Taxonomy of the Genus Halophila Thouars (Hydocharitaceae): A Review
Abstract
1. Introduction
2. Halophila Classification
| Genus Halophila Thouars |
| Halophila Thouars, Gen. Nov. Madagsc. 2. (1806). Type species: Halophila madagascariensis Steud. ex Doty et B.C. Stone. Barkania Ehrenb., Abh. Königl. Akad. Wiss. Berlin, 1832, I, 429 (1834). T: not designated. Lemnopsis Zoll., Syst. Verz. 1854, 1, 74–75, nom. illeg. non Zipp. (1829). T: not designated. |
| Key to sections | |
| 1. Erect lateral shoots (erect stems) extremely short or not distinct, bearing a pair of petiolate leaves; flowers mostly on rhizome nodes | 2 |
| 1: Erect lateral shoots (erected stems) elongated, distinct, bearing leaves in pseudo-whorls or in many pairs of sessile leaves; flowers on erect later shoot nodes | 5 |
| 2. Female flowers and fruits on extended floral shoots; styles 6 | Sect. 1. Australes |
| 2: Female flowers and fruits on rhizome nodes; styles 3 | 3 |
| 3. Leaf blade margin entire; surface glabrous | Sect. 2. Halophila |
| 3: Leaf margin serrulate; surface hairy or spines, rarely glabrous | 4 |
| 4. Dioecious; leaf blade distinctly linear; petiole sheathing lopsidedly | Sect. 3. Stipulaceae |
| 4: Monoecious; leaf blade ovate to elliptic; petioles not sheathing | Sect. 4. Decipientes |
| 5. Monoecious; minute plants having lateral shoots with 1–2 nodes, bearing a pseud-whorl of 6–10 leaves at the top; leaf blade without cross veins | Sect. 5. Microhalophila |
| 5: Dioecious; lateral shoots with numerous leaf blade-bearing nodes or 2 nodes bearing a pseudo-whorl of leaves at top; leaf blade with cross veins | 6 |
| 6. Lateral erect shoots with 2 scales at the base; having numerous leaf blade-bearing nodes or a pseudo-whorl of leaves at each node | 7 |
| 6: Lateral erect shoots with 2 scales at the base and 2 other scales around halfway up; bearing a pseudo-whorl of leaves at top | Sect. 6. Americanae |
| 7. Lateral erect shoots stiff, wiry; leaves in 10–20 pairs distichously arranged on each lateral shoot; leaf blade with basal folding | Sect. 7. Spinulosae |
| 7: Lateral erect shoots soft; leaves in 2 or 3 pseudo-whorls at each node on lateral shoot; blade not basal folding | Sect. 8. Tricostatae |
| Sect. 1. Australes |
| Halophila sect. Australes J. Kuo Type species: Halophila australis Doty & B.C. Stone. Dioecious. Male flowers form on the rhizome node; female flowers form on an erect extended lateral shoot. Leaf blade margin entire, surface glabrous. Dioca. Flores masculi ad geniculum rhizomatis facti sed feminei in surculum erectum productum laterale facti. Foliorum lamina integra, glabra. One species endemic to the temperate Australian south coast including Tasmania. |
| Halophila australis Doty & B.C. Stone, Brittonia, 1966, 18, 306. (Figure S1). T: Australia Victoria, Queencliff, Jan. 1922, Lucas s.n., holo: NSW!, iso: NSW! Halophila ovalis subsp. australis (Doty et B.C. Stone) Hartog, Verh. Kon Ned. Akad. Wentensch. Afd. Naturk. Sect. 2, 1970, 59, 251. |
| Sect. 2. Halophila |
| Halophila sect. Barkania Asch., Nuovo Giorn. Bot. Ital., 1871, 3, 301. Type species: Halophila madagascariensis (Steudel) Doty & B.C. Stone. Barkania Ehrenb., Abh. Königl. Akad. wiss. Berlin, 1832, 1, 429 (1834). T: not designated. Halophila sect. Typicae Ostenf., Bot. Tidsskr., 1902, 24, 240. T: not designated. |
| Key to the Species | |
| 1. Leaf blade distinctly ovate to elliptic | 2 |
| 1: Leaf blade oblong to spatulate | 6 |
| 2. Leaf blade greater than 30 mm in length; cross veins 12–28 | 3 |
| 2: Leaf blade, less than 25 mm in length; cross veins less than 12 | 5 |
| 3. Leaf blade with cross veins (16-) 18–28; ½ blade width:distance between intramarginal veins and blade margin ratio 20–25: 1 | H. major |
| 3: Leaf blade with cross veins 12–16; ½ blade width:distance between intramarginal veins and blade margin ratio 12–16: 1 | 4 |
| 4. Leaf blade with cross veins 12–16 (-18), ascending angle at 40o | H. ovalis |
| 4: Leaf blades with cross veins (8-) 9–14 (-15), sharply ascending at 20–30o. Endemic to Fiji, Samoa and Tonga | H. bullosa |
| 5. Space between leaf margin and intramarginal vein, and space between two adjacent cross veins narrow; cross veins rarely branched | H. minor |
| 5: Space between leaf margin and intramarginal vein, and between two adjacent cross veins wide; cross veins never branched | H. gaudichardii |
| 6. Leaf blade with cross veins more than 12 | 7 |
| 6: Leaf blade with cross veins less than 10 | 9 |
| 7. Blade extremely linear, 8–16 mm length, width 1.5–3 mm, L:W ratio 6–20:1 | H. linearis |
| 7: Blade elongate to spatulate, more than 30 mm in length, more than 4 mm in width; L:W ratio 7–8:1 | 8 |
| 8. Blade 35–40 mm long, 4–7 mm wide, L:W ratio 5–7:1; restricted to estuarine | H. ramuramathiana |
| 8: Blade up to 30 mm long, 7 mm wide, L:W ratio 3–4: 1; endemic to Tanegashima, Japan | H. mikii |
| 9. Intramarginal space, 0.5–1 mm wide. Endemic to temperate Japan and Southern Korean Peninsular | H. nipponica |
| 9: Intramarginal space narrow, less than 0.3 mm | 10 |
| 10. Blade spatulate to narrowly obovate | 11 |
| 10: Blade elliptic to oblong not spatulate | 12 |
| 11. Blade 12–16 mm long, 1.5–4.5 mm wide, L:W ratio 3.5–4; cross veins 6–7 | H. okinawensis |
| 11: Blade 20–30 mm long, 2.5–6 mm wide; cross veins 10–16. Endemic to Hawaii Islands | H. hawaiiana |
| 12. Blade 5–25 mm long, 1–4 mm wide; cross veins 5–8. Intramarginal space 0.15 mm. Endemic to Southern Florida | H. johnsonii |
| 12: Blade less than 15 mm in length; 3 mm width; cross veins 7, no branched; intramarginal veins extremely narrow, 01–0.2 mm. | H. madagascariensis |
| 1. Halophila major (Zoll.) Miq., Fl. Ind. Bat. 1855, 3, 230. (Figure S2). |
| T: Indonesia, Sumbawa, Kambing in the Bay of Bima, Oct 1847, H Zollinger 3430 (lecto: P, iso-lect BO, L, U, Z, fide: Kuo et al. 2006: 136–137). Lemnopsis major Zoll., Syst. Verz., 1854, 1, 75. T: not designated. Halophila ovalis var. major Asch., Linnaea, 1868, 35, 174. T: not designated. Halophila euphlebia Mak., Bot. Mag. Tokyo, 1912, 26, 208, Figure XV. T: Japan, Shikoku Is., Tokushima Pref., Haifu Gun, Shishikui Mura, 22 Aug 1906, S Nikai 1613; holo: TI!, iso: NS!. |
| 2. Halophila ovalis (R. Br.) Hook. f. Fl. Tasman, 1858, 2, 45. (Figure S3). |
| T: Australia, Queensland, at the inner entrance to Thirsty Sound (22°20′ S; 149°55′ E) southern part of Long Island, 24 Sept 1802, R Brown 5816, holo: K!; iso: BM! Caulinia ovalis R. Br. (1810) Prod. Fl. Nov. Holl., 1810, 1, 339. T: not designated. |
| 3. Halophila bullosa (Setch.) J. Kuo, n. comb. (Figure S4). |
| Halophila ovalis var. bullosa Setch.: Setchell, Carnegie Inst. Washington Pub. 1924, 341, 114–115. Figure 6. T: Samoa, Tutuila Island. Pago Pago Harbour, Aua Beach, ca 1 m, in white coral sand, patches, 12 June 1920, WA Setchell 114 (lecto: UC!; isolecto: BISH!, C!, S! US! fide: Smith, 1979, 128). Halophila ovalis ssp. bullosa (Setch.) Hartog, Verh. Kon Ned. Akad. Wentensch. Afd. Naturk. Sect. 2, 1970, 251. |
| 4. Halophila minor (Zoll.) den Hartog, Fl. Males. I, 1957, 5, 410, f. 17b. (Figure S5). |
| T: Indonesia, Lesser Sunda Islands, Flores, near Bari, 12 July 1847, H. Zollinger 3334, holo: BM; iso: P, L, fide: Kuo et al. Acta Phytotax. Geobot., 2006, 57, 134. Lemnopsis minor Zoll., in Zoll. Syst. Verz., 1854, 1, 75. T: not designated. Halophila lemnopsis Miq., Fl. Ind. Bat., 1855, 3, 230, nom. illeg. Halophila ovalis var. minor (Zoll.) Asch., Linnaea, 1868, 35, 175. T: not designated. |
| 5. Halophila gaudichardii J. Kuo, Acta Phytotax. Geobot., 2006, 57, 145, Figures 8, 15. (Figure S6). |
| T: Marianna Island, sine loc. exact, C. Gaudichard (lectoholo: P; iso: K-drawings of the type, L 5395235524, fide: Kuo et al., 2006: 146). Halophila ovata Gaudichard in Freycin., Voy. Bot., 1827, Pl. 40, f. 1. T: not designated. Halophila ovalis var. ovata Asch., Linnaea, 1868, 35, 173. T: not designated. |
| 6. Halophila ramamurthiana (Ravikumar & Ganesan) J. Kuo, n. com. (Figure S7). |
| Halophila ovalis (Br.) Hook. f. ssp. ramamurthiana Ravikumar & Ganesan, Aquat. Bot. 1990, 36, 351. T: India, Tamil Nadu State, South Arcot, district, Marakkaam (Kaliveli Tank), to a depth of ca. 2 m, 29 March 1987, Parthasarathy & Ravikumar 85440 (CAL, MH, not seen). |
| 7. Halophila mikii J. Kuo, Acta Phytotax. Geobot, 2006, 57, 140, Figures 5, 13. (Figure S8). |
| T: Japan, Kagoshima Pref., Tanegashima Island, Kumage Gun, Sumiyoshi, 27 Oct 1921, Z. Tashiro (KYO). |
| 8. Halophila linearis den Hartog, Act. Bot. Neerl. 1957, 6, 46, F. 1. (Figure S9). |
| T: Mozambique, Inhaca Is., west coast, exposed at spring ebbtide, July 1937, E Cohen 20652 (holo: BM! iso: L!) Halophila ovalis ssp. linearis (Hartog) Hartog: Verh. Kon Ned. Akad. Wentensch. Afd. Naturk. Sect. 2, 1970, 59, 251. |
| 9. Halophila nipponica J. Kuo, Acta Phytotax. Geobot., 2006, 57, 141, Figures 6, 13. (Figure S10). |
| T: Japan, Chiba Pref., Okinoshima, Teteyama, 12 Aug.,1994, Y. Hirano s.n. (TNS- holo; TI, MAK, UWA-iso). Halophila japonica Uchimura et Faye, Bull. Natn. Sci. Mus. Tokyo, Ser. B., 2006, 32, 131, Figs. 1–12. T: Japan, Kanagawa Pref., Yokosuka City, Odawa Bay (35°13′16” N; 139°37′17” E), 7 June 2005, M. Uchimura s.n. holo, iso.: TNS (not seen). Halophila nipponica subsp. notoensis Ohba et Miyata, Seagrasses of Japan, 2007, 15, 103, Pl. 68, 69.T: Japan, Ishikawa Pref., Kashima-gun, Notojima-machi, Sowaji, 14 Oct 2005, T Ohba & M Miyata s.n. (holo: CBM, not seen). No Latin description. Halophila x tanabensis Ohba et Miyata, Seagrasses of Japan, 2007, 17, 107, Pl 73. No Latin description. T: Japan, Wakayama Pref., Shirahama-cho, Eura, 5 July 2006, M Miyata (holo: CBM, not seen). |
| 10. Halophila okinawensis J. Kuo, Acta Phytotax. Geobot., 2006, 57, 144, Figures 7, 14. (Figure S11). |
| T: Japan, Okinawa Pref., Okinawa Isl., ca. 100 m from causeway, Southern Kin Bay, 8–9 m, 22 May 1997, Z. Kanamoto s.n., holo: TNS; iso: TI, MAK, UWA. |
| 11. Halophila hawaiiana Doty et B.C. Stone, Brittonia, 1966, 18, 303–305, Figures 1. (Figure S12). |
| T: U.S.A., Hawaiian Is., Maui Is., Kihei, 9 Sept. 1955, T Matsus. s.n. (Holo. BISH, lost. Hawaiian Is., Maui Is., Kalama Park, ca. 100 from shore, ca. 1 m of water at low tide, binds sand, 26 Aug 1955, MS Doty 13009 (L, neotype here designated). Halophila ovalis ssp. hawaiiana (Doty & Stone) Hartog, Hartog, Verh. Kon Ned. Akad. Wentensch. Afd. Naturk. Sect. 2, 1970, 59, 251. |
| 12. Halophila johnsonii Eiseman, Aquat. Bot., 1980, 9, 15–19, Figure 1. (Figure S13). |
| T: U.S.A., Florida, Bessie Cove, Hutchinson Island, Martin Co. (27°15’ N;80°12’ W), 23 May 1975, NJ Eiseman 3411, holo: HBFH; iso: HBFH, TEX, US (not seen). |
| 13. Halophila madagascariensis Steud. ex Doty et B.C. Stone, Taxon, 1967, 17, 417, Figure 1. (Figure S14). |
| T: Madagascar, exact locality unknown (in littoris orientalis sbmarinus), Du Petit Thouars (holo: P!, iso: BM!). Halophila madagascariensis Steud., Nom. ed. 2, 1840, 1, 720 (nomen). |
| Sect. 3. Stipulaceae |
| Halophila sect. Stipulaceae J. Kuo Type species: Halophila stipulacea (Forrsk.) Asch. |
| Key to the species | |
| 1. Leaf blade up to 60 cm long, 10 mm wide, surface glabrous, minute hairs, occasionally bullate, cross veins 10–40 | 1. H. stipulacea |
| 1: Leaf blade 35 mm long, 5 mm wide, surface papillous, not hairy, bullate; cross veins less than 14 | 2. H. balfourii |
| 1. Halophila stipulacea (Forrsk.) Asch. Sitz.-Ber. Ges. Natirf. Fr. Berlin, 1867, 3. (Figure S15). |
| T: Neotype: Eritrea, Massaua, G. Schweinfurth 8, 26 Jan 1891(G), isoneotype (P), file: Ferrer-Gallego & Boisset, Taxon, 2015, 64, 1935. Zostera bullata Délile, Fl. Aegypt. 1813, Ill, 75, 145, Pl. 53, f.6. T: Lect.: ‘a Suez sur la sable, la plante croissant au fond de la me rest verte et a la port d’un potamogrton (MPU), file: Ferrer-Gallego & Boisset, Taxon, 2015, 64, 1935. |
| 2. Halophila balfourii Solereder Beih. Bot. Centralbl. 1913, 1, 47. (Figure S16). |
| T: Lectotype: Rodriguez, Dr. I.B. Balfour. Aug.-Dec. 1874 (BM, isolecto. P, L, K), file: Ferrer-Gallego & Boisset, Taxon, 2015, 64, 1035. |
| Sect. 4. Decipientes |
| Halophila sect. Decipientes J. Kuo Type species: Halophila decipiens Ostenf. |
| Key to the species | |
| 1. Male and female flowers on the same spathes; petiole shorter than blade | 1. H. decipiens |
| 1: Male and female flowers on separate spathes of the same rhizome (plant) | 2 |
| 2. Petiole shorter than the blade; blade surface usually with stiff hairs | 2. H. capricorni |
| 2: Petiole as long as the blade; blade surface glabrous | 3. H. sulawesii |
| 1. Halophila decipiens Ostenf. Bot. Tidsskr. 1902, 24, 260. (Figure S17). |
| T: Off Kohdat, Gulf of Thailand, Feb, 1900, J. Schmidt 540; holo: C!; iso.: L!. Halophila decipiens var. pubescens Hartog, Fl. Males., 1957, 5, 411. T: not designated. |
| 2. Halophila capricorni Larkum, Aquat. Bot., 1995, 51, 320. (Figure S18). |
| T: Australia, Steve’s Bommie, One Tree Is., Great Barrier Reef, Qld., 13 Nov. 1990, A.W.D. Larkum s.n.; holo: NSW; iso: AD, SYD, UWA. |
| 3. Halophila sulawesii J. Kuo, Aquat. Bot. 2007, 87, 171. (Figure S19). |
| T: Indonesia, southern Sulawesi, the Supermodel Archipelago, Samalona Is., north side, 15 m, 4 Aug. 1989, E. Verheij 0388 (L, holo, iso). |
| Sect. 5. Microhalophila |
| Halophila sect. Microhalophila Asch., Nuov. Giorn. Bot. Ital., 1871, 3, 302. Type species: Halophila beccarii Asch. Halophila sect. Pusillae Ostenf., Bot. Tidsskr., 1902, 24, 240. Type species: Halophila beccarii Asch. |
| Halophila beccarii Asch., Nuov. Giorn. Bot. Ital., 1871, 3, 302. (Figure S20). |
| T: Malaysia, Borneo, Sarawak, in the mouth of the river Bintula, O. Beccari 11826 (= P.B. 3666). |
| Sect. 6 Americanae |
| Halophila sect. Americanae Ostenf., Bot. Tidsskr., 1902, 24, 260. Type species: Halophila engelmannii Asch. |
| Key to the species | |
| 1. Petiole extreme short, Blade oblong with pointed tip, cross veins 6–8 | 1. H. engelmannii |
| 1: Petiole 2–5 mm long, blade ovate with a rounded tip, cross veins 3–5 | 2. H. baillonis |
| 1. Halophila engelmannii Asch. in Neumayer, Anl. wiss. Beob. Reisen, 1875, 1, 368. (Figure S21). |
| T: U.S.A. Florida, near Apalachicola, deep water, Sept 1865, Dr. Chapman, received in a letter from Dr. Gray (NY, lectotypes, here designed). |
| 2. Halophila baillonis Asch. ex Dickie in Hook. f., J. Linn. Soc. 1874, 14, 317. (Figure S22). |
| T: Virgin Is., St. Thomas, Challenger Expedition, 1873, Dr. Moseley s.n. (K). Halophila aschersonii Ostenf., Bot. Tidsskr. 1901, 24, 239. T: St. Croix, Christiansted, Lagune, 26 Feb 1892, H. Lassen s.n. (C); St. Croix, Christiansted, Lagune, 1906, F. Børgese s.n. (C). |
| Sect. 7. Spinulosae |
| Halophila sect. Spinulosae Ostenf. Bot. Tidsskr. 1902, 24, 240. Type species: Halophila spinulosa (R.Br.) Asch. Halophila sect. Aschersonia Hartog, Fl. Males. I, 1957, 5, 408. Type species: Halophila spinulosa (R.Br.) Asch. |
| Halophila spinulosa (R.Br.) Asch. in G.B. von Neumayer, Anl. Wis. Beobacht. Reisen, 1875, 368. (Figure S23). |
| Caulinia spinulosa R.Br., Prodr. Fl. Nov. Holl., 1810, 339. T: Australia, Queensland, R. Brown Iter Austral. 1815; holo: BM n.v. (Lost). Neotype: Australia, Queensland, Port Denison, 1871, F. Kliner s.n. (MEL 3807); iso-neotypes: Port Denison, 1871, F. Kliner s.n. (MEL 3808-3812), here designated. |
| Sect. 8. Tricostatae |
| Halophila sect. Tricostatae M. Greenway, Aquat. Bot., 1979, 7, 67. Type species: H. tricostata M. Greenway |
| Halophila tricostata M. Greenway, Aquat. Bot., 1979, 7, 68. (Figure S24). |
| T: Australia, Queensland, Lizard Is., Cook District, 20–30 m, 1 Dec 1978, M. Greenway s.n. (BRI!). |
3. Discussion
3.1. New Sections in the Genus Halophila
3.2. Typifications in Halophila
3.3. New Combinations in Halophila
3.4. Deep Water Halophila
3.5. Species Identification in Halophila
3.6. Concluding Remarks
4. Materials and Methods
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Den Hartog, C.; Kuo, J. Taxonomy and biogeography. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherland, 2006; pp. 1–23. [Google Scholar]
- Ascherson, P. Vorabeiten zu einer ubersicht der phanerogamen Meergewächse. Linnaea 1868, 35, 152–208. [Google Scholar]
- Ascherson, P.; Gürke, M. Hydrocharitaceae. In Die naturlichen Planzenfamilien; Engler, A., Prantl, K., Eds.; W. Engelmann: Leipez, Germany, 1889; Volume 2, pp. 238–258. [Google Scholar]
- Rydberg, P.A. Family Elodeaceae. In North America Flora; New York Botanic Garden: New York, NY, USA, 1909; Volume 17, pp. 67–68. [Google Scholar]
- Den Hartog, C. Hydrocharitaceae. In Fl. Malesia; Noordhoff-Kolff N.V.: Jakarta, Indonesia, 1957; Volume 5, pp. 40–413. [Google Scholar]
- Den Hartog, C. The Sea-Grasses of the World. Verh. Kon. Ned. Akad. Wetensch. Afd. Natururk. Sect. 2 1970, 59, 1–275. [Google Scholar]
- Nikai, T. Ordines, Familiae, Tribi, Genera, Sections, Species, Varietates, Formae et Combinations Novae; Prof. Nikai-Takenoshin adhuc ut nova edita: Tokyo, Japan, 1943. [Google Scholar]
- Ascherson, P. Plantae Phanerogamae marine in Archipelago Indico et Mari Rubro. Neuo. Giornale. Bot. Ital. 1871, 3, 299–302. [Google Scholar]
- Ostenfeld, C.H. Halophila Aschersonii, n. sp. Bot. Tidssk. 1902, 24, 239–240. [Google Scholar]
- Ostenfeld, C.H.; Meeresgraser, I. Marine Hydrocharitaceae. Pflanzenareale 1927, 1, 35–38. [Google Scholar]
- Greenway, M. Halophila tricostata (Hydrocharitaceae), a new species of seagrass from the Great Barrier Reef Region. Aquat. Bot. 1979, 7, 67–70. [Google Scholar] [CrossRef]
- Du Thouars, L.M.A.P. Genera nova Madagascariensia Secundum Methodum Jussiaeanam Disposita; Académie des Sciences: Paris, France, 1806; p. 29. [Google Scholar]
- Forrskål, P. Flora Aegyptiaca-Arabica; Hauniae: Copenhagen, Denmark, 1775. [Google Scholar]
- Brown, R. Prodromus Florae Novae Holaniae et Insulae Van-Diemen; Taylor: London, UK, 1810. [Google Scholar]
- Gaudichard, C. Voyage Autour du Monde, par Louis de Freycinet. Botanique, Chez Pillet Aini; Imprimeur-Libraire: Paris, France, 1826; pp. 429–430. [Google Scholar]
- Steudel, E.T. Nomenclator Botanicus, seu, Synonimia Plangarum Univarsalis, Enumerans Ordine Alphabertico Nomina Phanerogamis Imposita, 2nd ed.; Cottae, J.G., Ed.; Stuttgaritae & Tubingae, Typis et sumptibus: Paris, France, 1840; p. 810. [Google Scholar]
- Zollinger, H. Systematisches Verzeichniss der im Indifchen Hrelolpdf in den Jahren 1842–1848 Gesammelten Sovie der aus Japan Empfangenen Pflanzen, Druck Und Verlag von E; Kiesling: Zurich, Switzerland, 1854; pp. 74–75. [Google Scholar]
- Miquel, F.A.W. Flora van Nederlandsch Indie. drede Deel. Fried; Fleischer: Amsterdam, The Netherlands, 1885; Volume 3, pp. 229–230. [Google Scholar]
- Ascherson, P. Die Geographische Verbreitung der Seegräser. In Geographische Mittheiungen; Pettermann, A., Ed.; Gotha: Justus Prthees, Germany, 1871; Volume 17, pp. 241–248. [Google Scholar]
- Hooker, J.D. On the marine algae of St. Thomas and the Bermuda, and on Halophila baillonis Asch. Contributions to the botany of the expedition of H.M.S. Challenger. J. Linn. Soc. Bot. 1874, 14, 311–317. [Google Scholar] [CrossRef]
- Ascherson, P. Die geographische Verbreitung der Seegräser. In Anleitung zu Wissenschaltlichen Beobachtungen auf Reisen, mit Besondre Rückscicht auf die Bedürfinisse der Kaiserlichen Marine; Neumayer, G., Ed.; Robert Oppenheim Verlag: Berlin, Germany, 1875; pp. 359–373. [Google Scholar]
- Ascherson, P. Die geographische Verbreitung der Seegräser. In Anleitung zu Wissenschaltlichen Beobachtungen auf Reisen, 3rd ed.; Neumayer, G., Ed.; Robert Oppenheim Verlag: Berlin, Germany, 1906; Volume 2, pp. 389–413. [Google Scholar]
- Ostenfeld, C.H. Hydrocharitaceae, Lemnaceae, Pontederiaceae, Potamogetonaceae, Gentianaceae (Limnanthemum), Nymphaceae. Bot. Tidsskr. 1902, 24, 260–263. [Google Scholar]
- Ostenfeld, C.H. On Halophila ovata Gaudichaud, a neglected species. Philippine J. Sci. 1909, 4, 67–68. [Google Scholar]
- Makino, T. Observations on the flora of Japan. Bot. Mag. Tokyo 1912, 26, 208–222. [Google Scholar] [CrossRef]
- Solereder, H. Systematisch-anatomische Untersuchung des Blattes der Hydrocharitacen. Beih. Bot. Zbl. 1913, 30, 24–104. [Google Scholar]
- Setchell, W.A. American Samoa; Carnegie Institute Washington Publication: Washington, DC, USA, 1924; Volume 341, pp. 114–115. [Google Scholar]
- Setchell, W.A. Marine plants and Pacific palaeogeography. In Proceedings of the 5th Pacific Science Congress; University of Toronto Press: Toronto, ON, Canada, 1934; pp. 3117–3131. [Google Scholar]
- Den Hartog, C. Two new species of Hydrocharitaceae. Acta Bot. Neerl. 1957, 6, 46–47. [Google Scholar] [CrossRef]
- Doty, M.S.; Stone, B.C. Two new species of Halophila (Hydrocharitaceae). Brittonia 1966, 18, 303–306. [Google Scholar] [CrossRef]
- Doty, M.S.; Stone, B.C. Typification for the generic name Halophila Thouars. Taxon 1967, 16, 414–418. [Google Scholar] [CrossRef]
- De la Bathie, H.F. Hydrocharitaceae. In Flore de Madagascar et des Comores; Humber, H., Ed.; Imprimerie Officielle: Mégrine, Tunisia, 1946; pp. 1–16. [Google Scholar]
- Sachet, M.-H.; Fosberg, F.R. Remarks on Halophila (Hydrocharitaceae). Taxon 1973, 22, 439–443. [Google Scholar] [CrossRef]
- Eiseman, N.J.; McMillan, C. A new species of seagrass, Halophila johnsonii, from the Atlantic coast of Florida. Aquat. Bot. 1984, 9, 15–19. [Google Scholar] [CrossRef]
- Ravikumar, K.; Ganesan, R. A new subspecies of Halophila ovalis (R.Br.) J.D. Hook. (Hydrocharitaceae) from the eastern coast of Peninsular India. Aquat. Bot. 1990, 36, 351–358. [Google Scholar] [CrossRef]
- Larkum, A.W.D. Halophila capricorni (Hydrocharitaceae): A new species of seagrass from the Coral Sea. Aquat. Bot. 1995, 51, 319–328. [Google Scholar] [CrossRef]
- Robertson, E.L. Seagrasses. In The Marine Benthic Flora of Southern Australia; Part I; Womersley, H.B.S., Ed.; Government Printer: Adelaide, Australia, 1984; pp. 57–122. [Google Scholar]
- Kuo, J. Taxonomic notes on Halophila ovata and H. minor. Biol. Mar. Medit. 2000, 7, 79–82. [Google Scholar]
- Kuo, J.; Den Hartog, C. Seagrass taxonomy and identification key. In Global Seagrass Research Methods; Short, F.T., Coles, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 31–58. [Google Scholar]
- Kuo, J.; Kanamoto, Z.; Iizumi, H.; Mukai, H. Seagrasses of the genus Halophila Thouars (Hydrocharitaceae) from Japan. Acta Phytotax. Geobot. 2006, 57, 129–154. [Google Scholar]
- Uchimura, M.; Faye, E.J.; Shimada, S.; Ogura, G.; Inoue, T.; Nakamura, Y. A taxonomic study of the seagrass genus Halophila (Hydrocharitaceae) from Japan: Description of a new species Halophila japonica sp. nov. and characterization of H. ovalis using morphological and molecular data. Bull. Natn. Sci. Mus. Tokyo 2006, 32, 129–150. [Google Scholar]
- Kuo, J. New monoecious seagrass Halophila sulawesii (Hydrocharitaceae) from Indonesia. Aquat. Bot. 2007, 87, 171–175. [Google Scholar] [CrossRef]
- Waycott, M.; Freshwater, D.W.; York, R.A.; Calladine, A.; Kenworthy, W.J. Evolutionary trends in the seagrass genus Halophila (Thouars): Insights from molecular phylogeny. Bull. Mar. Sci. 2002, 70, 1299–1308. [Google Scholar]
- Uchimura, M.; Faye, E.J.; Shimada, S.; Inoue, T.; Nakamura, Y. A reassessment of Halophila species (Hydrocharitaceae) diversity with special reference to Japanese representatives. Bot. Mar. 2008, 51, 258–268. [Google Scholar] [CrossRef]
- Shimada, S.; Watanabe, M.; Ichihara, K.; Uchimura, M. Morphological variations of seagrass species, Halophila nipponica (Hydrocharitaceae, Alismatales). Cost. Mar. Sci. 2012, 35, 85–90. [Google Scholar]
- Kim, Y.K.; Kim, S.H.; Yi, J.M.; Kang, C.-K.; Short, F.; Lee, K.S. Genetic identification and evolutionary trends of the seagrass Halophila nipponica in temperate coastal waters of Korea. PLoS ONE 2017, 12, e0177772. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.-V.; Höfler, S.; Papenbrock, J. New record of the seagrass species Halophila major (Zoll.) Miquel in Viet Nam evidence from leaf morphology and ITS analysis. Bot. Mar. 2013, 56, 313–321. [Google Scholar]
- Nguyen, X.-Y.; Bujang, J.S.; Papenbrock, J. Variability of leaf morphology and marker genes of members of Halophila complex collected in Viet Nam. Aquat. Bot. 2013, 110, 6–15. [Google Scholar]
- Nguyen, X.-V.; Thirunavukarassu, T.; Papenbrock, J. Genetic variation among Halophila ovalis (Hydrocharitaceae) and closely related seagrass species from the coast of Tamil Nadu, India—An AFLP fingerprint approach. Syst. Biodivers. 2014, 11, 467–476. [Google Scholar] [CrossRef]
- Nguyen, X.-Y.; Detcharoen, M.; Tutiprapas, P.; Soe-Htun, U.; Sidik, J.B.; Harah, M.Z.; Prathep, A.; Papenbrock, J. Genetic species identification and population structure of the Halophila (Hydrocharitaceae) from the Western Pacific to the Eastern Indian Ocean. BMC Evol. Biol. 2015, 14, 92–110. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Kletschkus, E.; Rupp-Schroder, S.I.; Shaffai, A.E.; Papenbrock, J. rDNA analysis of the Red Sea seagrass, Halophila, reveals vicariant evolutionary diversification. Syst. Biodivers. 2018, 16, 668–679. [Google Scholar] [CrossRef]
- Meñez, E.G.; Phillips, R.C.; Calumpong, H.P. Smithsonian Contribution to Marine Sciences, Number 21. In Seagrasses from the Philippines; Smithsonian Institution Press: Washington, DC, USA, 1983; p. 40. [Google Scholar]
- Phillips, R.C.; Meñez, E.G. Smithsonian Contributions to the Marine Sciences No. 34. In Seagrasses; Smithsonian Institution Press: Washington, DC, USA, 1988; p. 104. [Google Scholar]
- Ramamurthy, K.; Balakrishnan, N.P.; Ravikumar, K.; Ganesan, R. Flora of India-Series 4. In Seagrasses of Coromandel Coast., India; Botanical Survey of India: Coimbatore, India, 1992; p. 80. [Google Scholar]
- Waycott, M.; McKahon, K.; Mellors, J.; Calladine, A.; Kleine, D. A Guide to Tropical Seagrasses of the Indo-West Pacific; James Cook Univ.: Townsville, Australia, 2004; p. 72. [Google Scholar]
- Ohba, T.; Miyata, M. Seagrasses of Japan; Hokkaido University Press: Hokkaido, Japan, 2007; p. 116, (In Japanese and English). [Google Scholar]
- Van Tussenbrock, B.I.; Santos, M.G.B.; Wong, J.G.R.; Van Dijk, J.K.; Waycott, M. A Guide to the Tropical seagrasses of the Western Atlantic; Universidad Nacional Autonoma de Mexico: Mexico City, Mexico, 2010; p. 80, (In Mexican and English). [Google Scholar]
- Shaffai, A.E. Field Guide to Seagrasses of the Red Sea, 2nd ed.; Rouphael, A.A., Abdulla, A., Eds.; IUCN, Gland, Switzerland and Total Foundation: Courbevoie, France, 2016; p. 56. [Google Scholar]
- Bentham, G. Flora Australialiensis: A Description of the Plants of the Australian Territory; L. Reeve & Co.: London, UK, 1878; Volume 7, pp. 182–183. [Google Scholar]
- Von Mueller, F. Fragmenta Phytographiae Australiae; Auctoritate Gubrtni Coloniae Victoriae: Melbourne, Australia, 1874; Volume 6, p. 219. [Google Scholar]
- Balfour, B. On the genus Halophila. Bot. Soc. Edinburgh Trans. 1879, 13, 290–343. [Google Scholar] [CrossRef]
- Kuo, J. Halophila balfourii Solereder (Hydrocharitaceae)—An overlooked seagrass species. Plants 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- McMillan, C.; Bridges, K.W. Systematic implications of bullate leaves and isozymes for Halophila from Fiji and West Samoa. Aquat. Bot. 1982, 12, 173–188. [Google Scholar] [CrossRef]
- Singh, S.; Southgate, P.C.; Lal, M.M. Staminate and pistillate flowers and fruit of Halophila ovalis subsp. bullosa (Setchell). Hartog. Aquat. Bot. 2020, 166. (in press). [CrossRef]
- Bandeira, S.O.; Bjork, M. Seagrass research in the eastern Africa regional emphasis on diversity, ecology and ecophysiology. S. Afr. J. Bot. 2001, 67, 420–425. [Google Scholar] [CrossRef]
- Bandeira, S.O. Diversity and distribution of seagrasses around Inhace Is., Southern Mozambique. S. Afr. J. Bot. 2002, 68, 191–198. [Google Scholar] [CrossRef]
- Calumpong, H.P.; Stacion, J.S.; Lucanas, J.R.; Cadiz, P.L. Halophila tricostata Greenway (Hydrocharitaceae): An unreported seagrass from the Philippines. In Proceedings of the Poster presented at the World Seagrass Conference, Phuket, Thailand, 20–25 November 2010. [Google Scholar]
- Fortes, M.D. Seagrasses: A Resource Unknown in the ASEAN Region. In ICLARM Education Series 5; International Center for Living Aquatic Resources Management: Manila, Philippines, 1990; p. 46. [Google Scholar]
- Erftemeijer, P.L.A.; Stapel, J. Primary production of deep-water Halophila ovalis meadows. Aquat. Bot. 1999, 65, 71–82. [Google Scholar] [CrossRef]
- Lee Long, W.J.; Mellors, J.E.; Coles, R.G. Seagrasses between Cape York and Hervey Bay, Queensland, Australia. Aust. J. Mar. Freshwater Res. 1993, 44, 19–31. [Google Scholar]
- Lee Long, W.J.; Coles, R.G.; McKenzie, L.J. Deepwater seagrasses in northereastern Australia—How deep, how meaningful? In Seagrass Biology: Proceedings of an International Workshop; Kuo, J., Phillips, R.C., Walker, D.I., Kirkman, H., Eds.; The University of Western Australia: Nedlands, Australia, 1996; pp. 41–50. [Google Scholar]
- McDermid, K.; Gregoritza, M.C.; Reves, J.W.; Freshwater, D.W. Morphological and genetic variation in the endemic seagrass Halophila hawaiiana (Hydrocharitaceae) in the Hawaiian Archipelago. Pacific Sci. 2003, 57, 199–209. [Google Scholar] [CrossRef]
- Short, F.T.; Moore, G.E.; Peyton, K.A. Halophila ovalis in the tropical Atlantic Ocean. Aquat. Bot. 2010, 93, 141–146. [Google Scholar] [CrossRef]
- Waycott, M.; Procaccini, G.; Les, D.H.; Reusch, T.B.H. Seagrass evolution, ecology and conservation: A genetic perspective. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherland, 2006; pp. 25–50. [Google Scholar]
- Liu, S.Y.V.; Kumara, T.P.; Hsu, C.-H. Genetic identification and hybridization in the seagrass genus Halophila (Hydorcharitaceae) in Sri Lankan waters. PeerJ 2020, 8, e10027. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, D.; Guo, G.; Gao, L. Plant phylogenomics based on genome-partitioning strategies: Progress and prospects. Plant Divers. 2018, 40, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, M.; Faye, E.J.; Shimada, S.; Arai, S.; Inoue, T.; Nakamura, Y. A re-evaluation of the taxonomic status of Halophila euphlebia Makino (Hydrocharitaceae) based on morphological features and ITS sequence data. Bot. Mar. 2006, 49, 111–121. [Google Scholar] [CrossRef]
- Tuntiprapas, P.; Shimada, S.; Pongparadon, S.; Prarhwp, A. Is Halophila major (Zoll.) Miquel a big H. ovalis (R. Brown) J.D. Hooker? An evaluation based on age, morphology, and ITS sequence. Sci. Asia 2015, 41, 79–86. [Google Scholar] [CrossRef]
- Kurniawan, F.; Imran, Z.; Darus, R.F.; Anggraeni, F.; Damar, A.; Sumuddin, A.; Kamal, M.M. A new record of seagrass species Halophila major (Zoll.) Miquel (1855) from Indonesia. Aquat. Bot. 2020, 161, 103171. [Google Scholar] [CrossRef]
- Muta Harah, Z.; Japar Sidik, B.; Aziz, B.A. Flowering, fruiting and seedling of annual Halophila beccarii Aschers. in Peninsular Malaysia. Bull. Mar. Sci. 2002, 71, 1199–1205. [Google Scholar]
- Short, F.T.; Fernndez, E.; Vernon, A.; Gaeckle, J.L. Occurrence of Halophila baillonii meadows in Belize, Central America. Aquat. Bot. 2006, 85, 249–251. [Google Scholar] [CrossRef]
- Bujang, J.S.; Zakaria, M.H.; Awing, S.A.; Nojma, S.; Ogawa, H. Seed coat sculpturing in Halophila. Cost. Mar. Sci. 2006, 30, 240–242. [Google Scholar]
- Fortes, M.D.; Ooi, J.L.S.; Tan, Y.M.; Prathep, A.; Bujang, J.S. Seagrass in southeast Asia: A review of status, knowledge gaps, and a road map for conservation. Mar. Bot. 2018, 61, 269–288. [Google Scholar] [CrossRef]
- Short, F.T.; Pollodoro, B.; Livingstone, S.R.; Capener, K.E.; Bandeira, S.; Bujang, J.S.; Calumpong, H.P.; Carruthers, J.B.; Coles, R.G.; Dennison, W.C.; et al. Extinction risk assessment of the world’s seagrass species. Biol. Cons. 2011, 144, 1961–1971. [Google Scholar] [CrossRef]

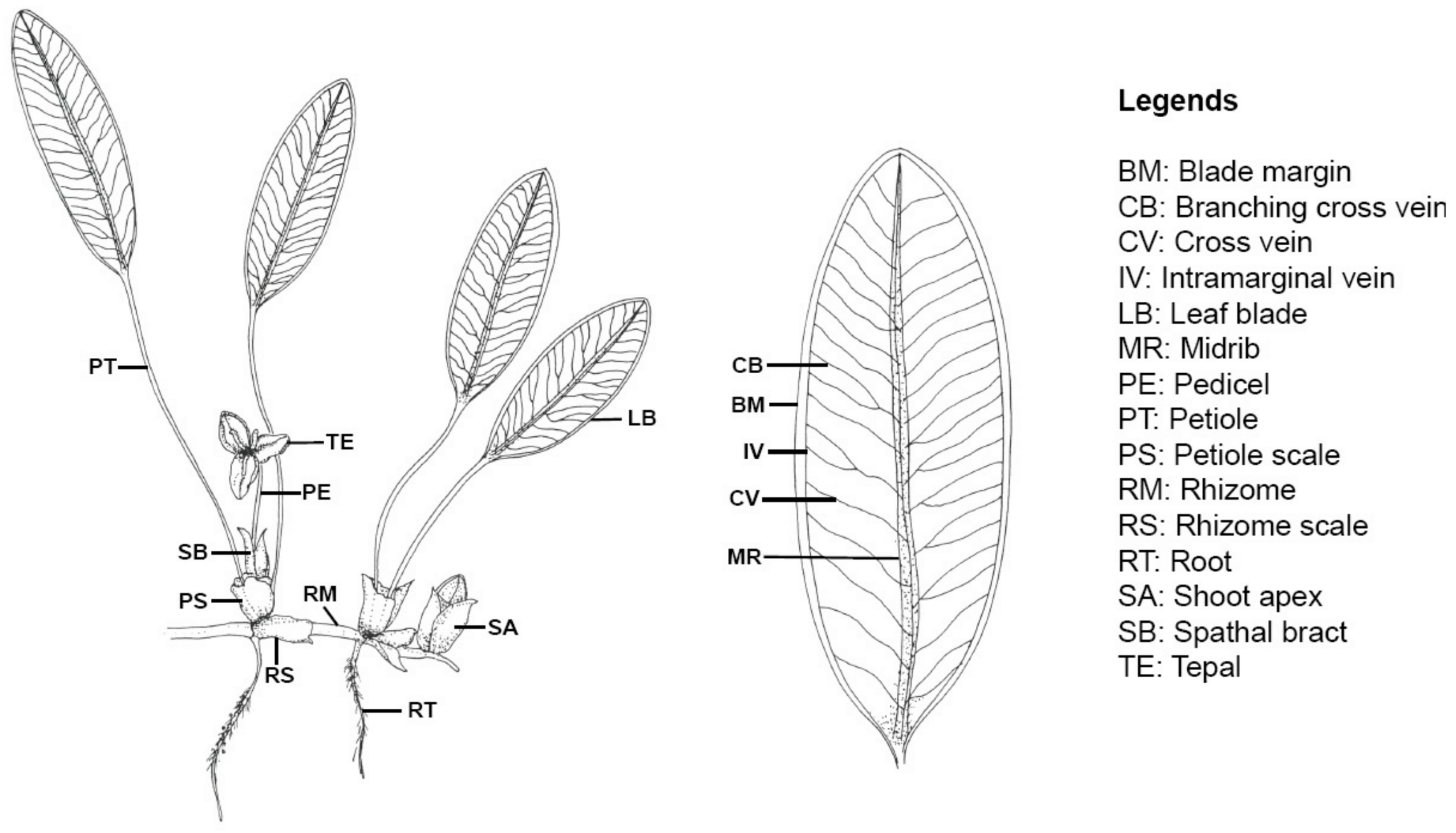
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, J. Taxonomy of the Genus Halophila Thouars (Hydocharitaceae): A Review. Plants 2020, 9, 1732. https://doi.org/10.3390/plants9121732
Kuo J. Taxonomy of the Genus Halophila Thouars (Hydocharitaceae): A Review. Plants. 2020; 9(12):1732. https://doi.org/10.3390/plants9121732
Chicago/Turabian StyleKuo, John. 2020. "Taxonomy of the Genus Halophila Thouars (Hydocharitaceae): A Review" Plants 9, no. 12: 1732. https://doi.org/10.3390/plants9121732
APA StyleKuo, J. (2020). Taxonomy of the Genus Halophila Thouars (Hydocharitaceae): A Review. Plants, 9(12), 1732. https://doi.org/10.3390/plants9121732




