Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review
Abstract
:1. Introduction
1.1. Nonthermal Plasma Generation Methods
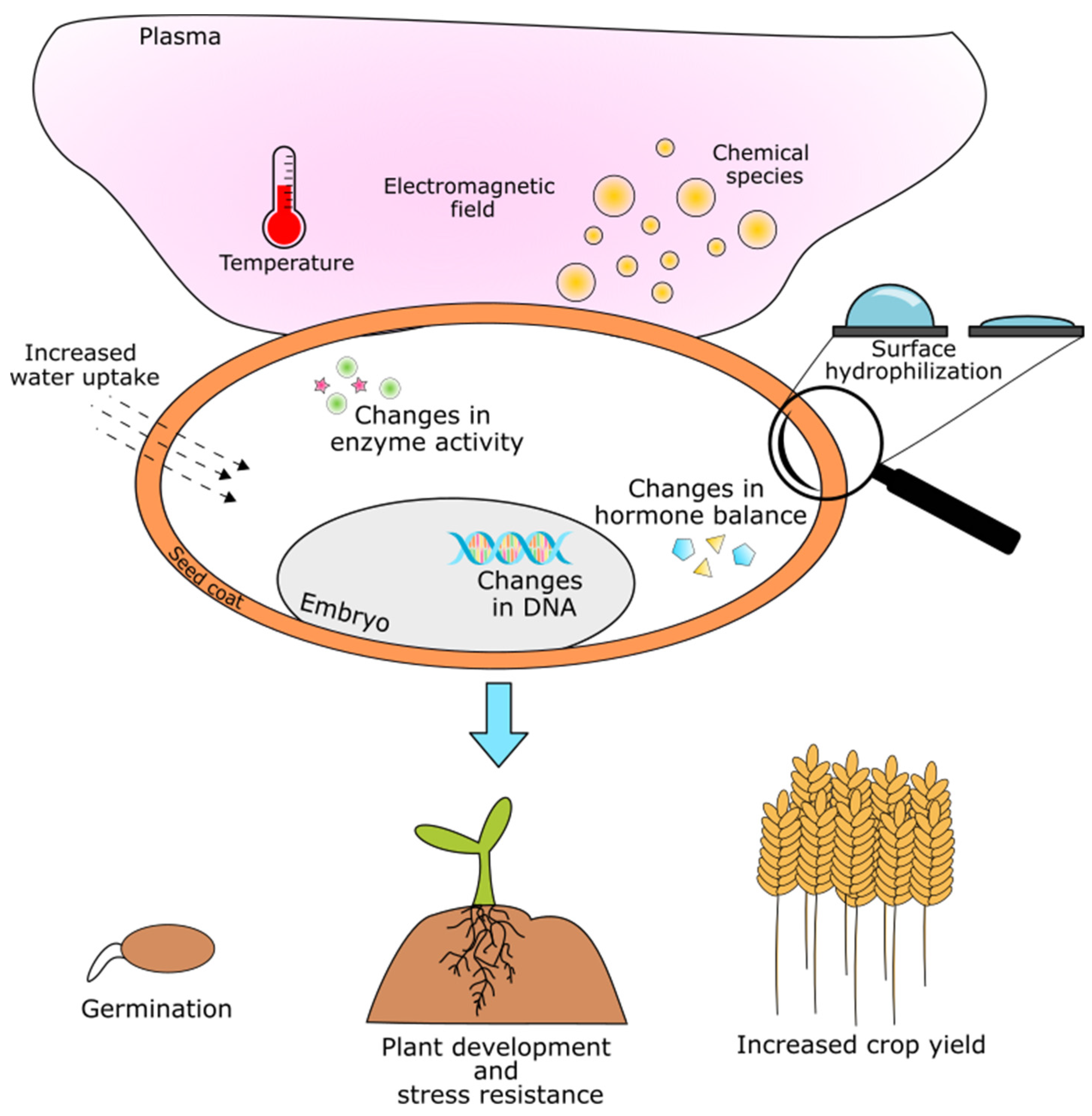
Direct and Indirect Effects of Nonthermal Plasma Treatment
2. Nonthermal Plasma Effects on Seeds
2.1. DNA Methylation and Demethylation and DNA Damage
2.2. Gene Expression and Protein Synthesis
2.3. The Effects on Enzyme Activity
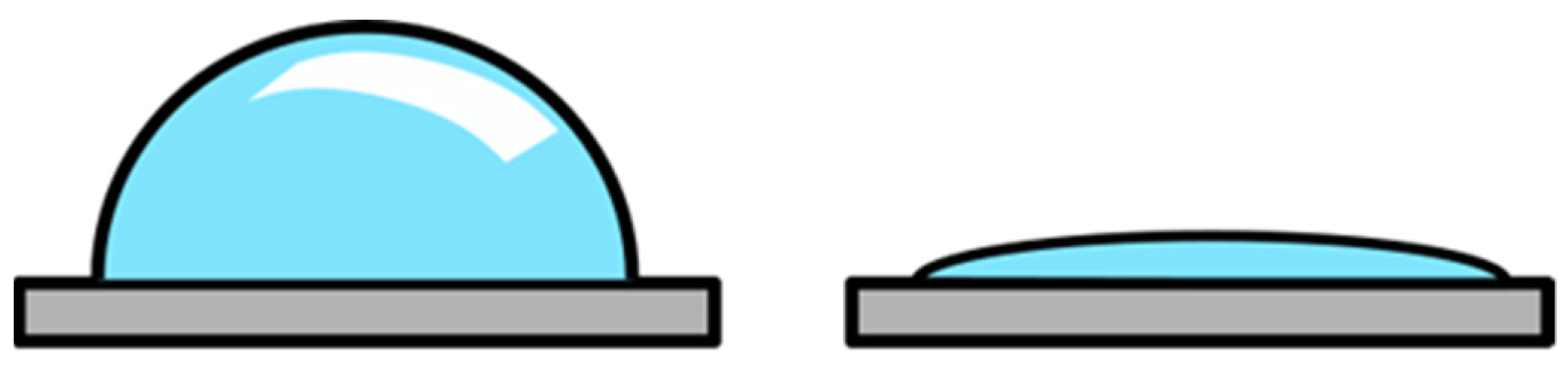
2.4. Morphological and Chemical Changes of the Seed Coat
2.5. Plant Hormone Balance
2.6. Germination and Seedling Growth Parameters
2.7. Resistance to Stress
2.8. Transfer of the Traits Induced by Nonthermal Plasma to the Next Generation
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crookes, W. Experiments on the Dark Space in Vacuum Tubes. Proc. R. Soc. A Math. Phys. Eng. Sci. 1907, 79, 98–117. [Google Scholar] [CrossRef] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Misra, N.N.; Schluter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture, 1st ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Frank-Kamenetskii, D.A.; Frank-Kamenetskii, D.A. Introduction. In Plasma: The Fourth State of Matter; Springer: Berlin, Germany, 1972; pp. 1–8. [Google Scholar]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated with Salmonella Biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold Plasma Decontamination of Foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.-D.; Polak, M.; Masur, K.; von Woedtke, T.; Winter, J.; Reuter, S. Plasma Processes and Plasma Sources in Medicine. Contrib. Plasma Phys. 2012, 52, 644–654. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sajib, S.A.; Rahi, M.S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, M.A.; Talukder, M.R.; Kabir, A.H. Mechanisms and Signaling Associated with LPDBD Plasma Mediated Growth Improvement in Wheat. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, T.; Meng, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Air Atmospheric Dielectric Barrier Discharge Plasma Induced Germination and Growth Enhancement of Wheat Seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiate). LWT Food Sci. Technol. 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ardebili, N.O.; Ardebili, Z.O.; Shafaati, M.; Ghoranneviss, M. Non-thermal Plasma Induced Expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) Growth and Resistance Against Salt Stress. Plasma Chem. Plasma Process. 2018, 38, 29–44. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, N.; Ono, R.; Nakano, R.; Shiratani, M.; Tashiro, K.; Kuhara, S.; Yasuda, K.; Hagiwara, H. DNA Microarray Analysis of Plant Seeds Irradiated by Active Oxygen Species in Oxygen Plasma. Plasma Med. 2016, 6, 459–471. [Google Scholar] [CrossRef]
- Nakano, R.; Tashiro, K.; Aijima, R.; Hayashi, N. Effect of Oxygen Plasma Irradiation on Gene Expression in Plant Seeds Induced by Active Oxygen Species. Plasma Med. 2016, 6, 303–313. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym. 2018, 15, 1600056. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [Green Version]
- Iranbakhsh, A.; Ardebili, Z.O.; Ardebili, N.O.; Ghoranneviss, M.; Safari, N. Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology. Acta Physiol. Plant. 2018, 40, 154. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.-F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of Surface-Borne Microorganisms and Increased Germination of Seed Specimen by Cold Atmospheric Plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of Atmospheric-Pressure N2, He, Air, and O2 Microplasmas on Mung Bean Seed Germination and Seedling Growth. Nat. Publ. Gr. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of Cold Atmospheric Pressure Plasma on Pea Seeds: DNA Damage of Seedlings and Optical Diagnostics of Plasma. Plasma Chem. Plasma Process. 2020, 1–14. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Tomeková, J.; Gálová, E.; Zahoranová, A. Cold Atmospheric Pressure Plasma Can Induce Adaptive Response in Pea Seeds. Plasma Chem. Plasma Process. 2019, 39, 475–486. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Hou, J.; Shao, H.; Dong, Y.; Jiang, J. Improving Seed Germination and Peanut Yields by Cold Plasma Treatment. Plasma Sci. Technol. 2016, 18, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with Radio-frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1800148. [Google Scholar] [CrossRef]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Amelioration of Photosynthesis and Quality of Wheat under Non-thermal Radio Frequency Plasma Treatment. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Li, R.; Yan, J. Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment. Arch. Biochem. Biophys. 2018, 655, 37–42. [Google Scholar] [CrossRef] [PubMed]
- De Groot, G.J.J.B.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1700068. [Google Scholar] [CrossRef]
- Shapira, Y.; Bormashenko, E.; Drori, E. Pre-germination plasma treatment of seeds does not alter cotyledon DNA structure, nor phenotype and phenology of tomato and pepper plants. Biochem. Biophys. Res. Commun. 2019, 519, 512–517. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 3–10. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Li, J.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Aguirre, D. Advances in Cold Plasma Applications for Food Safety and Preservation, 1st ed.; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128149218. [Google Scholar]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Coulombe, S.; Leask, R.L. Effects of non-thermal plasmas on DNA and mammalian cells. Plasma Process. Polym. 2010, 7, 899–909. [Google Scholar] [CrossRef]
- Lazović, S.; Maletić, D.; Leskovac, A.; Filipović, J.; Puač, N.; Malović, G.; Joksić, G.; Petrović, Z.L. Plasma induced DNA damage: Comparison with the effects of ionizing radiation. Appl. Phys. Lett. 2014, 105, 124101. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 32. [Google Scholar] [CrossRef] [Green Version]
- Zaplotnik, R.; Vesel, A. Effect of VUV radiation on surface modification of polystyrene exposed to atmospheric pressure plasma jet. Polymers 2020, 12, 1136. [Google Scholar] [CrossRef]
- Golda, J.; Biskup, B.; Layes, V.; Winzer, T.; Benedikt, J. Vacuum ultraviolet spectroscopy of cold atmospheric pressure plasma jets. Plasma Process. Polym. 2020, 17, 1900216. [Google Scholar] [CrossRef] [Green Version]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, F.E. Free Radicals and Antioxidant System in Seed Biology. In Advances in Seed Biology; InTech: London, UK, 2017; p. 167. [Google Scholar]
- Ranki, H.; Sopanen, T. Secretion of α-Amylase by the Aleurone Layer and the Scutellum of Germinating Barley Grain. Plant Physiol. 1984, 75, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R.R. The role of hormones during seed development and germination. Plant Horm. Biosynth. Signal Transduct. Action 2010, 549–573. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.L.; Owen, M.J. Hydrophobic recovery of plasma-treated polydimethylsiloxane. J. Adhes. 1995, 54, 33–45. [Google Scholar] [CrossRef]
- Junkar, I.; Modic, M.; Mozetič, M.; Vesel, A.; Hauptman, N.; Cvelbar, U. Modification of PET surface properties using extremely non-equilibrium oxygen plasma. Plasma Process. Polym. 2009, 6, 667–675. [Google Scholar] [CrossRef]
- Junkar, I. Plasma treatment of amorphous and semicrystalline polymers for improved biocompatibility. Vacuum 2013, 98, 111–115. [Google Scholar] [CrossRef]
- Modic, M.; Junkar, I.; Vesel, A.; Mozetic, M. Aging of plasma treated surfaces and their effects on platelet adhesion and activation. Surf. Coatings Technol. 2012, 213, 98–104. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotová, N.; Hrušková, I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Holc, M.; Primc, G.; Iskra, J.; Titan, P.; Kovač, J.; Mozetič, M.; Junkar, I. Effect of oxygen plasma on sprout and root growth, surface morphology and yield of garlic. Plants 2019, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, A.Y.; Sarani, A.; Leys, C. The influence of water vapor content on electrical and spectral properties of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2011, 20. [Google Scholar] [CrossRef]
- Alves Junior, C.; de Oliveira Vitoriano, J.; Layza Souza da Silva, D.; de Lima Farias, M.; Bandeira de Lima Dantas, N. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 33722. [Google Scholar] [CrossRef]


| Seed Type | Plasma Parameters | Exposure Time | Result Summary | Ref. |
|---|---|---|---|---|
| Arabidopsis thaliana | DBD, 10 kHz, 10 kV, atmospheric pressure, air | 15 min | Plasma pretreated seeds germinated faster, but the final germination rate was not significantly increased; germination was improved under salinity conditions as germination decrease caused by salinity stress was partially restored. | [25] |
| Arabidopsis thaliana, Raphanus sativus | RF, 13,56 MHz, 60 W, 20–80 Pa, O2 | No significant impact on plant growth, but the gene expression patterns were changed, also when comparing the first and the second generation of seeds. | [26] | |
| Arabidopsis thaliana, Raphanus sativus | RF, 13.56 MHz, 60 W, 20–80 Pa, Ar, O2 | 5, 15, 30 and 60 min | An enhanced seedling growth (at 80 Pa for 10 or 20 min) and changed gene expression pattern; growth enhancement was not inherited by the second-generation plants. | [27] |
| Barley (Hordeum vulgare) | SDBD (AC), 30 kHz, 400 W, atmospheric pressure, N2 and air | 10, 20, 40 and 80 s | Denser and longer roots and higher shoots in plasma-exposed seedlings; an increase in GABA levels. | [28] |
| Beans (Phaseolus vulgaris) | RF, 10 MHz, 20 W, 6.7 × 10−2 Pa, air | 2 min | Faster initial germination, but the final germination rate was the same; hydrophilization of the seed coat surface and accelerated water uptake. | [29] |
| Bell pepper (Capsicum annuum) | DBD (AC), 23 kHz, 11 kV, 80 W, atmospheric pressure, Ar | 60, 120 s | Growth-promoting and protecting effects on seedlings; inhibition and delay of nano ZnO toxicity and its negative effects on plant growth and differentiation. | [30] |
| Brown rice | DC, 1–3 kV, 800 Pa, air | 10 min | Increased germination rate, seedling length, water uptake, and GABA levels with optimum treatment at 3kV for 10 min. | [12] |
| Chickpea (Cicer arietinum) | surface microdischarge (SMD), complex 20-ms cycle, atmospheric pressure, air | 0.5–5 min | A noticeable increase in germination rate and root and shoot length. | [31] |
| Maize (Zea mays) | DCSBD, 14 kHz, 370 W, atmospheric pressure, air | 60, 120 s | No change in the germination rate, noticeably longer roots and bigger wet and dry biomass of plants after plasma treatment. | [32] |
| Maize (Zea mays) | DCSBD, 14 kHz, 20 kV, 400 W, atmospheric pressure, air | 30–300 s | No statistically significant changes in the germination rate; at longer exposure times, a decrease in germination rate; an increase in root length and shoot height. | [33] |
| Mung beans (Vigna radiata) | RF-CCP, 13.56 MHz, 40 and 60 W, 20 Pa, air | 10, 15 and 20 min | Faster germination, an increased germination rate, higher shoots, an increase in water-soluble sugars and higher amylase and phytase activity. | [22] |
| Mung beans (Vigna radiata) | microplasma, 9 kHz, 0–20 kV, 25 W, atmospheric pressure, N2, He, air and O2 | 10 min | Different effects depending on feed gas, higher germination rate in air and helium plasma, an increase in plant height for air plasma, no significant effects on seeds in O2 plasma. | [34] |
| Pea (Pisum sativum) | DBD, 14 kHz, 20 kV, 400 W, atmospheric pressure, air/N2/O2/N2 and O2 | 60, 180 and 300 s | More DNA damage than in nontreated samples; an ambient air plasma had the least damaging effects on seed DNA, compared to plasma treatment with different mixtures of O2 and N2. | [35] |
| Pea (Pisum sativum) | DCSBD, 14 kHz, 20 kV, atmospheric pressure, air | 60–300 s | No increase in DNA damage. After the application of DNA-damage agent Zeocin, an increase in DNA damage. The plasma-treated seeds had a lower level of DNA damage than untreated seeds. | [36] |
| Pea (Pisum sativum) | DCSBD, 14 kHz, 10 kV, 370 W, atmospheric pressure, air | 60–600 s | Increased biosynthesis of auxin and cytokinins as well as their catabolites and conjugates; a noticeable increase in germination rate and root and shoot length. | [21] |
| Peanut (Arachis hypogaea) | RF-CCP, 13.56 MHz, 60–140 W, 150 Pa, He | 15 s | Improved germination rate and peanut yield after plasma treatment of seeds at 120 W. | [37] |
| Quinoa (Chenpodium quinoa) | RF and DBD, 1 kHz, 8.2 kV, 6.4 W, 500 and 0.1 mbar, air | 10, 30, 60, 180 and 900 s | A higher germination rate in seeds treated for 10 s with RF plasma or 180 or 900 s by DBD plasma. A drastically changed chemistry of the seed coat outer layer | [13] |
| Rapeseed (Brassica napus) | RF-CCP, 13.56 MHz, 100 W, 150 Pa, He | 15 s | Increased germination rate and shoot and root growth. Germination rate, germination index and vigor of seeds exposed to drought stress and treated with plasma was increased, along with plant growth and a decrease in MDA content compared to untreated seeds exposed to drought | [15] |
| Sunflower (Helianthus annuus) | RF-EMF, 5.28 MHz, atmospheric pressure, air | 5, 10 and 15 min | No significant changes in the germination rate of plasma and EMF treated seeds; noticeable changes in phytohormone balance; after short exposure of seeds to RF-CP or RF-EMF a long-term effect on gene expression in leaves, mostly stimulating expression of proteins involved in photosynthesis and its regulation. | [38] |
| RF-CP, 200 Pa, air | 2, 5 or 7 min | |||
| Soybean (Glycine max) | 60 kHz, 10.8–22.1 kV, 3.4–15.6 W, atmospheric pressure, Ar | 12, 24, 48, 60, 120 and 180 s | A slightly higher germination rate and enhanced root and shoot growth; changes in DNA methylation level, an increased SOD, POD and CAT enzyme activity. | [24] |
| Wheat (Triticum aestivum) | SDBD (AC), 50 Hz, atmospheric pressure, air | 5, 15 and 20 min | The germination rate unchanged; an enhanced root growth (especially in seedlings treated with plasma for 15 min). | [39] |
| Wheat (Triticum aestivum) | DBD, 50 Hz, 13 kV, atmospheric pressure, air | 4 min | Increased germination rate, root length and shoot height; noticeable changes in the expression of genes LEA1, SnRK2 and P5CS; increased proline and soluble sugar levels in normal water conditions and in seedlings exposed to drought conditions. A decrease in MDA content in seeds under drought stress. An increase in SOD, POD and CAT enzyme activity. | [40] |
| Wheat (Triticum aestivum) | RF, 3 × 109 MHz, 60, 80 and 100 W, 150 Pa, He | 1–5 shots of high voltage nanosecond | No changes in the germination rate and speed; enhanced root and shoot growth and an increased yield. | [41] |
| Wheat (Triticum aestivum) | DBD (AC), 50 kHz, 13 kV, 1.5 W, atmospheric pressure, air | 1, 4, 7, 10 and 13 min | Increased root and shoot growth, an increase in proline and soluble sugar levels. With increased exposure time a noticeable decline of MDA content and an increase in SOD and POD enzyme activity; increased germination rate. | [20] |
| Wheat (Triticum aestivum) | DBD, 50 kHz, 80 kV, atmospheric pressure, air | 30, 60 and 80 s | Improved seed germination rate and an increase in root growth. Shoots longer only in seeds treated with plasma for 30 s and no retention time, and seeds treated with plasma for 60 s and retention time of 24 h. | [42] |
| Wheat (Triticum aestivum) | DBD (AC), 50 kHz, 13 kV, atmospheric pressure, air, O2, N2, Ar | In seeds, treated with air, N2 or Ar plasma an increase in root length; no increase in seeds treated with O2 plasma. | [18] | |
| Wheat (Triticum aestivum) | LPDBD, 5 kHz, 4.5 kV, 45 W, 10 torr, Ar/O2 or Ar/air | 90 s | Shorter roots and longer shoots compared to the control seedlings; an elevated SOD enzymatic activity in roots and a higher level of H2O2 in roots and shoots in seedlings treated with O2/Ar plasma; with Ar/air plasma an increase in CAT activity in leaves. | [19] |
| Wheat (Triticum aestivum) | RF, 13.56 MHz, 80 W, 0.1 mbar, air | 60, 120, 180 and 240 s | With Plasma treatment for 180 s a higher wheat yield and increased plant photosynthesis. | [43] |
| Maize, wheat, soybeans, tobacco | RF-CCP, 13.56 MHz, 50–1000 W, 30–200 Pa, air | 5–90 s | An increase in the germination rate of maize and seedling growth of wheat; SOD and POD enzymatic activity also increased; an increase in the yield of tobacco (20%) and soybean (4%). | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. https://doi.org/10.3390/plants9121736
Starič P, Vogel-Mikuš K, Mozetič M, Junkar I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants. 2020; 9(12):1736. https://doi.org/10.3390/plants9121736
Chicago/Turabian StyleStarič, Pia, Katarina Vogel-Mikuš, Miran Mozetič, and Ita Junkar. 2020. "Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review" Plants 9, no. 12: 1736. https://doi.org/10.3390/plants9121736





