Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas
Abstract
1. Introduction
2. Results
2.1. Growth Parameters Affected by Salinity and Drought
2.2. Ionic Concentrations
2.3. Physiological Parameters and Water Relations
3. Discussion
3.1. Growth Parameters Affected by Salinity and Drought
3.2. Chemical Parameters Affected by Salinity and Drought
3.3. Physiological Parameters Affected by Salinity and Drought
4. Materials and Methods
4.1. Experimental Design and Crop Establishment
4.2. Measurement of Growth and Chemical Attributes
4.3. Determination of Physiological Attributes
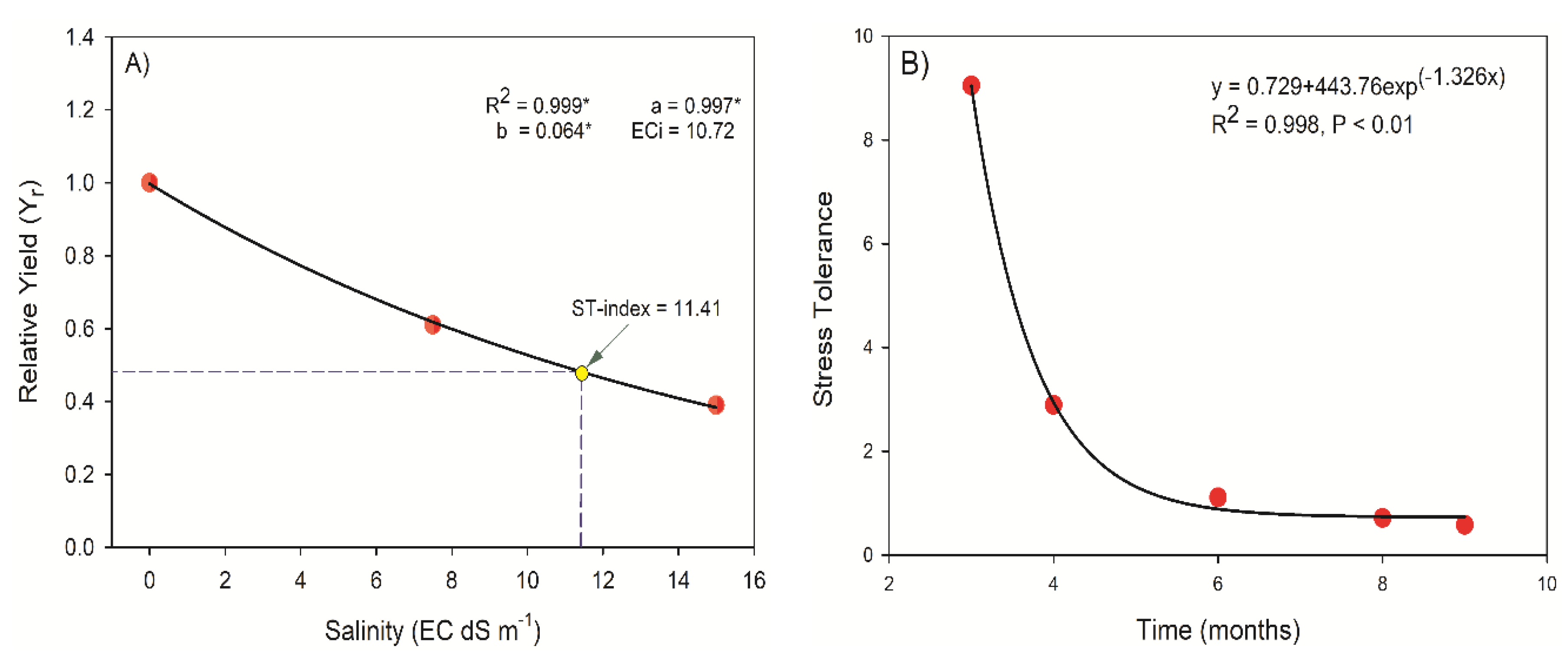
4.4. Quantification of Stress Tolerance
4.4.1. Stress Response Models and Salinity-Tolerance Index
4.4.2. Stress Tolerance Determination
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Argus, R.E.; Colmer, T.D.; Grierson, P.F. Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-Enriched Animal Manure Alleviates the Adverse Effects of Salt Stress on Growth, Physiology and Nutrients Homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Mostofa, M.G.; Akter, M.M.; Srivastava, A.K.; Sayed, M.A.; Hasan, M.S.; Tran, L.S.P. Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: Oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 2017, 187, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Dai, H.; Zheng, W.; Cao, F.; Zhang, G.; Sun, D.; Wu, F. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2013. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Hussain, T.; Koyro, H.W.; Huchzermeyer, B.; Khan, M.A. Eco-physiological adaptations of Panicum antidotale to hyperosmotic salinity: Water and ion relations and anti-oxidant feedback. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 212, 30–37. [Google Scholar] [CrossRef]
- Divakara, B.N.; Upadhyaya, H.D.; Wani, S.P.; Gowda, C.L.L. Biology and genetic improvement of Jatropha curcas L.: A review. Appl. Energy 2010, 87, 732–742. [Google Scholar] [CrossRef]
- Jingura, R.M.; Matengaifa, R.; Musademba, D.; Musiyiwa, K. Characterisation of land types and agro-ecological conditions for production of Jatropha as a feedstock for biofuels in Zimbabwe. Biomass Bioenergy 2011, 35, 2080–2086. [Google Scholar] [CrossRef]
- Maes, W.H.; Trabucco, A.; Achten, W.M.J.; Muys, B. Climatic growing conditions of Jatropha curcas L. Biomass Bioenergy 2009, 33, 1481–1485. [Google Scholar] [CrossRef]
- King, A.J.; He, W.; Cuevas, J.A.; Freudenberger, M.; Ramiaramanana, D.; Graham, I.A. Potential of Jatropha curcas as a source of renewable oil and animal feed. J. of Exp. Bot. 2009, 60, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Achten, W.M.J.; Maes, W.H.; Reubens, B.; Mathijs, E.; Singh, V.P.; Verchot, L.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bioenergy 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Yaron, B.; Zieslin, N.; Halevy, A.H. Response of Baccara roses to saline irrigation. J. Am. Soc. Hort. Sci. 1969. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301229927 (accessed on 10 October 2020).
- Steppuhn, H.; Van Genuchten, M.T.; Grieve, C.M. Root-zone salinity: I. Selecting a product-yield index and response function for crop tolerance. Crop Sci. 2005, 45, 209–220. [Google Scholar] [CrossRef]
- Naveed, M.; Ramzan, N.; Mustafa, A.; Samad, A.; Niamat, B.; Yaseen, M.; Ahmad, Z.; Hasanuzzaman, M.; Sun, N.; Shi, W.; et al. Alleviation of Salinity Induced Oxidative Stress in Chenopodium quinoa by Fe Biofortification and Biochar—Endophyte Interaction. Agronomy 2020, 10, 168. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shabala, S. Control of xylem Na + loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiol. Plant. 2019, 165, 619–631. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Akhtar, J.; Qureshi, R.H. Pot study on wheat growth in saline and waterlogged compacted soil: II. Root growth and leaf ionic relations. Soil Tillage Res. 2004, 77, 179–187. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and functional adaptations in plants for salinity tolerance. In Plant Adaptation and Phytoremediation; Springer Netherlands: Dordrecht, The Netherlands, 2010; ISBN 9789048193707. [Google Scholar]
- Aboukheira, A.A.A.S. Jatropha curcas Global Diversity View project Innovative Irrigation Practices and Technologies for Alfalfa Production in Egypt View project. Biomass Bioenergy 2009, 33, 1343–1350. [Google Scholar] [CrossRef]
- Díaz-López, L.; Gimeno, V.; Lidón, V.; Simón, I.; Martínez, V.; García-Sánchez, F. The tolerance of Jatropha curcas seedlings to NaCl: An ecophysiological analysis. Plant Physiol. Biochem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Qureshi, R.H.; Ahmad, N. Nutrient availability in a calcareous saline-sodic soil during vegetative bioremediation. Arid Soil Res. Rehabil. 1997, 11, 343–352. [Google Scholar] [CrossRef]
- Marcar, N.; Crawford, D.; Proceedings, P.L.-A. The potential of trees for utilisation and management of salt-affected land. In “Productive Use of Saline Land”; Davidson, N., Galloway, R., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1993. [Google Scholar]
- Noreen, S.; Ashraf, M. Alleviation of adverse effects of salt stress on sunflower (helianthus annuus l.) by exogenous application of salicylic acid: Growth and photosynthesis. Pakistan J. Bot. 2008, 40, 1657–1663. [Google Scholar]
- Ashraf, M.Y.; Sarwar, G. Salt tolerance potential in some members of Brassicaceae physiological studies on water relations and mineral contents. In Prospects for Saline Agriculture; Springer: Dordrecht, The Netherlands, 2002; pp. 237–245. [Google Scholar]
- Cheeseman, J.M. Mechanisms of Salinity Tolerance in Plants. Plant Physiol. 1988, 87, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Soda, N.; Ephrath, J.E.; Dag, A.; Beiersdorf, I.; Presnov, E.; Yermiyahu, U.; Ben-Gal, A. Root growth dynamics of olive (Olea europaea L.) affected by irrigation induced salinity. Plant Soil 2017. [Google Scholar] [CrossRef]
- Bernstein, N.; Meiri, A.; Zilberstaine, M. Root Growth of Avocado is More Sensitive to Salinity than Shoot Growth. J. Am. Soc. Hortic. Sci. 2004, 129, 188–192. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Hennion, N.; Durand, M.; Vriet, C.; Doidy, J.; Maurousset, L.; Lemoine, R.; Pourtau, N. Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol. Plant. 2019, 165, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Chang, J.; Cheong, B.E.; Natera, S.; Roessner, U. Morphological and metabolic responses to salt stress of rice (Oryza sativa L.) cultivars which differ in salinity tolerance. Plant Physiol. Biochem. 2019. [Google Scholar] [CrossRef]
- Carillo, P.; Raimondi, G.; Kyriacou, M.C.; Pannico, A.; El-Nakhel, C.; Cirillo, V.; Colla, G.; De Pascale, S.; Rouphael, Y. Morpho-physiological and homeostatic adaptive responses triggered by omeprazole enhance lettuce tolerance to salt stress. Sci. Hortic. (Amsterdam). 2019. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Y.; Xu, G. Interactive effects of potassium and sodium on root growth and expression of K/Na transporter genes in rice. Plant Growth Regul. 2009, 57, 271–280. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, M.; Newman, I.A.; Mendham, N.J.; Zhang, G.; Shabala, S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Shabala, S. Regulation of potassium transport in leaves: From molecular to tissue level. Ann. Bot. 2003, 92, 627–634. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants. 2002. Available online: https://www.researchgate.net/publication/316582636_Marschner’s_Mineral_Nutrition_of_Higher_Plants (accessed on 10 October 2020).
- Adolf, V.I.; Jacobsen, S.E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013. [Google Scholar] [CrossRef]
- Kotula, L.; Khan, H.A.; Quealy, J.; Turner, N.C.; Vadez, V.; Siddique, K.H.M.; Clode, P.L.; Colmer, T.D. Salt sensitivity in chickpea (Cicer arietinum L.): Ions in reproductive tissues and yield components in contrasting genotypes. Plant Cell Environ. 2015. [Google Scholar] [CrossRef]
- Geressu Asfaw, K. Effects of Salinity on Seedling Biomass Production and Relative Water Content of Twenty Sorghum (Sorghum biolor L. Moench) Accessions. Asian J. Agric. Sci. 2011, 3, 242–249. [Google Scholar] [CrossRef][Green Version]
- Saeed, Z.; Naveed, M.; Imran, M.; Bashir, M.A.; Sattar, A.; Mustafa, A.; Hussain, A.; Xu, M. Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 2019, 14, e0213016. [Google Scholar] [CrossRef]
- Senadheera, P.; Tirimanne, S.; Maathuis, F.J.M. Long Term Salinity Stress Reveals Variety Specific Differences in Root Oxidative Stress Response. Rice Sci. 2012. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Singh, S.K.; Hoyos-Villegas, V.; Ray, J.D.; Smith, J.R.; Fritschi, F.B. Quantification of leaf pigments in soybean (Glycine max (L.) Merr.) based on wavelet decomposition of hyperspectral features. Field Crop. Res. 2013, 149, 20–32. [Google Scholar] [CrossRef]
- Adem, G.D.; Roy, S.J.; Zhou, M.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and Heat Stress Injury to Two Cool-Season Turfgrasses in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Velagaleti, R.R.; Kramer, D.; Marsh, S.S.; Reichenbach, N.G.; Fleischman, D.E. Some Approaches to Rapid and Pre-Symptom Diagnosis of Chemical Stress in Plants; ASTM International: West Conshohocken, PA, USA, 1990; pp. 333–345. [Google Scholar] [CrossRef]
- Rangani, J.; Parida, A.K.; Panda, A.; Kumari, A. Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte Salvadora persica L. Front. Plant Sci. 2016. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahbaz, M. Assessment of genotypic variation in salt tolerance of early CIMMYT hexaploid wheat germplasm using photosynthetic capacity and water relations as selection criteria. Photosynthetica 2003. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, P.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 2008, 46, 107–114. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO 2 concentration around leaves. Plant Cell Environ. 2007. [Google Scholar] [CrossRef]
- Asrar, H.; Hussain, T.; Hadi, S.M.S.; Gul, B.; Nielsen, B.L.; Khan, M.A. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ. Exp. Bot. 2017, 135, 86–95. [Google Scholar] [CrossRef]
- Ben-Asher, J.; van Dam, J.; Feddes, R.A.; Jhorar, R.K. Irrigation of grapevines with saline water. II. Mathematical simulation of vine growth and yield. Agric. Water Manag. 2006. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, N.K.; Gupta, S.; Hasegawa, H. Effect of NaCl salinity on photosynthetic rate, transpiration rate, and oxidative stress tolerance in contrasting wheat genotypes. Photosynthetica 2005. [Google Scholar] [CrossRef]
- Ali-Dinar, H.M.; Ebert, G.; Lüdders, P. Growth, Chlorophyll Content, Photosynthesis and Water Relations in Guava (Psidium guajava L.) Under Salinity and Different Nitrogen Supply Wachstum, Chlorophyllgehalt, Photosynthese und Wasserstatus von Guaven (Psidium guajava L.) bei Salinität und unterschiedlicher Stickstoffversorgung. Available online: https://www.pubhort.org/ejhs/1999/file_3735.pdf (accessed on 10 October 2020).
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant. Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000. [Google Scholar] [CrossRef]
- Plaut, Z.; Meinzer, F.C.; Federman, E. Leaf development, transpiration and ion uptake and distribution in sugarcane cultivars grown under salinity. Plant Soil 2000. [Google Scholar] [CrossRef]
- Lafitte, R. Relationship between leaf relative water content during reproductive stage water deficit and grain formation in rice. Field Crop. Res. 2002. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: Stomata vs. Metabolism and the role of ATP. Ann. Bot. 2002. [Google Scholar] [CrossRef]
- Faisalabad, Punjab, Pakistan Today, Tonight & Tomorrow’s Weather Forecast | AccuWeather. Available online: https://www.accuweather.com/en/pk/faisalabad/260626/weather-forecast/260626 (accessed on 7 October 2020).
- Richards, L.A.; Allison, L.; Bernstein, C.A.; Bower, J.W.; Brown, M.; Fireman, J.T.; Hatcher, H.E.; Hayward, G.A.; Pearson, R.C.; Reeve, L.E.; et al. Diagnosis and Improvement of Saline and Alkali Soils; United States Salinity Laboratory Staff: Washington, DC, USA, 1954. [Google Scholar]
- Sairam, R.K.; Rao, K.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002. [Google Scholar] [CrossRef]
- Teulat, B.; Zoumarou-Wallis, N.; Rotter, B.; Ben Salem, M.; Bahri, H.; This, D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003. [Google Scholar] [CrossRef] [PubMed]
- Steppuhn, H.; van Genuchten, M.T.; Grieve, C.M. Root-Zone Salinity. Crop Sci. 2005, 45, 221–232. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. Adapt. Food Crop. Temp. Water Stress 1993, 410, 257–270. [Google Scholar]
- Steel, R. Analysis of variance I: The one-way classification. Princ. Proced. Stat. A Biom. Approach 1997, 139–203. [Google Scholar]


| Treatments | Plant Height (cm) | Stem Diameter (cm) | No. of Branches | No. of Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (months) | Duration (months) | Duration (months) | Duration (months) | |||||||||
| 3 | 6 | 9 | 3 | 6 | 9 | 3 | 6 | 9 | 3 | 6 | 9 | |
| Control | 62.6 ± 0.93a | 68.80 ± 0.86a | 72.00 ± 1.58a | 1.82 ± 0.06d | 1.93 ± 0.03a | 2.40 ± 0.18a | 12.20 ± 0.37a | 14.80 ± 0.86a | 16.40 ± 0.86a | 11.40 ± 0.68a | 14.20 ± 0.86a | 15.80 ± 0.86a |
| Drought | 33.4 ± 1.81b | 36.20 ± 1.74b | 41.40 ± 1.58b | 1.07 ± 0.06bc | 1.20 ± 0.11c | 1.37 ± 0.05bc | 9.80 ± 0.37b | 12.20 ± 0.73b | 13.60 ± 0.51b | 7.40 ± 0.81b | 10.20 ± 0.37b | 13.20 ± 0.58b |
| 7.5 dS m−1 (Well-watered | 21.00 ± 1.00c | 22.60 ± 1.03c | 26.80 ± 0.97c | 1.58 ± 0.08b | 1.63± 0.08b | 1.68 ± 0.07b | 9.60 ± 0.68b | 10.80 ± 0.86b | 13.20 ± 1.02bc | 7.20 ± 0.80b | 10.20 ± 0.86b | 12.40 ± 1.33b |
| 7.5 dS m−1+Drought | 13.85 ± 1.92d | 14.67 ± 0.93d | 17.20 ± 1.58d | 1.04 ± 0.07c | 1.15 ± 0.04c | 1.48 ± 0.19bc | 7.20 ± 0.66c | 8.00 ± 0.55c | 11.20 ± 0.37cd | 6.60 ± 0.51b | 7.60 ± 0.51c | 9.40 ± 0.51c |
| 15 dS m−1 (Well-watered) | 12.89 ± 1.07d | 15.81 ± 0.97d | 18.63 ± 1.58d | 1.08 ± 0.06c | 1.14 ± 0.06c | 1.16 ± 0.01cd | 6.80 ± 0.58c | 7.20 ± 0.66cd | 10.00 ± 0.71d | 6.20 ± 0.58b | 6.80 ± 0.66c | 7.60 ± 0.51cd |
| 15 dS m−1+Drought | 10.75 ± 1.95d | 13.70 ± 0.93d | 16.44 ± 1.58d | 0.56 ± 0.08d | 0.84 ± 0.07d | 1.08 ± 0.06d | 4.80 ± 0.97d | 5.20 ± 0.37d | 7.60 ± 0.75e | 4.00 ± 0.55c | 4.40 ± 0.51d | 5.60 ± 0.51e |
| Treatment | Na+ | K+ | K+: Na+ Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | |
| Control | 0.12e | 0.14e | 0.16e | 1.62a | 0.68a | 0.55a | 13.06a | 4.72a | 3.49a |
| 7.5 dS m−1 (well-watered) | 0.60d | 0.66d | 0.79d | 0.89c | 0.48c | 0.41b | 1.483c | 0.73c | 0.52c |
| 15 dS m−1 (well-watered) | 1.29b | 1.51b | 1.89b | 0.83c | 0.43cd | 0.38b | 0.643c | 0.29c | 0.20c |
| Drought | 0.16e | 0.18e | 0.19e | 1.24b | 0.57b | 0.49a | 7.949b | 3.28b | 2.53b |
| 7.5 dS m−1+Drought | 1.05c | 1.12c | 1.25c | 0.87c | 0.46c | 0.39b | 0.829c | 0.41c | 0.31c |
| 15 dS m−1+Drought | 1.84a | 2.06a | 2.32a | 0.75d | 0.36d | 0.27c | 0.408c | 0.18c | 0.12c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; ul-Hassan, M.; Sun, N.; et al. Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas. Plants 2020, 9, 1574. https://doi.org/10.3390/plants9111574
Abrar MM, Saqib M, Abbas G, Atiq-ur-Rahman M, Mustafa A, Shah SAA, Mehmood K, Maitlo AA, ul-Hassan M, Sun N, et al. Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas. Plants. 2020; 9(11):1574. https://doi.org/10.3390/plants9111574
Chicago/Turabian StyleAbrar, Muhammad Mohsin, Muhammad Saqib, Ghulam Abbas, Muhammad Atiq-ur-Rahman, Adnan Mustafa, Syed Atizaz Ali Shah, Khalid Mehmood, Ali Akbar Maitlo, Mahmood ul-Hassan, Nan Sun, and et al. 2020. "Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas" Plants 9, no. 11: 1574. https://doi.org/10.3390/plants9111574
APA StyleAbrar, M. M., Saqib, M., Abbas, G., Atiq-ur-Rahman, M., Mustafa, A., Shah, S. A. A., Mehmood, K., Maitlo, A. A., ul-Hassan, M., Sun, N., & Xu, M. (2020). Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas. Plants, 9(11), 1574. https://doi.org/10.3390/plants9111574







