Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening?
Abstract
:1. Introduction
2. Results and Discussion
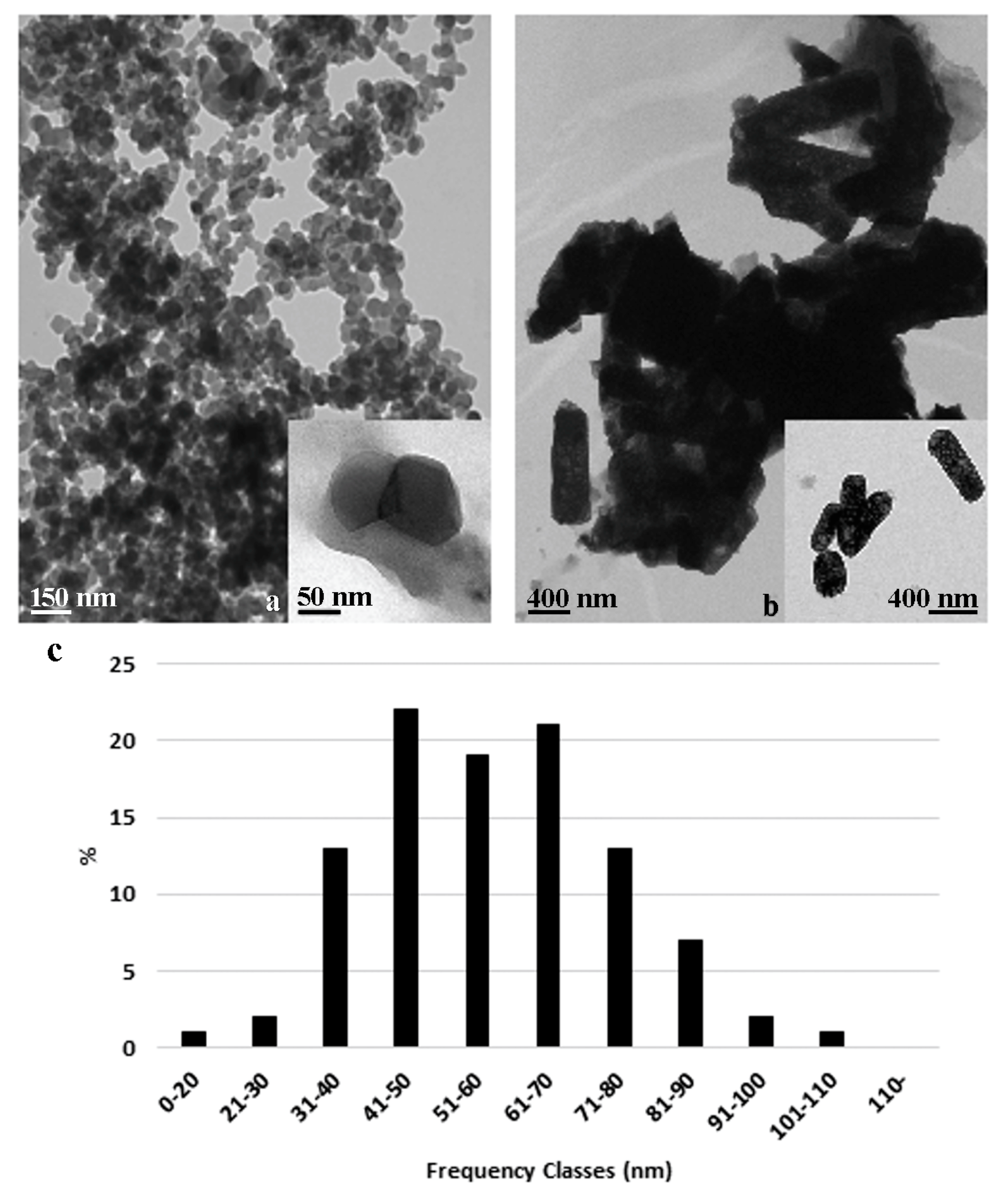
2.1. ZnO NPs and Bulk Characteristics
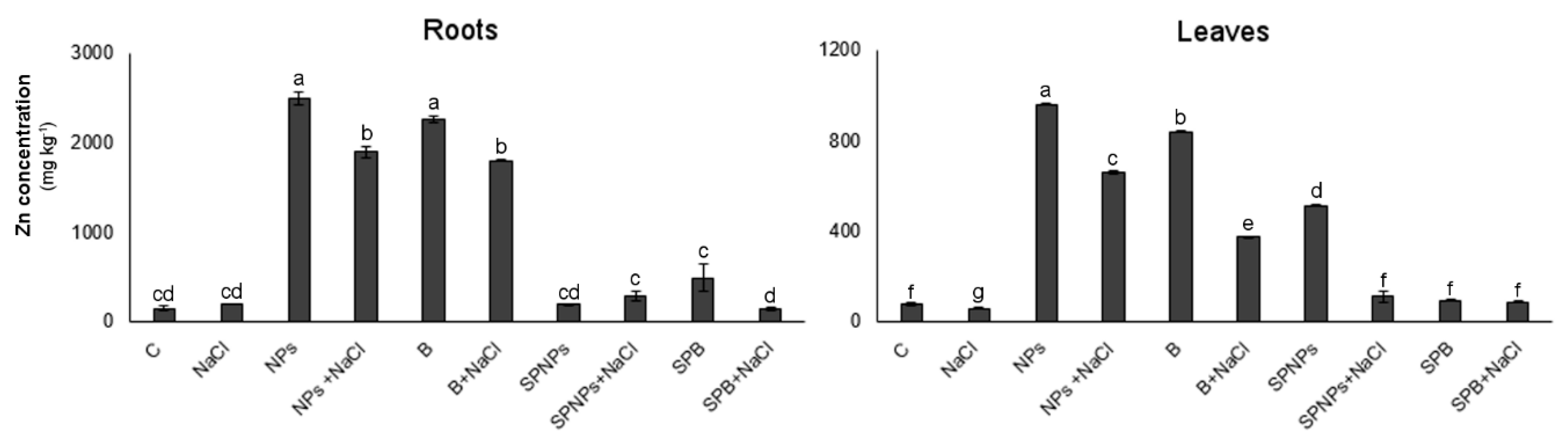
2.2. Zinc Concentration
2.3. Ion Concentration
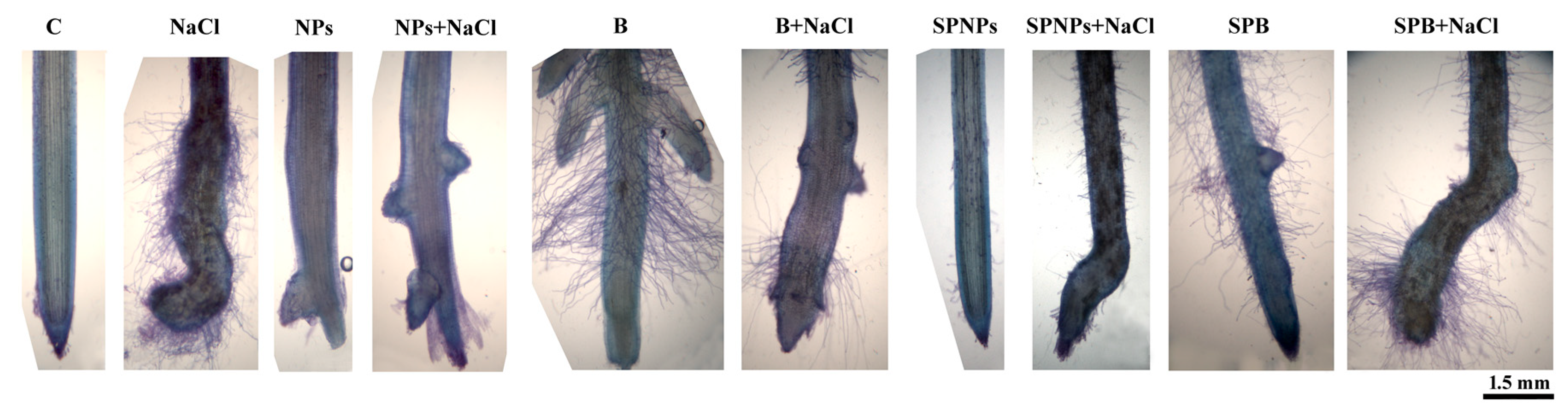
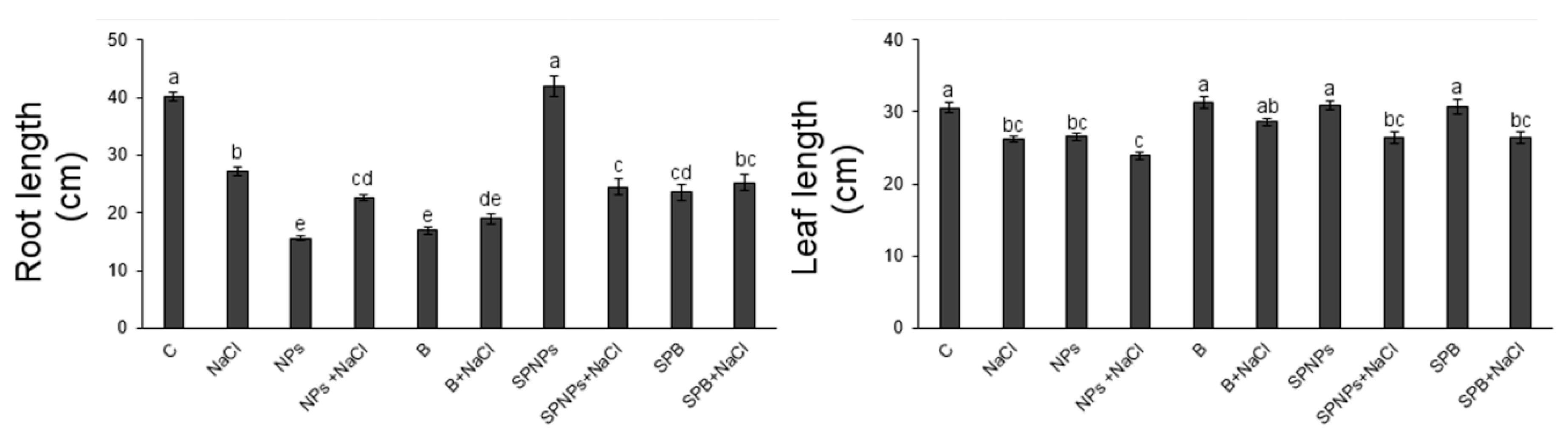
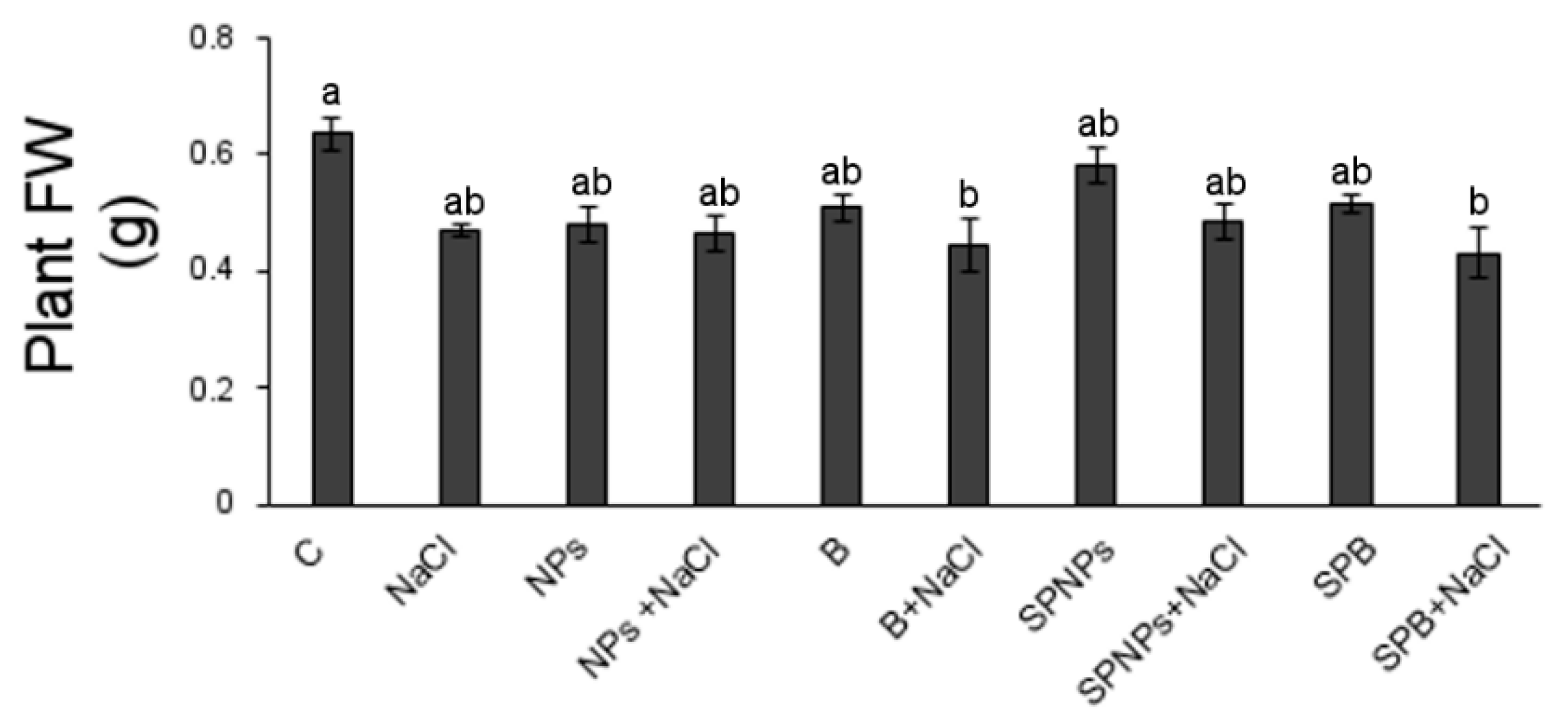
2.4. Plant Growth and Root Morphology
2.5. Relative Water Content, Pigments Concentration, and Chlorophyll Fluorescence
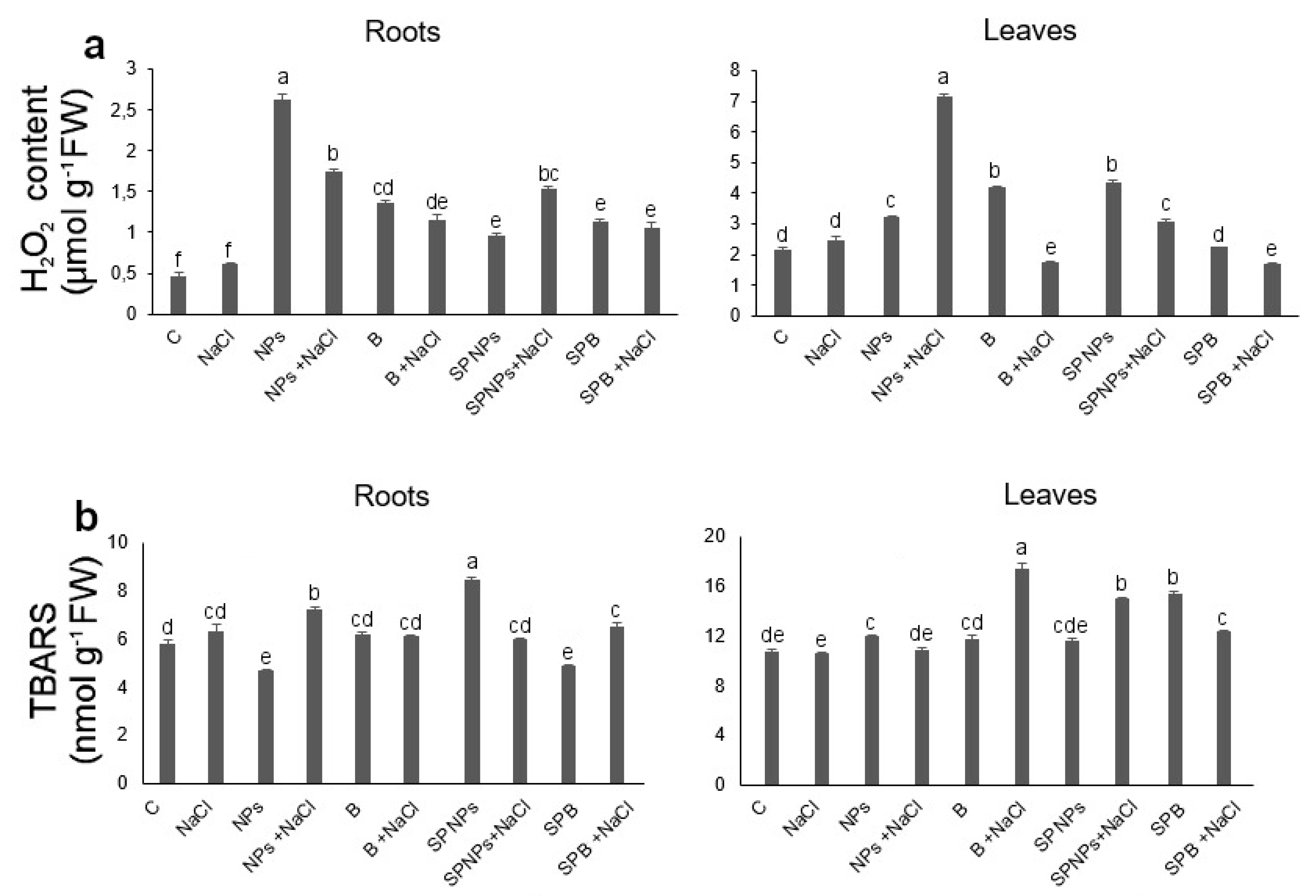
2.6. Oxidative Stress
3. Materials and Methods
3.1. ZnO Nanoparticles and Bulk Characterization
3.2. Plant Material and Experimental Design
3.3. Atomic Absorption Spectrometry Analysis for Zinc Concentration Determination
3.4. Ion Concentration
3.5. Seedling Growth, Root Morphology, and Relative Water Content
3.6. Pigment Concentration and Photosynthetic Efficiency
3.7. Hydrogen Peroxide and TBARS Determination
3.8. Histochemical Analyses
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, S.; Sharma, M.K.; Kumar, N.; Neelam. Impact of salinity and zinc application on growth, physiological and yield traits in wheat. Curr. Sci. 2019, 116, 1324–1330. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef] [Green Version]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of zinc application on growth, absorption and distribution of mineral nutrients under salinity stress in soybean (Glycine max L.). J. Plant Nutr. 2014, 37, 2255–2269. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants–An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.H.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in Habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars. Environ. Pollut. 2020, 265, 115045. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Haripriya, P.; Stella, P.M.; Anusuya, S. Foliar spray of zinc oxide nanoparticles improves salt tolerance in finger millet crops under glass-house condition. SCIOL Biotechnol. 2018, 1, 20–29. [Google Scholar]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- García-Gómez, C.; García, S.; Obrador, A.F.; González, D.; Babín, M.; Fernández, M.D. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotox. Environ. Saf. 2018, 160, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Prakhash, M.G.; Chung, I.M. Determination of zinc oxide nanoparticles toxicity in root growth in wheat (Triticum aestivum L.) seedlings. Acta Biol. Hung. 2016, 67, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Mishra, R.M.; Singh, S.; Singh, S.; Vishwakarma, K.; Sharma, S.; Singh, V.P.; Singh, P.K.; Prasad, S.M.; Dubey, N.K.; et al. Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: Implication of the ascorbate–glutathione cycle. Front. Plant Sci. 2017, 8, 1. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles–A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Viezcas, J.; Castillo-Michel, H.; Servin, A.; Peralta-Videa, J.; Gardea-Torresdey, J. Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina (velvet mesquite) treated with ZnO nanoparticles. Chem. Eng. J. 2011, 170, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, antioxidative enzymes in tomato plants. Biol. Plantarum 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Ficco, D.B.M.; Giuzio, L.; Pompa, M.; Cattivelli, L.; Flagella, Z. Durum wheat salt tolerance in relation to physiological, yield and quality characters. Cereal Res. Commun. 2011, 39, 525–534. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S. Durum wheat seedlings in saline conditions: Salt spray versus root-zone salinity. Estuar. Coast. Shelf Sci. 2016, 169, 173–181. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6, 1242. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. Ion exchange properties of roots and ionic interactions within the root apoplasm: Their role in ion accumulation by plants. Bot. Rev. 1980, 46, 75–99. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Shariatmadari, H.; Karimian, N.; Kalbasi, M.; van der Zee, S.E.A.T.M. Cadmium and zinc in saline solutions and their concentrations in wheat. Soil Sci. Soc. Am. J. 2006, 70, 582–588. [Google Scholar] [CrossRef]
- Saleh, J.; Maftoun, M. Interactive effects of NaCl levels and zinc sources and levels on the growth and mineral composition of rice. J. Agric. Sci. Technol. 2008, 10, 325–336. [Google Scholar]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effects of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J. Agric. Sci. Technol. 2016, 18, 1013–1025. [Google Scholar]
- Alpaslan, M.; Inal, A.; Güneş, A.; Çikili, Y.; Öscan, H. Effect of zinc treatment on the alleviation of sodium and chloride injury in tomato (Lycopersicum esculentum (L.) Mill. cv. Lale) grown under salinity. Turk. J. Bot. 1999, 23, 1–6. [Google Scholar]
- Darko, E.; Gierczik, K.; Hudák, O.; Forgó, P.; Pál, M.; Türkösi, E.; Kovács, V.; Dulai, S.; Majláth, I.; Molnár, I.; et al. Differing metabolic responses to salt stress in wheat-barley addition lines containing different 7H chromosomal fragments. PLoS ONE 2017, 12, e0174170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Wang, C.M.; Zhang, J.L.; Liu, X.S.; Li, Z.; Wu, G.Q.; Cai, J.Y.; Flowes, T.J.; Wang, S.M. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ. 2009, 32, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signaling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Hamada, A.M.; El-Enany, A.E. Effect of NaCl salinity on growth, pigment and mineral element contents, and gas exchange of broad bean and pea plants. Biol. Plantarum 1994, 36, 75–81. [Google Scholar] [CrossRef]
- Anderson, A.J.; McLean, J.E.; Jacobson, A.R.; Britt, D.W. CuO and ZnO nanoparticles modify interkingdom cell signaling processes relevant to crop production. J. Agric. Food Chem. 2018, 66, 6513–6524. [Google Scholar] [CrossRef]
- Yang, K.Y.; Doxey, S.; McLean, J.E.; Britt, D.; Watson, A.; Al Qassy, D.; Jacobson, A.; Anderson, A.J. Remodeling of root morphology by CuO and ZnO nanoparticles: Effects on drought tolerance for plants colonized by a beneficial pseudomonad. Botany 2018, 96, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodriguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolis of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Cadiz, N.M.; Davies, M.S. Effects of cadmium, lead and zinc on root meristem, root hair formation, xylogenesis and development of lateral root primordia in Ocimum sanctum L. and Festuca rubra L. cv. Merlin. In Biology of Root Formation and Development. Basic Life Sciences; Altman, A., Waisel, Y., Eds.; Springer: Boston, MA, USA, 1997; Volume 65, pp. 275–276. [Google Scholar]
- Forino, L.M.; Ruffini Castiglione, M.; Bartoli, G.; Balestri, M.; Andreucci, A.; Tagliasacchi, A.M. Arsenic-induced morphogenic response in roots of arsenic hyperaccumulator fern Pteris vittata. J. Hazard Mater. 2012, 235–236, 271–278. [Google Scholar] [CrossRef]
- Sun, X.; Guo, L. Relationship between cadmium-induced root subapical hair development and ethylene biosynthesis in oilseed rape seedlings. Acta Biol. Cracoviensia Ser. Bot. 2013, 55, 68–75. [Google Scholar] [CrossRef]
- Burssens, S.; Himanen, K.; van de Cotte, B.; Beeckman, T.; Van Montagu, M.; Inzé, D.; Verbruggen, N. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta 2000, 211, 632–640. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Qados, A.M.S.A. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 1243. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Wang, S.; Yang, D.; Zou, Z.; Li, J. Physiological effects of MgO and ZnO nanoparticles on the Citrus maxima. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 243–253. [Google Scholar] [CrossRef]
- Kukreja, S.; Kumar, N.; Sharma, S.K.; Sharma, S.K.; Unvi, V.; Sharma, P.K. Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol. Plantarum 2005, 49, 305–308. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Koscielniak, J.; Zuk-Golaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. J. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Redondo-Gomez, S.; Andrades-Moreno, L.; Mateos-Naranjo, E.; Parra, R.; Valera-Burgos, J.; Aroca, R. Synergic effect of salinity and zinc stress on growth and photosynthetic responses of the cordgrass, Spartina densiflora. J. Exp. Bot. 2011, 62, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Moe-Lange, J.; Hennet, L.; Feldman, L.J. Salt stress affects the redox status of Arabidopsis root meristems. Front. Plant Sci. 2016, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.; Bueno, P.; Marín, M.C.; Rodríguez-Rosales, M.P.; Belver, A.; Venema, K.; Donaire, J.P. Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol. 2002, 156, 409–415. [Google Scholar] [CrossRef]
- Jorhem, L. Determination of metals in foods by atomic absorption spectrometry after dry ashing: NMKL collaborative study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Spanò, C.; Bottega, S.; Sorce, C.; Bartoli, G.; Ruffini Castiglione, M. TiO2 nanoparticles may alleviate cadmium toxicity in co-treatment experiments on the model hydrophyte Azolla filiculoides. Environ. Sci. Pollut. Res. 2019, 26, 29872–29882. [Google Scholar] [CrossRef] [PubMed]
- Camusso, M.; Polesello, S. Determinazione di cloruro, nitrato, solfato mediante cromatografia ionica. Not. Metod. Anal. IRSA 1999, 1, 1–14. (in Italian). [Google Scholar]
- Mosello, R.; Bianchi, M.; Geiss, H.; Marchetto, A.; Serrini, G.; Serrini-Lanza, G.; Tartari, G.; Munthau, H. AQUACON-MedBas Project. Subproject No. 5. Freshwater Analysis: Intercomparison 2000; EUR 18075 EN; European Commission: Brussels, Belgium, 1998; p. 66. [Google Scholar]
- Balestri, M.; Bottega, S.; Spanò, C. Response of Pteris vittata to different cadmium treatments. Acta Physiol. Planctarum 2014, 36, 767–775. [Google Scholar] [CrossRef]
- Sorce, C.; Bottega, S.; Spanò, C. Seasonal and microclimatic influences on the ecophysiology of Mediterranean coastal dune plants. Estuar. Coast. Shelf Sci. 2019, 219, 317–327. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S.; Ruffini Castiglione, M.; Pedranzani, H.E. Antioxidant response to cold stress in two oil plants of the genus Jatropha. Plant Soil Environ. 2017, 63, 271–276. [Google Scholar]
- Giorgetti, L.; Spanoò, C.; Muccifora, S.; Bellani, L.; Tassi, E.; Bottega, S.; Di Gregorio, S.; Siracusa, G.; Sanitaà di Toppi, L.; Ruffini Castiglione, M. An integrated approach to highlight biological responses of Pisum sativum root to nano-TiO2 exposure in a biosolid-amended agricultural soil. Sci. Total Environ. 2019, 650, 2705–2716. [Google Scholar] [CrossRef]


| Na+ in Root (mg kg−1) | K+ in Root (mg kg−1) | Cl− in Root (mg kg−1) | K+/Na+ in Root | Na+ in Leaf (mg kg−1) | K+ in Leaf (mg kg−1) | Cl− in Leaf (mg kg−1) | K+/Na+ in Leaf | |
|---|---|---|---|---|---|---|---|---|
| C | 189.78 ± 2.61 g | 462.78 ± 41.59 h | 270.53 ± 9.12 i | 2.44 ± 0.23 d | 1795.0 ± 29.44 f | 12,535.0 ± 21.79 f | 2496.0 ± 49.13 g | 6.99 ± 0.06 d |
| NaCl | 20,911 ± 63.88 b | 5640 ± 119.1 g | 30,834 ± 311.8 b | 0.27 ± 0.01 e | 33,993 ± 529.8 b | 16,135 ± 680.9 de | 48,503 ± 514.9 c | 0.48 ± 0.03 f |
| NPs | 1673.6 ± 25.21 f | 15,676 ± 186.0 a | 2650.0 ± 72.57 fg | 9.37 ± 0.25 a | 1052.0 ± 31.19 f | 18,680 ± 23.28 c | 1577.0 ± 79.17 g | 17.79 ± 0.51 b |
| NPs + NaCl | 22,368 ± 76.56 a | 9113.1 ± 321.10 c | 33,669 ± 188.7 a | 0.41 ± 0.01 e | 28,066 ± 33.30 c | 23,141 ± 145.5 b | 45,895 ± 471.3 d | 0.82 ± 0.01 f |
| B | 1365.3 ± 32.51 fg | 12,467 ± 204.0 b | 1792.0 ± 15.50 h | 9.14 ± 0.09 a | 1654.0 ± 30.57 ef | 35,890 ± 118.2 a | 2493.0 ± 47.42 g | 21.72 ± 0.41 a |
| B + NaCl | 16,997 ± 421.8 d | 7342.4 ± 37.51 e | 11,064 ± 83.43 e | 0.43 ± 0.01 e | 36,781 ± 189.6 a | 16,125 ± 48.85 de | 53,108 ± 106.6 b | 0.44 ± 0.00 f |
| SPNPs | 2040.0 ± 15.85 f | 6526.0 ± 53.98 f | 3056.0 ± 27.42 f | 3.20 ± 0.04 c | 2871.0 ± 8.82 e | 14,461 ± 722.3 e | 4439.0 ± 76.02 f | 5.04 ± 0.25 e |
| SPNPs + NaCl | 18,360 ± 455.0 c | 6754.6 ± 89.38 ef | 24,761 ± 121.6 c | 0.37 ± 0.01 e | 35,883 ± 107.0 a | 16,607 ± 369.6 d | 56,495 ± 692.5 a | 0.46 ± 0.01 f |
| SPB | 1879.4 ± 27.61 f | 7680.4 ± 135.0 d | 2528.0 ± 18.61 gh | 4.09 ± 0.09 b | 1607.0 ± 31.55 ef | 24,992 ± 68.41 b | 2064.0 ± 27.18 g | 15.56 ± 0.30 c |
| SPB + NaCl | 11,367 ± 298.0 e | 9887.0 ± 10.05 c | 16,403 ± 187.5 d | 0.87 ± 0.02 e | 23,792 ± 495.6 d | 24,865 ± 335.4 b | 38,645 ± 524.1 e | 1.05 ± 0.01 f |
| Leaf Number | Total Chlorophyll (mg g−1FW) | Carotenoids (mg g−1FW) | Chla/Chlb | Fv/Fm | |
|---|---|---|---|---|---|
| C | 2.80 ± 0.09 a | 1.21 ± 0.07 | 0.21 ± 0.01 | 3.05 ± 0.02 a | 0.79 ± 0.00 |
| NaCl | 2.10 ± 0.07 b | 1.29 ± 0.08 | 0.22 ± 0.01 | 2.94 ± 0.10 ab | 0.80 ± 0.00 |
| NPs | 2.95 ± 0.05 a | 1.35 ± 0.04 | 0.19 ± 0.00 | 2.58 ± 0.05 bc | 0.80 ± 0.00 |
| NPs + NaCl | 2.30 ± 0.10 b | 1.28 ± 0.07 | 0.17 ± 0.01 | 2.49 ± 0.06 c | 0.79 ± 0.01 |
| B | 2.70 ± 0.10 a | 1.36 ± 0.11 | 0.20 ± 0.02 | 2.74 ± 0.03 bc | 0.79 ± 0.01 |
| B + NaCl | 2.20 ± 0.09 b | 1.44 ± 0.15 | 0.19 ± 0.02 | 2.58 ± 0.02 bc | 0.78 ± 0.01 |
| SPNPs | 2.80 ± 0.09 a | 1.39 ± 0.07 | 0.19 ± 0.01 | 2.65 ± 0.04 bc | 0.79 ± 0.01 |
| SPNPs + NaCl | 1.95 ± 0.05 b | 1.26 ± 0.10 | 0.17 ± 0.02 | 2.44 ± 0.14 c | 0.79 ± 0.00 |
| SPB | 2.25 ± 0.10 b | 1.45 ± 0.17 | 0.21 ± 0.03 | 2.53 ± 0.07 bc | 0.76 ± 0.01 |
| SPB + NaCl | 2.10 ± 0.07 b | 1.32 ± 0.08 | 0.19 ± 0.01 | 2.66 ± 0.08 bc | 0.79 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanò, C.; Bottega, S.; Bellani, L.; Muccifora, S.; Sorce, C.; Ruffini Castiglione, M. Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening? Plants 2020, 9, 1514. https://doi.org/10.3390/plants9111514
Spanò C, Bottega S, Bellani L, Muccifora S, Sorce C, Ruffini Castiglione M. Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening? Plants. 2020; 9(11):1514. https://doi.org/10.3390/plants9111514
Chicago/Turabian StyleSpanò, Carmelina, Stefania Bottega, Lorenza Bellani, Simonetta Muccifora, Carlo Sorce, and Monica Ruffini Castiglione. 2020. "Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening?" Plants 9, no. 11: 1514. https://doi.org/10.3390/plants9111514
APA StyleSpanò, C., Bottega, S., Bellani, L., Muccifora, S., Sorce, C., & Ruffini Castiglione, M. (2020). Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening? Plants, 9(11), 1514. https://doi.org/10.3390/plants9111514









