Google Scholar]
- Irisarri, P.; Zhebentyayeva, T.; Errea, P.; Pina, A. Differential expression of phenylalanine ammonia lyase (PAL) genes implies distinct roles in development of graft incompatibility symptoms in Prunus. Sci. Hortic. 2016, 204, 16–24. [Google Scholar] [CrossRef]

| Graft Type | Functional Grafts | Non-Functional Grafts | |||
|---|---|---|---|---|---|
| Days after grafting | 10 | 20 | 210 | 10 | 20 |
| Necrotic remnant | + | − | − | + + | + + + |
| Areas of non-adhesion | + | + | + | + + + | + + + |
| Undifferentiated cells | + + | + | − | + + | + |
| Xylem cells | + | + + + | + + + | + | + + + |
| Vascular connections | ± | + | + | − | − |
| Staining | Mounting | Microscopy Technique | Target |
|---|---|---|---|
| Safranin-Fast Green | Entellan | Bright field | General staining |
| Hematoxylin-Eosin | Entellan | Bright field | General staining |
| Lugol | No | Bright field | Starch |
| Ruthenium red | No | Bright field | Pectic polysaccharides |
| Sirofluor | No | Epifluorescence | Callose |
| Phloroglucinol | No | Bright field | Lignin |
| No | Entellan | Epifluorescence | Autofluorescence |
| No | Entellan | Polarization | Birefringent structures |
| Calcofluor White | No | Epifluorescence | Cellulose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, C.; Acebes, J.L.; Encina, A.; Álvarez, R. Histological Changes Associated with the Graft Union Development in Tomato. Plants 2020, 9, 1479. https://doi.org/10.3390/plants9111479
Frey C, Acebes JL, Encina A, Álvarez R. Histological Changes Associated with the Graft Union Development in Tomato. Plants. 2020; 9(11):1479. https://doi.org/10.3390/plants9111479
Chicago/Turabian StyleFrey, Carlos, José Luis Acebes, Antonio Encina, and Rafael Álvarez. 2020. "Histological Changes Associated with the Graft Union Development in Tomato" Plants 9, no. 11: 1479. https://doi.org/10.3390/plants9111479
APA StyleFrey, C., Acebes, J. L., Encina, A., & Álvarez, R. (2020). Histological Changes Associated with the Graft Union Development in Tomato. Plants, 9(11), 1479. https://doi.org/10.3390/plants9111479






