Genetic Improvement of Arundo donax L.: Opportunities and Challenges
Abstract
:1. Introduction
2. Genetic Variability
3. Clonal Selection
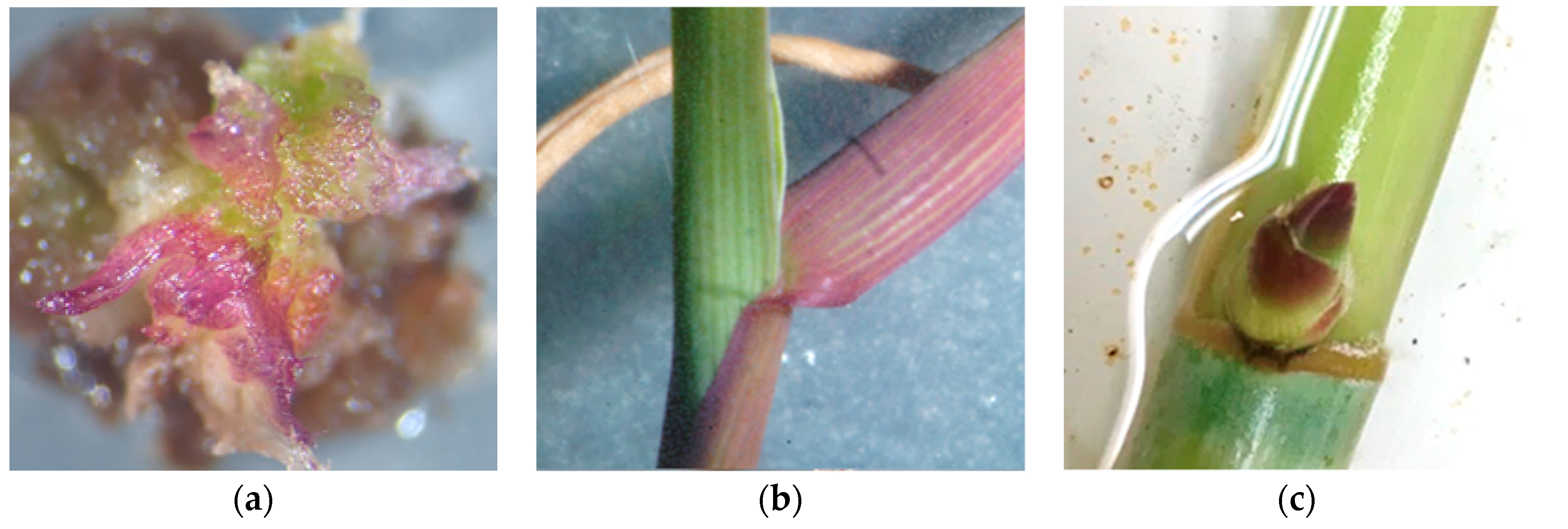
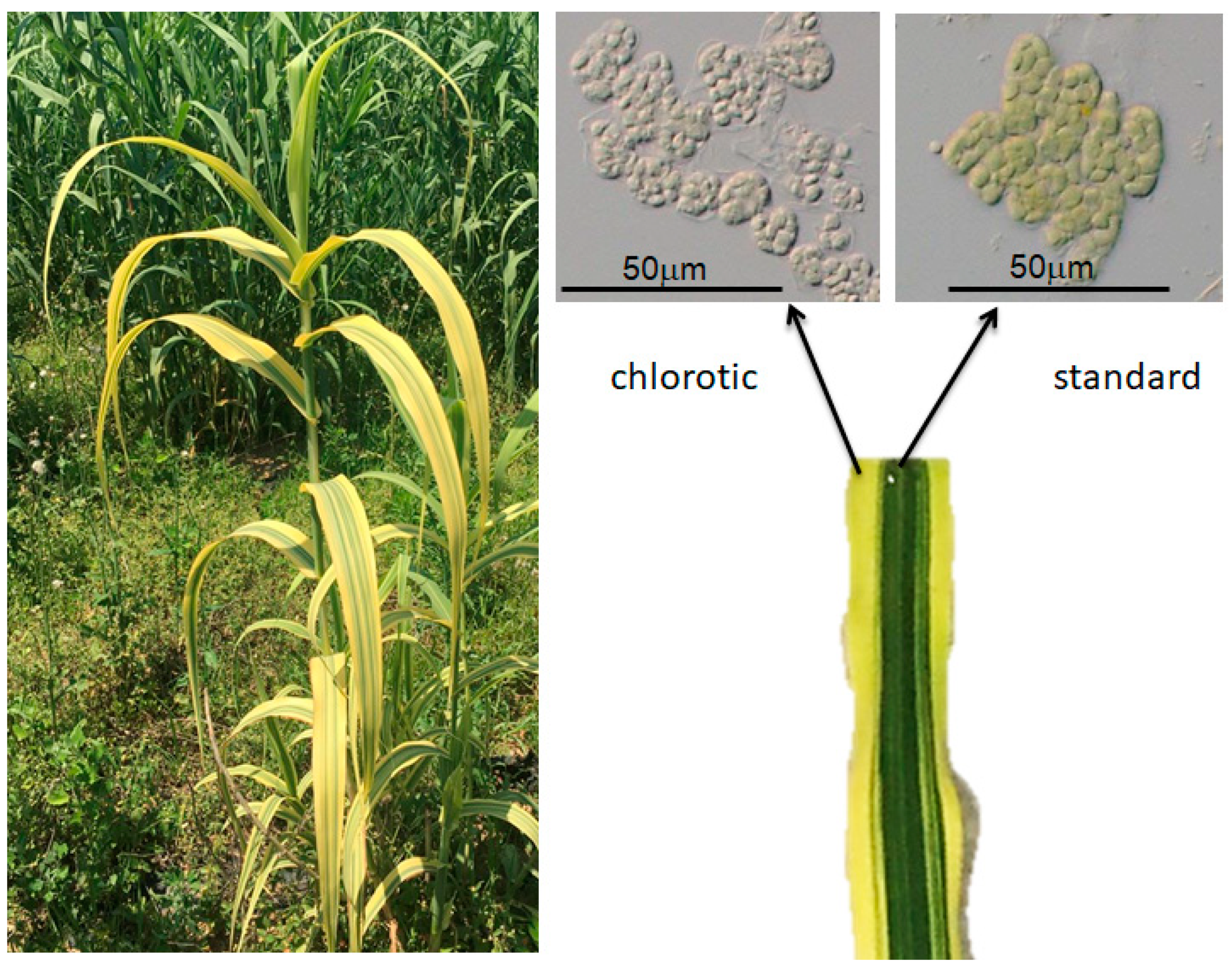
4. Somaclonal Variation and Mutagenesis
5. Genetic Engineering
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perdue, R.E. Arundo donax—Source of musical reeds and industrial cellulose. Econ. Bot. 1958, 12, 368–404. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Beinhoff, O. Analysis of lignocelluloses and lignins from Arundo donax L. and Miscanthus sinensis Anderss., and hydroliquefaction of Miscanthus. Biomass 1989, 18, 109–126. [Google Scholar] [CrossRef]
- Coffman, G.C.; Ambrose, R.F.; Rundel, P.W. Wildfire promotes dominance of invasive giant reed (Arundo donax) in riparian ecosystems. Biol. Invasions 2010, 12, 2723–2734. [Google Scholar] [CrossRef] [Green Version]
- Osbrink, W.; A Goolsby, J.; Thomas, D.B.; Mejorado, A.; Showler, A.T.; de León, A.P. Higher Ant Diversity in Native Vegetation Than in Stands of the Invasive Arundo, Arundo donax L., Along the Rio Grande Basin in Texas, USA. Int. J. Insect Sci. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, L.D.; Holt, J.S. Ecological correlates of invasion by Arundo donax in three southern California riparian habitats. Biol. Invasions 2007, 10, 591–601. [Google Scholar] [CrossRef]
- Frandsen, P.R. Team Arundo: Interagency Cooperation to Control Giant Cane (Arundo donax). In Springer Series on Environmental Management; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1997; pp. 244–248. [Google Scholar]
- Tucker, G.C.; Gordon, C. The genera of Arundinoideae (Graminaeae) in Southeastern United States. J. Arnold Arboretum 1990, 71, 145–717. [Google Scholar] [CrossRef]
- Pilu, R.; Manca, A.; Landoni, M. Arundo donax as an energy crop: Pros and cons of the utilization of this perennial plant. Maydica 2013, 58, 54–59. [Google Scholar]
- Bell, G. Ecology and Management of Arundo donax, and Approaches to Riparian Habitat Restoration in Southern California, Plant Invasions: Studies from North America and Europe; Brock, J.H., Wade, M., Pysek, P., Green, D., Eds.; Blackhuys Publishers: Leiden, The Netherlands, 1997; pp. 103–113. [Google Scholar]
- Di Tomaso, J.M.; Healey, E.A. Aquatic and riparian weeds of the west, University of California. Div. Agric. Nat. Res. 2003, 3421, 254–262. [Google Scholar]
- Balogh, E.; Herr, J.M.; Czakó, M.; Márton, L. Defective development of male and female gametophytes in Arundo donax L. (POACEAE). Biomass Bioenergy 2012, 45, 265–269. [Google Scholar] [CrossRef]
- Bucci, A.; Cassani, E.; Landoni, M.; Cantaluppi, E.; Pilu, R. Analysis of chromosome number and speculations on the origin of Arundo donax L. (Giant Reed). Cytol. Genet. 2013, 47, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Haddadchi, A.; Gross, C.L.; Fatemi, M. The expansion of sterile Arundo donax (Poaceae) in southeastern Australia is accompanied by genotypic variation. Aquat. Bot. 2013, 104, 153–161. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Rosato, M.; A Rossello, J.; Vila, B. Impact of polyploidy on fertility variation of Mediterranean Arundo L. (Poaceae). Comptes Rendus Biol. 2015, 338, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mariani, C.; Cabrini, R.; Danin, A.; Piffanelli, P.; Fricano, A.; Gomarasca, S.; di Candilo, M.; Grassi, F.; Soave, C. Origin, diffusion and reproduction of the giant reed (Arundo donax L.): A promising weedy energy crop. Ann. Appl. Biol. 2010, 157, 191–202. [Google Scholar] [CrossRef]
- Makde, C.A.N. Reproductive Behavior of Arundo donax L. Int. J. Res. Biosci. Agric. Technol. 2014, II. [Google Scholar] [CrossRef]
- Decruyenaere, J.G.; Holt, J.S. Seasonality of clonal propagation in giant reed. Weed Sci. 2001, 49, 760–767. [Google Scholar] [CrossRef]
- Ceotto, E.; di Candilo, M. Shoot cuttings propagation of giant reed (Arundo donax L.) in water and moist soil: The path forward? Biomass Bioenergy 2010, 34, 1614–1623. [Google Scholar] [CrossRef]
- Boland, J.M. The importance of layering in the rapid spread of Arundo donax (Giant Reed). Madroño 2006, 53, 303–312. [Google Scholar] [CrossRef]
- Hanson, H.C.; Zohary, M. Plant Life of Palestine, Israel, and Jordan. J. Range Manag. 1962, 15, 339. [Google Scholar] [CrossRef]
- Arcidiacono, S.; Costa, R.; Marletta, G.; Pavone, P.; Napoli, M. Usi popolari delle piante selvatiche nel territorio di Villarosa (EN—Sicilia Centrale). Quad. Bot. Amb. Appl. 2010, 21, 95–118. [Google Scholar]
- Polunin, O.; Huxley, A. Flowers of the Mediterranean; Hogarth Press: London, UK, 1987. [Google Scholar]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Ahmad, R.; Liow, P.-S.; Spencer, D.F.; Jasieniuk, M. Molecular evidence for a single genetic clone of invasive Arundo donax in the United States. Aquat. Bot. 2008, 88, 113–120. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeven, A.C.; Wet, J.M.J. Dictionary of Cultivated Plants and Their Regions of Diversity; Hudoc: Wageningen, The Netherlands, 1982. [Google Scholar]
- Danin, A. Arundo (Gramineae) in the Mediterranean reconsidered. Willdenowia 2004, 34, 361. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Baumel, A.; Juin, M.; Vila, B. Revised systematics of Mediterranean Arundo (Poaceae) based on AFLP fingerprints and morphology. Taxon 2012, 61, 1217–1226. [Google Scholar] [CrossRef]
- Zecca, G.; Tommasi, N.; Grassi, F. Multiple evolutionary lineages detected in giant reed (Arundo donax L.): Applied and evolutionary perspectives. Ann. Appl. Biol. 2020, 176, 285–295. [Google Scholar] [CrossRef]
- Pizzolongo, P. Osservazioni cariologiche su Arundo donax e Arundo plinii. Annuali Bot. 1962, 27, 173–187. [Google Scholar]
- Hunter, A.W.S. A Karyosystematic investigation in Gramineae. Can. J. Res. 1934, 11, 213–241. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.; Abraham, A. Studies on the Cytology and Phylogeny of South Indian Grasses. Cytolpgia 1971, 36, 579–594. [Google Scholar] [CrossRef] [Green Version]
- Hardion, L.; Verlaque, R.; Fridlender, A.; Vila, B. Arundo. IAPT/IOPB chromosome data 11. Taxon 2011, 60, 1221. [Google Scholar]
- Facchini, P. La Canna Gentile per la Produzione della Cellulosa Nobile, l’impresa Agricolo Industriale di Torviscosa; Snia Viscosa: Milano, Italy, 1941. [Google Scholar]
- Jambor, A.; Török, Á. The Economics of Arundo donax—A Systematic Literature Review. Sustainability 2019, 11, 4225. [Google Scholar] [CrossRef] [Green Version]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; di Nasso, N.N.; Bonari, E. Comparison of Arundo donax L. and Miscanthus x giganteus in a long-term field experiment in Central Italy: Analysis of productive characteristics and energy balance. Biomass Bioenergy 2009, 33, 635–643. [Google Scholar] [CrossRef]
- Dahl, J.; Obernberger, I. Evaluation of the combustion characteristics of four perennial energy crops (Arundo donax, Cynara cardanculus, Miscanthus x Giganteus and Panicum virgatum). In Proceedings of the 2nd World Conference on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004. [Google Scholar]
- Jeguirim, M.; Trouvé, G. Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Bioresour. Technol. 2009, 100, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Xun, Z.; Rogers, P.L. Comparative evaluations of cellulosic raw materials for second generation bioethanol production. Lett. Appl. Microbiol. 2010, 51, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Adani, F.; Papa, G.; Schievano, A.; Cardinale, G.; D’Imporzano, G.; Tambone, F. Nanoscale Structure of the Cell Wall Protecting Cellulose from Enzyme Attack. Environ. Sci. Technol. 2011, 45, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Galletti, A.M.R.; Antonetti, C.; Ribechini, E.; Colombini, M.P.; di Nasso, N.N.; Bonari, E. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl. Energy 2013, 102, 157–162. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Carneiro, P.; Jerónimo, A.; Silva, V.; Cartaxo, F.; Faria, P. Improving Building Technologies with a Sustainable Strategy. Proc. Soc. Behav. Sci. 2016, 216, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, R. Trends for future applications of wet fractionation of green crops. In Proceedings of the Commission of the European Communities Forage Protein Conservation and Utilization Seminar, Dublin, Ireland, 15–18 September 1982. [Google Scholar]
- Di Nasso, N.; Roncucci, N.; Bonari, E. Seasonal dynamics of aboveground and belowgrownd biomass and nutrient accumulation and remobilization in giant reed (Arundo donax L.): A three-year study on marginal land. Bioenergy Res. 2013, 6, 725–736. [Google Scholar] [CrossRef]
- Nackley, L.L.; Kim, S.-H. A salt on the bioenergy and biological invasions debate: Salinity tolerance of the invasive biomass feed stock Arundo donax. GCB Bioenergy 2014, 7, 752–762. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Monti, A.; Cosentino, S.L. Conclusive Results of the European Project OPTIMA: Optimization of Perennial Grasses for Biomass Production in the Mediterranean Area. BioEnergy Res. 2015, 8, 1459–1460. [Google Scholar] [CrossRef] [Green Version]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Duarte, M.P.; Monti, A. Perennial Grass Production Opportunities on Marginal Mediterranean Land. BioEnergy Res. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Cantaluppi, E.; Adani, F. Giant cane (Arundo donax L.) for biogas production: The effect of two ensilage methods on biomass characteristics and biogas potential. Biomass Bioenergy 2016, 93, 131–136. [Google Scholar] [CrossRef]
- Khudamrongsawat, J.; Tayyar, R.; Holt, J.S. Genetic diversity of giant reed (Arundo donax) in the Santa Ana River, California. Weed Sci. 2004, 52, 395–405. [Google Scholar] [CrossRef]
- Tarin, D.; Pepper, A.E.; Goolsby, J.A.; Moran, P.J.; Arquieta, A.C.; Kirk, A.E.; Manhart, J.R. Microsatellites Uncover Multiple Introductions of Clonal Giant Reed (Arundo donax). Invasive Plant Sci. Manag. 2013, 6, 328–338. [Google Scholar] [CrossRef]
- Cantaluppi, E.; Cassani, E.; Puglisi, D.; Corno, L.; Munaro, M.; Landoni, M.; Adani, F.; Pilu, R. Study on the inflorescences of Arundo donax L. clones sampled in Italy. Braz. J. Bot. 2015, 39, 275–285. [Google Scholar] [CrossRef]
- Pilu, R.; Cassani, E.; Landoni, M.; Badone, F.C.; Passera, A.; Cantaluppi, E.; Corno, L.; Adani, F. Genetic characterization of an Italian Giant Reed (Arundo donax L.) clones collection: Exploiting clonal selection. Euphytica 2013, 196, 169–181. [Google Scholar] [CrossRef]
- Touchell, D.H.; Ranney, T.G.; Panthee, D.R.; Gehl, R.J.; Krings, A. Genetic Diversity, Cytogenetics, and Biomass Yields among Taxa of Giant Reeds (Arundo Species). J. Am. Soc. Hortic. Sci. 2016, 141, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Malone, J.M.; Virtue, J.G.; Williams, C.; Preston, C. Genetic diversity of giant reed (Arundo donax) in Australia. Weed Biol. Manag. 2017, 17, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Saltonstall, K.; Lambert, A.; Meyerson, L.A. Genetics and Reproduction of Common (Phragmites australis) and Giant Reed (Arundo donax). Invasive Plant Sci. Manag. 2010, 3, 495–505. [Google Scholar] [CrossRef]
- De Stefano, R.; Cappetta, E.; Guida, G.; Mistretta, C.; Caruso, G.; Giorio, P.; Albrizio, R.; Tucci, M. Screening of giant reed (Arundo donax L.) ecotypes for biomass production under salt stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 152, 911–917. [Google Scholar] [CrossRef]
- Guarino, F.; Cicatelli, A.; Brundu, G.; Improta, G.; Triassi, M.; Castiglione, S. The use of MSAP reveals epigenetic diversity of the invasive clonal populations of Arundo donax L. PLoS ONE 2019, 14, e0215096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docimo, T.; de Stefano, R.; de Palma, M.; Cappetta, E.; Villano, C.; Aversano, R.; Tucci, M. Transcriptional, metabolic and DNA methylation changes underpinning the response of Arundo donax ecotypes to NaCl excess. Planta 2019, 251. [Google Scholar] [CrossRef] [PubMed]
- Janin, G.; Letzelter, B. Cannes de Provence: Determination de l’ echantillon moyen d’ une tige en vue d’ une selection clonale (Arundo donax). Papeterie 1977, 9, 379–384. [Google Scholar]
- Cosentino, S.L.; Copani, V.; D’Agosta, G.M.; Sanzone, E.; Mantineo, M. First results on evaluation of Arundo donax L. clones collected in Southern Italy. Ind. Crop. Prod. 2006, 23, 212–222. [Google Scholar] [CrossRef]
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Scordia, D.; Testa, G.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Physiological responses of Arundo donax ecotypes to drought: A common garden study. GCB Bioenergy 2016, 9, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Danelli, T.; Cantaluppi, E.; Tosca, A.; Cassani, E.; Landoni, M.; Bosio, S.; Adani, F.; Pilu, R. Influence of Clonal Variation on the Efficiency of Arundo donax Propagation Methods. J. Plant Growth Regul. 2019, 38, 1449–1457. [Google Scholar] [CrossRef]
- Pompeiano, A.; Vita, F.; Miele, S.; Guglielminetti, L. Freeze tolerance and physiological changes during cold acclimation of giant reed (Arundo donax L.). Grass Forage Sci. 2013, 70, 168–175. [Google Scholar] [CrossRef]
- Di Nasso, N.; Roncucci, N.; Bonari, E. Giant reed (Arundo donax L.) as energy crop in Central Italy: A review. Ital. J. Agron. 2013, 8, 10–17. [Google Scholar]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and Water Stress Effects on Biomass Production in Different Arundo donax L. Clones. BioEnergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef] [Green Version]
- Phenotypic differences determine drought stress responses in ecotypes of Arundo donax adapted to different environments. J. Exp. Bot. 2017, 68, 2439–2451. [CrossRef]
- Haworth, M.; Marino, G.; Cosentino, S.L.; Brunetti, C.; de Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Della-Rocca, G.; Centritto, M.; Parenti, A.; Monti, A. Giant reed genotypes from temperate and arid environments show different response mechanisms to drought. Physiol. Plant. 2018, 163, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Riggi, E.; Avola, G.; Marino, G.; Haworth, M.; Cosentino, S.; Centritto, M. Open field experiment for the evaluation of Arundo donax ecotypes ecophysiology and yield as affected by soil water content. Ind. Crop. Prod. 2019, 140, 111630. [Google Scholar] [CrossRef]
- Fabbrini, F.; Ludovisi, R.; Alasia, O.; Flexas, J.; Douthe, C.; Carbó, M.R.; Robson, P.R.; Taylor, G.; Scarascia-Mugnozza, G.; Keurentjes, J.J.B.; et al. Characterization of phenology, physiology, morphology and biomass traits across a broad Euro-Mediterranean ecotypic panel of the lignocellulosic feed stock Arundo donax. GCB Bioenergy 2018, 11, 152–170. [Google Scholar] [CrossRef]
- Domokos-Szabolcsy, É.; Alladalla, N.A.; Alshaal, T.; Sztrik, A.; Márton, L.; El-Ramady, H. In vitro comparative study of two Arundo donax L. ecotypes’ selenium tolerance. Int. J. Hortic. Sci. 2014, 20, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Elhawat, N.; Alshaal, T.; Domokos-Szabolcsy, É.; El-Ramady, H.; Márton, L.; Czakó, M.; Kátai, J.; Balogh, P.; Sztrik, A.; Molnar, M.; et al. Phytoaccumulation potentials of two biotechnologically propagated ecotypes of Arundo donax in copper-contaminated synthetic wastewater. Environ. Sci. Pollut. Res. 2014, 21, 7773–7780. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Xiao, X.-Y.; Guo, Z.-H. Identification of indicators of giant reed (Arundo donax L.) ecotypes for phytoremediation of metal-contaminated soil in a non-ferrous mining and smelting area in southern China. Ecol. Indic. 2019, 101, 249–260. [Google Scholar] [CrossRef]
- Antal, G.; Fári, M.G.; Domokos-Szabolcsy, É. Obtention of new ornamental leaf variants of giant reed (Arundo donax L.) originated from somatic embryogenesis and their photosynthetic parameters. Int. J. Hortic. Sci. 2018, 24, 18–24. [Google Scholar] [CrossRef]
- Miller, P.; Miller, J. Gard. Dict., 8th ed.; Philip Miller: London, UK, 1768. [Google Scholar]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. Plant Gene Silencing 2000, 43, 59–68. [Google Scholar] [CrossRef]
- Lwin, A.K.; Bertolini, E.; Pè, M.E.; Zuccolo, A. Genomic skimming for identification of medium/highly abundant transposable elements in Arundo donax and Arundo plinii. Mol. Genet. Genom. 2016, 292, 157–171. [Google Scholar] [CrossRef]
- Marton, E. Method for Micropropagation of Monocots Based on Sustained Totipotent Cell Cultures. U.S. Patent 7,863,046 B2, 2011. Available online: https://patentimages.storage.googleapis.com/11/b7/6b/3530cc96ccb1c7/US7863046.pdf (accessed on 15 September 2020).
- Cavallaro, V.; Tringali, S.; Patanè, C. Large-scale in vitro propagation of giant reed (Arundo donax L.), a promising biomass species. J. Hortic. Sci. Biotechnol. 2011, 86, 452–456. [Google Scholar] [CrossRef]
- Antal, G.; Kurucz, E.; Fari, M.G.; Popp, J. Tissue culture and agamic propagation of winter-frost tolerant ‘longicaulis’. Environ. Eng. Manag. J. 2014, 13, 2709–2715. [Google Scholar] [CrossRef]
- Gubišová, M.; Čičková, M.; Klcova, L.; Gubiš, J. In vitro tillering—An effective way to multiply high-biomass plant Arundo donax. Ind. Crop. Prod. 2016, 81, 123–128. [Google Scholar] [CrossRef]
- Marton, E. Sustained Totipotent Culture of Selected Monocot Genera. U.S. 2002/0166149 A1. 2016. Available online: http://patentimages.storage.googleapis.com/pdfs/US7303916.pdf (accessed on 15 September 2020).
- Praveena, M.; Giri, C.C. Plant regeneration from immature inflorescence derived callus cultures of salt tolerant kallar grass (Leptochloa fusca L.). Physiol. Mol. Biol. Plants. 2012, 18, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Dhir, S.; Knowles, K.; Pagan, C.L.; Mann, J.; Dhir, S. Optimization and transformation of Arundo donax L. using particle bombardment. Afr. J. Biotechnol. 2010, 9, 6460–6469. [Google Scholar]
- Evangelistella, C.; Valentini, A.; Ludovisi, R.; Firrincieli, A.; Fabbrini, F.; Scalabrin, S.; Cattonaro, F.; Morgante, M.; Mugnozza, G.S.; Keurentjes, J.J.B.; et al. De novo assembly, functional annotation, and analysis of the giant reed (Arundo donax L.) leaf transcriptome provide tools for the development of a biofuel feedstock. Biotechnol. Biofuels 2017, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Ozudogru, E.; Roncasaglia, R.; da Silva, D.C.; Moreira, F.D.C.; Lambardi, M. Cryopreservation of embryogenic callus of Arundo donax L. Acta Hortic. 2016, 27–34. [Google Scholar] [CrossRef]
- Pigna, G.; Dhillon, T.; Dlugosz, E.M.; Yuan, J.S.; Gorman, C.; Morandini, P.; Lenaghan, S.C.; Stewart, C.N. Methods for suspension culture, protoplast extraction, and transformation of high-biomass yielding perennial grass Arundo donax. Biotechnol. J. 2016, 11, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Valli, F.; Trebbi, D.; Monti, A.; Tuberosa, R.; Salvi, S.; Zegada-Lizarazu, W. In vitrophysical mutagenesis of giant reed (Arundo donax L.). GCB Bioenergy 2017, 9, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Zegada-Lizarazu, W.; Salvi, S.; Monti, A. Assessment of mutagenized giant reed clones for yield, drought resistance and biomass quality. Biomass Bioenergy 2020, 134, 105501. [Google Scholar] [CrossRef]
- Li, W.; Li, K.; Zhang, Q.; Zhu, T.; Zhang, Y.; Shi, C.; Liu, Y.; Xia, E.; Jiang, J.; Shi, C. Improved hybrid de novo assembly and annotation of African wild rice, Oriza longistaminata, from Illumina and PacBio sequencing reads. Plant Genome 2020, 13, e20001. [Google Scholar] [CrossRef] [Green Version]
- Siadjeu, C.; Pucker, B.; Viehover, P.; Albach, D.; Weisshaar, B. High contiguity de novo genome sequence assembly of trifoliate yam (Discorea dumetorum) using long read sequencing. Genes 2020, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Sheng, J.; Zhu, F.; Wei, T.; Zhao, L.; Hu, X.; Zheng, X.; Zhou, F.; Hu, Z.; Diao, Y.; et al. Genetic, transcriptional, and regulatory landscape of monolignol biosynthesis pathway in Miscantus x giganteus. Biotechnol. Biofuels 2020, 13, 179. [Google Scholar] [CrossRef]
- Fu, C.; Xiao, X.; Xi, Y.; Ge, Y.; Chen, F.; Bouton, J.; Dixon, R.A.; Wang, Z.-Y. Downregulation of Cinnamyl Alcohol Dehydrogenase (CAD) Leads to Improved Saccharification Efficiency in Switchgrass. BioEnergy Res. 2011, 4, 153–164. [Google Scholar] [CrossRef]
- Xu, B.; Escamilla-Treviño, L.L.; Sathitsuksanoh, N.; Shen, Z.; Shen, H.; Zhang, Y.-H.P.; Dixon, R.A.; Zhao, B. Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Park, J.-J.; Yoo, C.G.; Flanagan, A.; Pu, Y.; Debnath, S.; Geun, Y.C.; Ragauskas, A.J.; Wang, Z.-Y. Defined tetra-allelic gene disruption of the 4-coumarate:coenzyme A ligase 1 (Pv4CL1) gene by CRISPR/Cas9 in switchgrass results in lignin reduction and improved sugar release. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sablok, G.; Fu, Y.; Bobbio, V.; Laura, M.; Rotino, G.L.; Bagnaresi, P.; Allavena, A.; Velikova, V.; Viola, R.; Loreto, F.; et al. Fuelling genetic and metabolic exploration of C 3 bioenergy crops through the first reference transcriptome of A rundo donax L. Plant Biotechnol. J. 2014, 12, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Scartazza, A.; Vita, F.; Alpi, A.; Guglielminetti, L. Arundo donax L. response to low oxygen stress. Environ. Exp. Bot. 2015, 111, 147–154. [Google Scholar] [CrossRef]
- Shaheen, S.; Ahmad, R.; Mahmood, Q.; Pervez, A.; Shah, M.M.; Hafeez, F. Gene expression and biochemical response of giant reed under Ni and Cu stress. Int. J. Phytoremediat. 2019, 21, 1474–1485. [Google Scholar] [CrossRef]
- Shiliang, H.; Stragliati, L.; Bellini, E.; Ricci, A.; Saba, A.; di Toppi, L.S.; Varotto, C. Evolution and functional differentiation of recently diverged phytochelatin synthase genes from Arundo donax L. J. Exp. Bot. 2019, 70, 5391–5405. [Google Scholar] [CrossRef] [Green Version]
- Sicilia, A.; Santoro, D.F.; Testa, G.; Cosentino, S.L.; Piero, A.R.L. Transcriptional response of giant reed (Arundo donax L.) low ecotype to long-term salt stress by unigene-based RNAseq. Phytochemistry 2020, 177, 112436. [Google Scholar] [CrossRef]
- Sicilia, A.; Testa, G.; Santoro, D.F.; Cosentino, S.L.; Piero, A.R.L. RNASeq analysis of giant cane reveals the leaf transcriptome dynamics under long-term salt stress. BMC Plant Biol. 2019, 19, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Poli, M.; Sablok, G.; Wang, B.; Liang, Y.; La Porta, N.; Velikova, V.; Loreto, F.; Li, M.; Varotto, C. Dissection of early transcriptional responses to water stress in Arundo donax L. by unigene-based RNA-seq. Biotechnol. Biofuels 2016, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Spencer, D.F.; Tan, W.; Liow, P.-S.; Ksander, G.G.; Whitehand, L.C.; Weaver, S.; Olson, J.; Newhouser, M. Evaluation of Glyphosate for Managing Giant Reed (Arundo donax). Invasive Plant Sci. Manag. 2008, 1, 248–254. [Google Scholar] [CrossRef]
- Amaducci, S.; Perego, A. Field evaluation of Arundo donax clones for bioenergy production. Ind. Crop. Prod. 2015, 75, 122–128. [Google Scholar] [CrossRef]



| Sample Size | Markers (n) | Location | G/N | Nei’s Index | Reference |
|---|---|---|---|---|---|
| 97 | RAPD (14) | California | 0.460 | - | [54] |
| Isozyme (2) | USA | 0.092 | - | [54] | |
| 185 | SRAP (10) | USA | 0.011 | - | [24] |
| SRAP (10) | France | 0.050 | - | [24] | |
| 12 | ISSR (10) | Italy | 0.083 | 0.0566 | [15] |
| 122 | AFLP (6) | Asia-Middle East | - | 0.099 | [15] |
| AFLP (6) | Mediterranean | - | 0.0744 | [15] | |
| 16 | AFLP (6) | Mediterranean | - | 0.008 | [28] |
| 58 | ISSR (10) | Australia | - | 0.815 | [13] |
| 159 | ISSR (10) | Mexico-USA | - | 0.243 | [55] |
| 203 | ISSR (10) | Spain | - | 0.929 | [55] |
| 15 | ISSR (10 | Italy | 0.933 | - | [56] |
| 86 | SSR/STS (7) | Italy | 0.093 | - | [57] |
| 31 | ISSR (20) | USA-India-Nepal | 0.81 | - | [58] |
| 218 | AFLP | Australia | 0.94 | 0–0.192 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danelli, T.; Laura, M.; Savona, M.; Landoni, M.; Adani, F.; Pilu, R. Genetic Improvement of Arundo donax L.: Opportunities and Challenges. Plants 2020, 9, 1584. https://doi.org/10.3390/plants9111584
Danelli T, Laura M, Savona M, Landoni M, Adani F, Pilu R. Genetic Improvement of Arundo donax L.: Opportunities and Challenges. Plants. 2020; 9(11):1584. https://doi.org/10.3390/plants9111584
Chicago/Turabian StyleDanelli, Tommaso, Marina Laura, Marco Savona, Michela Landoni, Fabrizio Adani, and Roberto Pilu. 2020. "Genetic Improvement of Arundo donax L.: Opportunities and Challenges" Plants 9, no. 11: 1584. https://doi.org/10.3390/plants9111584
APA StyleDanelli, T., Laura, M., Savona, M., Landoni, M., Adani, F., & Pilu, R. (2020). Genetic Improvement of Arundo donax L.: Opportunities and Challenges. Plants, 9(11), 1584. https://doi.org/10.3390/plants9111584








