Increased [CO2] Causes Changes in Physiological and Genetic Responses in C4 Crops: A Brief Review
Abstract
:1. Introduction
2. C4 Plants of Commercial Importance
3. C4 Photosynthesis Mechanism and CO2
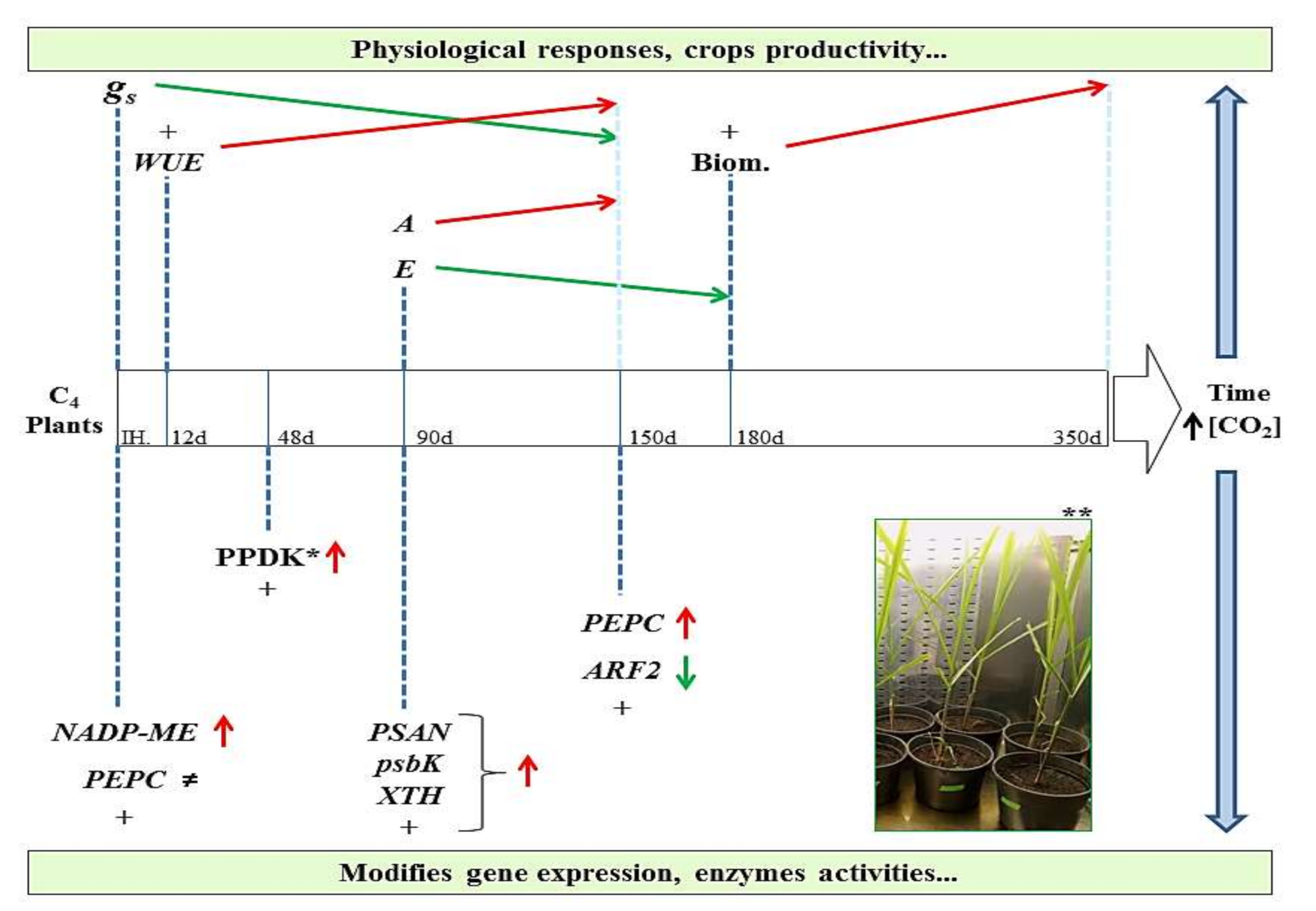
4. Physiological, Biochemical, and Gene Expression Changes
5. Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pacheco, M.R.P.D.S.; Helene, M.E.M. Atmosfera, fluxos de carbono e fertilização por CO2. Estud. Av. 1990, 4, 204–220. [Google Scholar] [CrossRef]
- Solomon, S.; Plattner, G.K.; Knutii, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intergovernmental Panel on Climate Change. AR4 Climate Change 2007: Impacts, Adaptation, and Vulnerability. 2007. Available online: https://www.ipcc.ch/report/ar4/wg2/ (accessed on 25 May 2020).
- Sonnemann, G.R.; Grygalashvyly, M. Effective CO2 lifetime and future CO2 levels based on fit function. Ann. Geophys. 2013, 31, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Misra, V.; Shrivastava, A.K.; Mall, A.K.; Solomon, S.; Singh, A.K.; Ansari, M.I. Can sugarcane cope with increasing atmospheric CO2 concentration? Aust. J. Crop Sci. 2019, 13, 780–784. [Google Scholar] [CrossRef]
- Butterfield, R.R.; Morison, J.I.L. Modelling the impact of climatic warming on winter cereal development. Agric. For. Meteorol. 1992, 62, 241–261. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Taub, D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nature Educ. Knowl. 2010, 3, 21. [Google Scholar]
- Prins, A.; Mukubi, J.M.; Pellny, T.K.; Verrier, P.J.; Beyene, G.; Lopes, M.S.; Emani, K.; Treumann, A.; Lelarge-Trouverie, C.; Noctor, G.; et al. Acclimation to high CO2 in maize is related to water status and dependent on leaf rank. Plant Cell Environ. 2011, 34, 314–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamim, H. Photosynthesis of C3 and C4 species in response to increased CO2 concentration and drought stress. Hayati. J. Biosci. 2011, 12, 131–138. [Google Scholar] [CrossRef]
- Mooney, H.A.; Canadell, J.; Chapin III, F.S.; Ehleringer, J.R.; Körner, C.; McMurtrie, R.E.; Parton, W.J.; Pitelka, L.F.; Schulze, E.-D. Ecosystem physiology responses global change. In The Terrestrial Biosphere and Global Change; Walker, B., Steffen, W., Canadell, J., Ingran, J., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 141–189. [Google Scholar]
- Vu, J.C.V.; Allen, L.H.; Gesch, R.W. Up-regulation of photosynthesis and sucrose metabolism enzymes in young expanding leaves of sugarcane under elevated growth CO2. Plant Sci. 2006, 171, 123–131. [Google Scholar] [CrossRef]
- Souza, A.P.; Gaspar, M.; Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y., Jr.; Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Vara Prasad, P.V.; Vu, J.C.V.; Boote, K.J.; Allen, L.H. Enhancement in leaf photosynthesis and regulation of Rubisco in the C4 sorghum plant at elevated growth carbon dioxide and temperature occur at early stages of leaf ontogeny. Funct. Plant Biol. 2009, 36, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbilow, J.; Romero, M.; Alborn, H.T.; et al. Effects of elevated [CO2] on maize defense against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 2014, 37, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.A.; McAuslane, H.J.; Alborn, H.T.; Allen, L.H.; Teal, P.E.A. Interactive effects of elevated [CO2] and drought on the maize phytochemical defense response against mycotoxigenic Fusarium verticillioides. PLoS ONE 2016, 11, e0159270. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Vodkin, L.O.; Walter, A.; Schurr, U. The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 2006, 142, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.; Bunce, J.A.; Sicher, R.C.; Zhu, X.; Gao, B.; Chen, G. An attempt to interpret a biochemical mechanism of C4 photosynthetic thermo-tolerance under sudden heat shock on detached leaf in elevated CO2 grown maize. PLoS ONE 2017, 12, e0187437. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.; Vicente, R.; Amador, A.; Araus, J.L. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Front. Plant Sci. 2016, 7, 1738. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.A.; Da Graca, J.P.; De Laia, M.L.; Nhani-Jr, A.; Galbiati, J.A.; Ferro, M.I.T.; Ferro, J.A.; Zingaretti, S.M. Sugarcane genes differentially expressed during water deficit. Biol. Plant 2011, 55, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Provart, N.J.; Gil, P.; Chen, W.; Han, B.; Chang, H.S.; Wang, X.; Zhu, T. Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiol. 2003, 132, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.A.; Laia, M.L.; Zingaretti, S.M. Analysis of gene expression profiles under water stress in tolerant and sensitive sugarcane plants. Plant Sci. 2009, 176, 286–302. [Google Scholar] [CrossRef]
- Iglesias, A.A.; Andreo, C.S. Kinetic and structural properties of NADP-malic enzyme from sugarcane leaves. Plant Physiol. 1990, 92, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Lara, M.V.; Andreo, C.S. C4 plants adaptation to high levels of CO2 and to drought environments. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Ed.; InTech: London, UK, 2011; pp. 415–428. [Google Scholar] [CrossRef] [Green Version]
- Instituto Brasileiro de Geografia e Estatística. Levantamento Sistemático da Produção Agrícola: Pesquisa Mensal de Previsão e Acompanhamento das Safras Agrícolas no ano Civil. 2017. Available online: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=76 (accessed on 2 June 2020).
- Lara, M.V.; Andreo, C.S. Photosynthesis in nontypical C4 species. In Handbook of Photosyntheis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis: London, UK, 2005; pp. 392–421. [Google Scholar]
- Soreng, R.J.; Peterson, P.M.; Romschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Filgueiras, T.S.; Davis, J.I.; Morrone, O. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Abdel-Rhman, M.M. Improvement the production of maize (Zea mays L.) crop by using particle bombardment. Int. Conf. Biol. Civ. Environ. Eng. 2015, 74–79. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento. Acompanhamento da safra brasileira de grãos. Safra 2019/20, nono levantamento; 2020. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 3 June 2020).
- Queiroz, V.A.V.; Carneiro, H.L.; Deliza, R.; Rodrigues, J.A.S.; Vasconcelhos, J.H.; Tardin, F.D.; Queiroz, L.R. Sorghum genotypes for cereal bar production. Pesq. Agropec. Bras. 2012, 47, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.H.; Maretzki, A. Sugarcane. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 643–669. [Google Scholar]
- Zuurbier, P.; Van de Vooren, J. Sugarcane Ethanol: Contributions to Climate Change Mitigation and the Environment; Wageningen Academic Publishers: Wageningen, The Netherlands, 2008; p. 256. [Google Scholar]
- Souza, S.P.; Nogueira, L.A.H.; Martinez, J.; Cortez, L.A.B. Sugarcane can afford a cleaner energy profile in Latin America & Caribbean. Renew. Energy 2018, 121, 164–172. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Cana-De-Açúcar. Safra 2020/21, Primeiro Levantamento; 2020. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 3 June 2020).
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Global alterations in areas of suitability for maize production from climate change and using a mechanistic species distribution model (CLIMEX). Sci. Rep. 2017, 7, 5910. [Google Scholar] [CrossRef]
- Hsiang, S.; Kopp, R.; Jina, A.; Rising, J.; Delgado, M.; Mohan, S.; Rasmussen, D.J.; Muir-Wood, R.; Wilson, P.; Oppenheimer, M.; et al. Estimating economic damage from climate change in the United States. Science 2017, 356, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Lemonnier, P.; Wedow, J.M. The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biol. J. 2020, 22, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmond, C.B.; Troughton, J.H.; Goodchild, D.J. Physiological, biochemical and structural studies of photosynthesis and photorespiration in two species of Atriplex. Z. Pflanzenphysiol. 1969, 61, 218–237. [Google Scholar]
- Schuler, M.L.; Mantegazza, O.; Weber, A.P.M. Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J. 2016, 87, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: Species number, evolutionary lineages, and hall of fame. J. Exp. Bot. 2016, 67, 4039–4056. [Google Scholar] [CrossRef] [Green Version]
- Edwards, G.E.; Franceschi, V.R.; Ku, M.S.B.; Voznesenskaya, E.V.; Pyankov, V.I.; Andreo, C.S. Compartmentation of photosynthesis in cells and tissues of C4 plants. J. Exp. Bot. 2001, 52, 577–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leegood, R.C. The regulation of C4 photosynthesis. Adv. Bot. Res. 1997, 26, 251–316. [Google Scholar] [CrossRef]
- Lambers, H.; Pons, T.L.; Chapin III, F.S. Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hatch, M.D.; Burnell, J.N. Carbonic anhydrate activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol. 1990, 61, 1041–1052. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, A.; Soni, J.K.; Pawar, N. Physiological response of C3, C4 and CAM plants in changeable climate. Pharm. Innov. J. 2017, 6, 70–79. [Google Scholar]
- Gutierrez, M.; Gracen, V.E.; Edwards, G.E. Biochemical and cytological relationships in C4 plants. Planta 1974, 119, 279–300. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D.; Kagawa, T.; Craig, S. Subdivision of C4-pathway species based on differing C4 decarboxylating systems and ultrastructural features. Aust. J. Plant Physiol. 1975, 2, 111–128. [Google Scholar] [CrossRef]
- Hatch, M.D. C4 photosynthesis: A unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta 1987, 895, 81–106. [Google Scholar] [CrossRef]
- Kanai, R.; Edwards, G.E. The biochemistry of C4 photosynthesis. In C4 Plant Biology; Sage, R.F., Monson, R.K., Eds.; Academic Press: London, UK, 1999; pp. 49–87. [Google Scholar]
- Ludwig, M. The roles of organic acids in C4 photosynthesis. Front. Plant Sci. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, C.L.D.; Furbank, R.T.; Hatch, M.D. Mechanism of C4 photosynthesis. A model describing the inorganic carbon pool in bundle sheath cells. Plant Physiol. 1989, 91, 1372–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations; six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Malan, C. Influence of Elevated CO2 on the Growth, Yield and Photosynthesis of Sugarcane. Bachelor’s Thesis, North-West University, Potchefstroom, South Africa, 2017. [Google Scholar]
- Kim, S.-H.; Sicher, R.C.; Bae, H.; Gitz, D.C.; Baker, J.T.; Timlin, D.J.; Reddy, V.R. Canopy photosynthesis, evapotranspiration, leaf nitrogen, and transcription profiles of maize in response to CO2 enrichment. Glob. Chang. Biol. 2006, 12, 588–600. [Google Scholar] [CrossRef]
- Manderscheid, R.; Erbs, M.; Weigel, H.-J. Interactive effects of free-air CO2 enrichment and drought stress on maize growth. Eur. J. Agron. 2014, 52, 11–21. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, R.; Li, Y.; Liu, X.; Wang, G.; Yin, K.; Jin, J.; Herbert, S.J. Warming and elevated CO2 alter the transcriptomic response of maize (Zea mays L.) at the silking stage. Sci. Rep. 2019, 9, 17948. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Allen, L.H. Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J. Plant Physiol. 2009, 166, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.W.; Brooks, T.J.; Adam, N.R.; Cousins, A.B.; Kimball, B.A.; Pinter, P.J., Jr.; LaMorte, R.L.; Triggs, J.; Ottman, M.J.; Leavitt, S.W.; et al. Elevated atmospheric CO2 improved sorghum plant water status by ameliorating the adverse effects of drought. New Phytol. 2001, 152, 231–248. [Google Scholar] [CrossRef]
- Conley, M.M.; Kimball, B.A.; Brooks, T.J.; Pinter, P.J., Jr.; Hunsaker, D.J.; Wall, G.W.; Adam, N.R.; LaMorte, R.L.; Matthias, A.D.; Thompson, T.L.; et al. CO2 enrichment increases water-use efficiency in sorghum. New Phytol. 2001, 151, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.P.; Cocuron, J.C.; Garcia, A.C.; Alonso, A.P.; Buckeridge, M.S. Changes in whole-plant metabolism during the grain-filling stage in sorghum grown under elevated CO2 and drought. Plant Physiol. 2015, 169, 1755–1765. [Google Scholar] [CrossRef]
- Rinaldi, M.; Rascio, A.; Garofalo, P. Sunflower and biomass sorghum photosynthesis response to CO2 enrichment. Rom. Agric. Res. 2015, 32, 1–10. [Google Scholar]
- Mina, U.; Kumar, R.; Gogoi, R.; Bhatia, A.; Harit, R.C.; Singh, D.; Kumar, A.; Kumar, A. Effect of elevated temperature and carbon dioxide on maize genotypes health index. Ecol. Indic. 2019, 105, 292–302. [Google Scholar] [CrossRef]
- Khanboluki, G.; Hosseini, H.M.; Holford, P.; Zadeh, B.M.; Milham, P.J. Effect of elevated atmospheric CO2 concentration on growth and physiology of wheat and sorghum under cadmium stress. Commun. Soil Sci. Plant Anal. 2018, 49, 2867–2882. [Google Scholar] [CrossRef]
- Ottman, M.J.; Kimball, B.A.; Pinter, P.J.; Wall, G.W.; Vanderlip, R.L.; Leavitt, S.W.; LaMorte, R.L.; Matthias, A.D.; Brooks, T.J. Elevated CO2 increases sorghum biomass under drought conditions. New Phytol. 2001, 150, 261–273. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity and yield of maize are not affected by open–air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Singels, A.; Jones, M.; Marin, F.; Ruane, A.C.; Thorburn, P. Predicting climate change impacts on sugarcane production at sites in Australia, Brazil and South Africa using the Canegro model. Sugar Tech 2014, 16, 347–355. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, J.; Yao, F.; Hao, C. Interactive effects of elevated CO2 concentration and irrigation on photosynthetic parameters and yield of maize in Northeast China. PLoS ONE 2014, 9, e98318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adishesha, K.; Janagoudar, B.S.; Amaregouda, A.; Shanawad, U.K.; Chandranaik, M. Morphological charaters of maize (Zea mays L.) genotypes to elevated carbon dioxide and temperature regimes. Int. J. Pure App. Biosci. 2017, 5, 163–170. [Google Scholar] [CrossRef]
- Sicher, R.C.; Barnaby, J.Y. Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Plant 2012, 144, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, U.N.; Burnett, R.B.; Kirkham, M.B.; Kanemasu, E.T. Effect of carbon dioxide on sorghum yield, root growth, and water use. Agric. Meteorol. 1986, 37, 109–122. [Google Scholar] [CrossRef]
- Stokes, C.J.; Inman-Bamber, N.G.; Everingham, Y.L.; Sexton, J. Measuring and modelling CO2 effects on sugarcane. Environ. Model. Softw. 2016, 78, 68–78. [Google Scholar] [CrossRef] [Green Version]
- McCormick, A.J.; Cramer, M.D.; Watt, D.A. Culm sucrose accumulation promotes physiological decline of mature leaves in ripening sugarcane. Field Crops Res. 2008, 108, 250–258. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzales, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.; Smeekens, S. Sugar perception and signaling-an update. Curr. Opin. Plant Biol. 2009, 12, 562–567. [Google Scholar] [CrossRef]
- Bowes, G. Facing the inevitable: Plants and increasing atmospheric CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 309–332. [Google Scholar] [CrossRef]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [CrossRef]
- Maroco, J.P.; Edwards, G.E.; Ku, M.S.B. Photosynthetic acclimation of maize to growth under elevated levels of carbon dioxide. Planta 1999, 210, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.B.; Adam, N.R.; Wall, G.W.; Kimball, B.A.; Pinter, P.J., Jr.; Leavitt, S.W.; LaMorte, R.L.; Matthias, A.D.; Ottman, M.J.; Thompson, T.L.; et al. Reduced photorespiration and increased energy-use efficiency in young CO2-enriched sorghum leaves. New Phytol. 2001, 150, 275–284. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Bernacchi, C.J.; Dohleman, F.G.; Ort, D.R.; Long, S.P. Will photosynthesis of maize (Zea mays) in the U.S. Corn Belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob. Chan. Biol. 2004, 10, 1–12. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yong, Z.H.; Liao, Y.; Zhang, D.Y.; Chen, Y.; Zhang, H.B.; Chen, J.; Zhu, J.G.; Xu, D.Q. Photosynthetic acclimation in rice leaves to free-air CO2 enrichment related to both ribulose-1,5-bisphosphate carboxylation limitation and ribulose-1,5-bisphosphate regeneration limitation. Plant Cell Physiol. 2005, 46, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin response factor 2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Tian, L.; Sun, M.X.; Huang, X.Y.; Zhu, J.; Guan, Y.F.; Jia, Q.S.; Yan, Z.N. Auxin response factor17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol. 2013, 162, 720–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, P.O.; Lee, I.C.; Kim, J.; Kim, H.J.; Ryu, J.S.; Woo, H.R.; Nam, H.G. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 2010, 61, 1419–1430. [Google Scholar] [CrossRef]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Guo, B.; Cai, Y.; Zhang, H.; Luo, S.; Gallois, P. Transcriptome analysis identifies differentially expressed genes in maize leaf tissues in response to elevated atmospheric [CO2]. J. Plant Interact. 2018, 13, 373–379. [Google Scholar] [CrossRef]

| Plant | [CO2] Applied 1 | Experimental Conditions 2 | Reference |
|---|---|---|---|
| Zea mays | AC: 370 µmol mol−1 | Soil–plant–atmosphere research chambers | [60] |
| EC: 750 µmol mol−1 | |||
| AC: 350 µL L−1 | CEC | [9] | |
| EC: 700 µL L−1 | |||
| AC: 378 µL L−1 | FACE | [61] | |
| EC: 550 µL L−1 | |||
| AC: 394 ppm | OTCs | [19] | |
| EC: 566 ppm | |||
| AC: 400 ppm | OTCs | [62] | |
| EC: 550 ppm | |||
| Saccharum spp. | AC: 360 µmol mol−1 | TGG | [12] |
| EC: 720 µmol mol−1 | |||
| AC: 370 ppm | OTCs | [13] | |
| EC: 720 ppm | |||
| AC: 360 µmol mol−1 | TGG | [63] | |
| EC: 720 µmol mol−1 | |||
| AC: 400 ppm | OTCs | [59] | |
| EC: 750−800 ppm | |||
| Sorghum bicolor | AC: 370 µmol mol−1 | FACE | [64] |
| EC: 570 µmol mol−1 | |||
| AC: 370 µmol mol−1 | FACE | [65] | |
| EC: 570 µmol mol−1 | |||
| AC: 400 µmol mol−1 | OTCs | [66] | |
| EC: 800 µmol mol−1 |
| Plant | Growth Parameters | Elevated [CO2] | Effects * |
|---|---|---|---|
| Zea mays | Leaf area | 550 ppm | (↑) 1 (↑) 2 |
| Plant height | 550 ppm | (↑) 1 (↑) 2 | |
| Total masses | 70 Pa–550 ppm | (↑) 3 (=) 4 | |
| Saccharum spp. | Leaf area | 720 µmol mol−1 | (↑)5 |
| Plant height | 720 ppm | (↑)6 | |
| Total masses | 720 ppm | (↑)6 | |
| Sorghum bicolor | Leaf area | 795–900 µ L−1 | (↑) 7 (↑) 8 |
| Plant height | 795 µ L−1 | (=) 7 | |
| Total masses | 900 µ L−1 | (↑) 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.G.d.; Alves, R.d.C.; Zingaretti, S.M. Increased [CO2] Causes Changes in Physiological and Genetic Responses in C4 Crops: A Brief Review. Plants 2020, 9, 1567. https://doi.org/10.3390/plants9111567
Silva RGd, Alves RdC, Zingaretti SM. Increased [CO2] Causes Changes in Physiological and Genetic Responses in C4 Crops: A Brief Review. Plants. 2020; 9(11):1567. https://doi.org/10.3390/plants9111567
Chicago/Turabian StyleSilva, Renan Gonçalves da, Rita de Cássia Alves, and Sonia Marli Zingaretti. 2020. "Increased [CO2] Causes Changes in Physiological and Genetic Responses in C4 Crops: A Brief Review" Plants 9, no. 11: 1567. https://doi.org/10.3390/plants9111567







