Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (APTI), Dust Amount and Elemental Concentration of Metals
Abstract
1. Introduction
2. Results
2.1. Dust Amount
2.2. APTI and Elemental Concentration
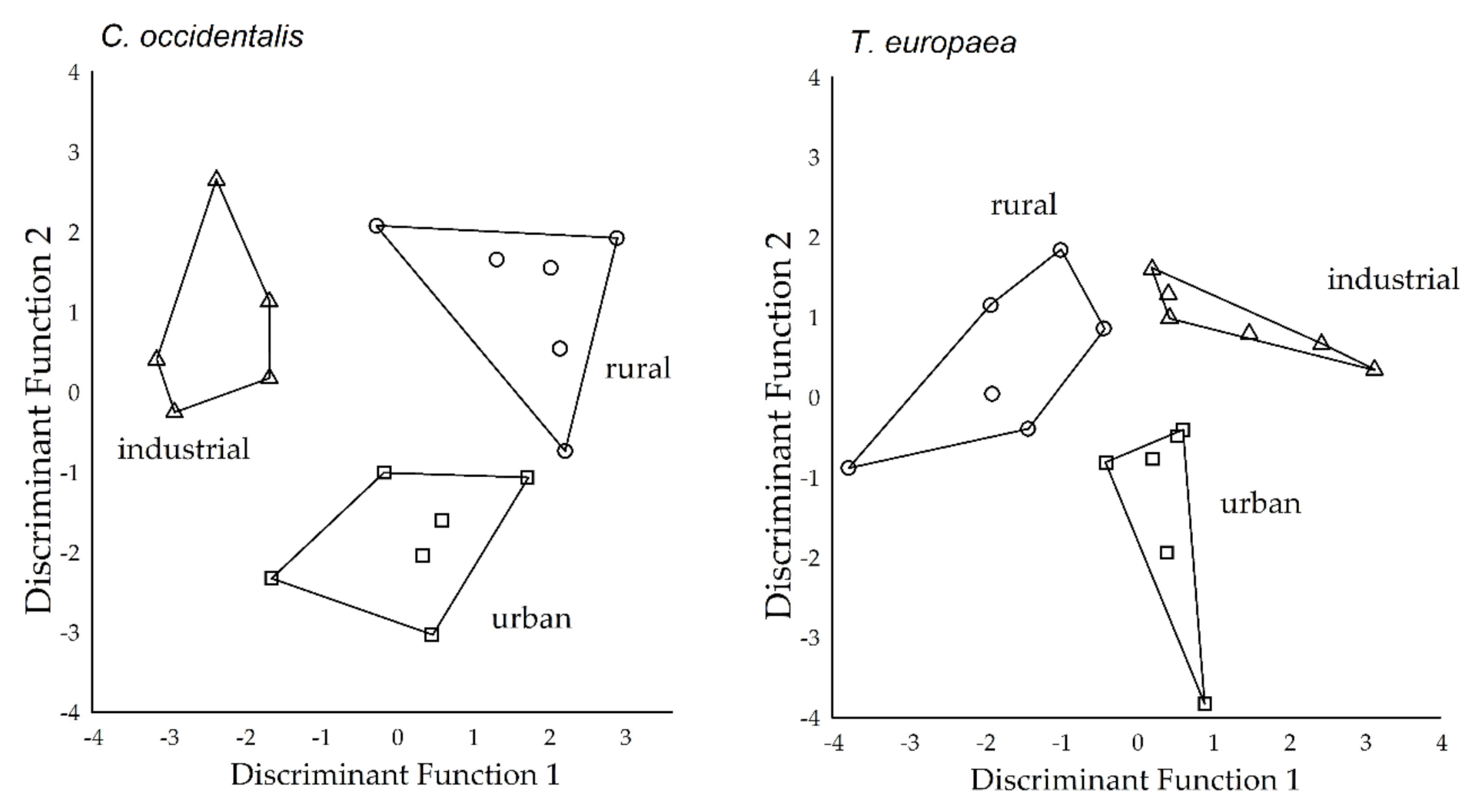
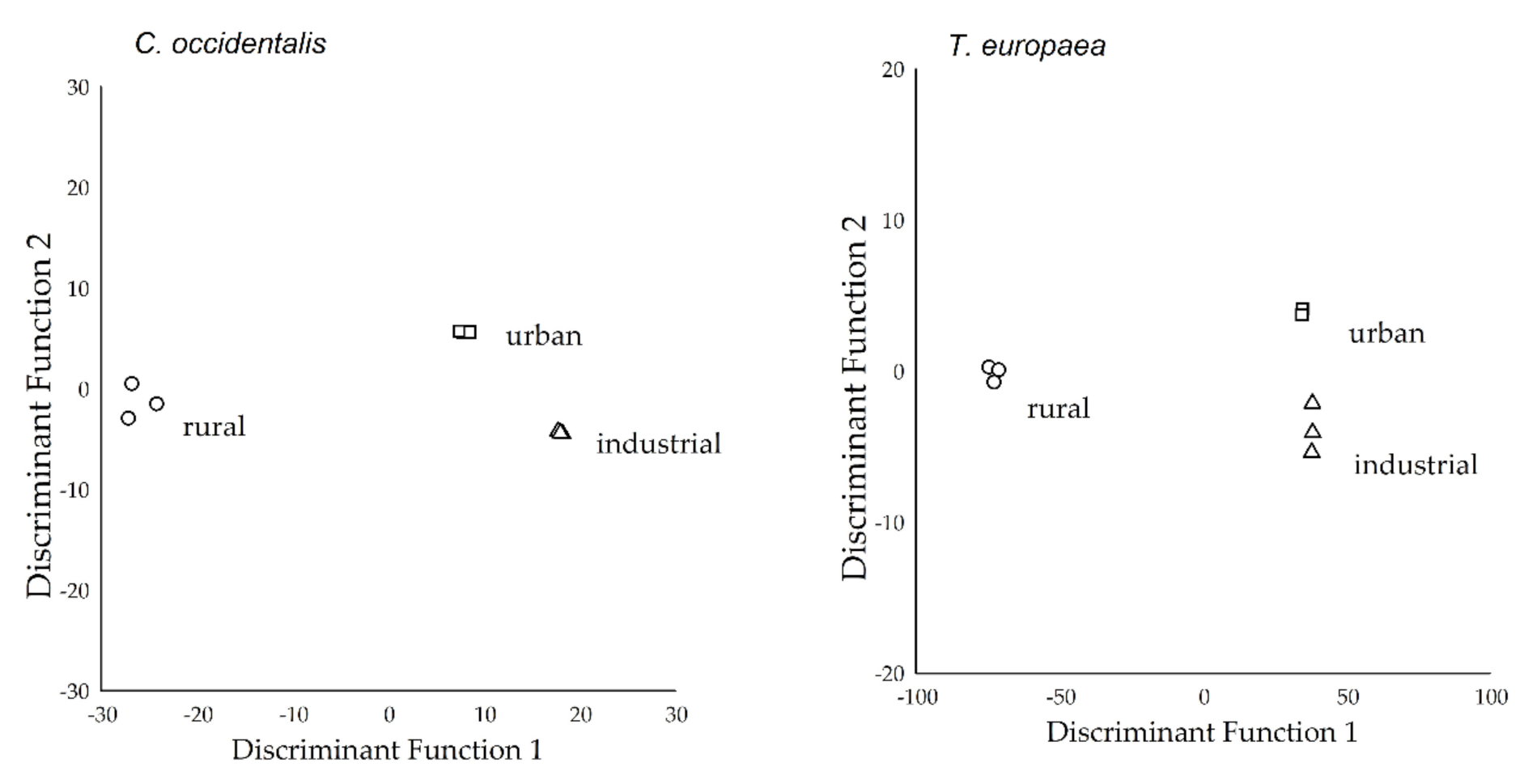
2.3. Separation of Study Sites Based on Deposited Dust and APTI Parameters
2.4. Separation of Study Sites Based on the Elemental Concentrations of Leaf Tissue
2.5. Correlation between APTI and the Measured Environmental Parameters
3. Discussion
4. Materials and Methods
4.1. Study Areas and Sample Collection
4.2. Analysis of Dust Samples
4.3. Air Pollution Tolerance Index
4.4. Analysis of the Elemental Concentration of Leaves
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alhowaish, A. Eighty years of urban growth and socioeconomic trends in Dammam Metropolitan Area, Saudi Arabia. Habitat Int. 2015, 50, 90–98. [Google Scholar] [CrossRef]
- Sierra-Vargas, M.P.; Tamayo, L.F.T. Air pollution: Impact and prevention. Respirology 2012, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Mafuyai, G.M.; Eneji, I.S.; Sha’Ato, R. Concentration of Heavy Metals in Respirable Dust in Jos Metropolitan Area, Nigeria. Open J. Air Pollut. 2014, 3, 10–19. [Google Scholar] [CrossRef]
- Costa, F.G.E.; Pereir, M.D.L. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; IntechOpen: Rijeka, Croatia, 2012; pp. 227–247. [Google Scholar]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Trzęsicki, M.; Barczyk, G. Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere 2017, 183, 471–482. [Google Scholar] [CrossRef]
- Saadullah, K.L.; Mudassir, A.Z.; Atta, M.S.; Muhammed, F.; Gulam, R.S.; Waris, A.; Leghari, S.K.; Zaid, M.A.; Sarangzai, A.M.; Faheem, M.; et al. Effect of road side dust pollution on the growth and total chlorophyll contents in Vitis vinifera L. (grape). Afr. J. Biotechnol. 2014, 13, 1237–1242. [Google Scholar] [CrossRef]
- Bharti, S.K.; Trivedi, A.; Kumar, N. Air pollution tolerance index of plants growing near an industrial site. Urban Clim. 2018, 24, 820–829. [Google Scholar] [CrossRef]
- Mirecki, N.; Agic, R.; Šunić, L.; Milenkovic, L.; Ilic, Z. Transfer factor as indicator of heavy metals content in plants. Fresen. Environ. Bull. 2015, 24, 4212–4219. [Google Scholar]
- Feng, W.; Guo, Z.; Xiao, X.; Peng, C.; Shi, L.; Ran, H.; Xu, W. Atmospheric deposition as a source of cadmium and lead to soil-rice system and associated risk assessment. Ecotoxicol. Environ. Saf. 2019, 180, 160–167. [Google Scholar] [CrossRef]
- Lei, Y.; Duan, Y.; He, D.; Zhang, X.; Chen, L.; Li, Y.; Gao, G.; Tian, G.; Zheng, J. Effects of Urban Greenspace Patterns on Particulate Matter Pollution in Metropolitan Zhengzhou in Henan, China. Atmosphere 2018, 9, 199. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef]
- Singh, S.; Rao, D.; Agrawal, M.; Pandey, J.; Naryan, D. Air pollution tolerance index of plants. J. Environ. Manag. 1991, 32, 45–55. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; Alharbi, B.H.; Al-Shamsi, M.A.; Alshahrani, T.S.; Al-Namazi, A.A.; Alharbi, S.F.; Alotaibi, F.S.; Qian, Y. Assessing the response of five tree species to air pollution in Riyadh City, Saudi Arabia, for potential green belt application. Environ. Sci. Pollut. Res. 2020, 27, 29156–29170. [Google Scholar] [CrossRef]
- Molnár, V.É.; Simon, E.; Tóthmérész, B.; Ninsawat, S.; Szabo, S. Air pollution induced vegetation stress—The Air Pollution Tolerance Index as a quick tool for city health evaluation. Ecol. Indic. 2020, 113, 106234. [Google Scholar] [CrossRef]
- Yadav, R.; Pandey, P. Assessment of Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API) of Roadside Plants for the Development of Greenbelt in Urban Area of Bathinda City, Punjab, India. Bull. Environ. Contam. Toxicol. 2020, 105, 906–914. [Google Scholar] [CrossRef]
- Schoolmaster, D.R., Jr.; Grace, J.B.; Schweiger, W.E. A general theory of multimetric indices and their properties. Methods Ecol. Evol. 2012, 3, 773–781. [Google Scholar]
- Karmakar, D.; Padhy, P.K. Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Suspended particulate matter deposition and its impact on urban trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Varga, T.; Barnucz, P.; Major, I.; Lisztes-Szabó, Z.; Jull, A.J.T.; László, E.; Pénzes, J.; Molnár, M. Fossil Carbon Load in Urban Vegetation for Debrecen, Hungary. Radiocarbon 2019, 61, 1199–1210. [Google Scholar] [CrossRef]
- Varga, T.; Jull, A.J.T.; Lisztes-Szabó, Z.; Molnár, M. Spatial Distribution of 14C in Tree Leaves from Bali, Indonesia. Radiocarbon 2019, 62, 235–242. [Google Scholar] [CrossRef]
- Simon, E.; Baranyai, E.; Braun, M.; Cserháti, C.; Fábián, I.; Tóthmérész, B. Elemental concentrations in deposited dust on leaves along an urbanization gradient. Sci. Total. Environ. 2014, 490, 514–520. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, T.; Kumari, M. Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci. Total. Environ. 2020, 722, 137622. [Google Scholar] [CrossRef]
- Chauhan, A. Photosynthetic pigment changes in some selected trees induced by automobile exhaust in Dehradun, Uttarakhand. N. Y. Sci. J. 2010, 3, 45–51. [Google Scholar]
- Giri, S.; Shrivastava, D.; Deshmukh, K.; Dubey, P. Effect of Air Pollution on Chlorophyll Content of Leaves. Curr. Agric. Res. J. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Iqbal, M.; Shafiq, M.; Zaidi, S.; Athar, M. Effect of automobile pollution on chlorophyll content of roadside urban trees. Glob. J. Environ. Sci. Manag. 2015, 1, 283–296. [Google Scholar]
- Mukherjee, A.; Agrawal, M. Pollution Response Score of Tree Species in Relation to Ambient Air Quality in an Urban Area. Bull. Environ. Contam. Toxicol. 2015, 96, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.T. Biomonitoring Study of Air Pollution with Betula pendula Roth from Plovdiv, Bulgaria. Ecol. Balk. 2011, 3, 1–11. [Google Scholar]
- Rai, P.K. Particulate matter tolerance of plants (APTI and API) in a biodiversity hotspot located in a tropical region: Implications for eco-control. Part. Sci. Technol. 2019, 38, 193–202. [Google Scholar] [CrossRef]
- Gholami, A.; Mojiri, A.; Amini, H. Investigation of the Air Pollution Tolerance Index (APTI) using some plant species in Ahvaz region. J. Anim. Plant Sci. 2016, 26, 475–480. [Google Scholar]
- Zhang, P.; Liu, Y.-J.; Chen, X.; Yang, Z.; Zhu, M.-H.; Li, Y.-P. Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicol. Environ. Saf. 2016, 132, 212–223. [Google Scholar] [CrossRef]
- Simon, E.; Braun, M.; Vidic, A.; Bogyo, D.; Fábián, I.; Tóthmérész, B. Air pollution assessment based on elemental concentration of leaves tissue and foliage dust along an urbanization gradient in Vienna. Environ. Pollut. 2011, 159, 1229–1233. [Google Scholar] [CrossRef]
- Mansour, R.S. The pollution of tree leaves with heavy metal in Syria. Int. J. Chem. Tech. Res. 2014, 6, 2283–2290. [Google Scholar]
- Hariram, M.; Sahu, R.; Elumalai, S.P. Impact Assessment of Atmospheric Dust on Foliage Pigments and Pollution Resistances of Plants Grown Nearby Coal Based Thermal Power Plants. Arch. Environ. Contam. Toxicol. 2017, 74, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Ogunkunle, C.O.; Suleiman, L.B.; Oyedeji, S.; Awotoye, O.O.; Fatoba, P.O. Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agrofor. Syst. 2015, 89, 447–454. [Google Scholar] [CrossRef]
- Kircsi, A.; Szegedi, S. The development of the urban heat island studied on temperature profiles in Debrecen. ACTA Climat. Chorol. 2003, 36–37, 63–69. [Google Scholar]
- Pásztor, L.; Laborczi, A.; Takács, K.; Szatmári, G.; Illés, G.; Fodor, N.; Négyesi, G.; Bakacsi, Z.; Szabó, J. Spatial distribution of selected soil features in Hajdú-Bihar county represented by digital soil maps. Landsc. Environ. 2016, 10, 203–213. [Google Scholar] [CrossRef]
- Négyesi, G.; Lóki, J.; Buró, B.; Bertalan-Balázs, B.; Pásztor, L. Wind erosion researches in Hungary—Past, present and future possibilities. Hung. Geogr. Bull. 2019, 68, 223–240. [Google Scholar] [CrossRef]
- Dobos, E.; Borbély-Kiss, I.; Kertész, Z.; Szabó, G.; Koltay, E. Comparison of Debrecen fine fraction aerosol data with others collected in a European collaboration. ATOMKI Annu. Rep. 2007, 25, 1–4. [Google Scholar]
- Varga, G. Changing nature of Saharan dust deposition in the Carpathian Basin (Central Europe): 40 years of identified North African dust events (1979–2018). Environ. Int. 2020, 139, 105712. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. Released. IBM SPSS Statistics for Windows. Version 20.0; IBM Corp.: Armonk, NY, USA, 2011. [Google Scholar]

| Function | C. occidentalis | T. europaea | ||
|---|---|---|---|---|
| DF1 | DF2 | DF1 | DF2 | |
| Eigenvalue | 3.3 | 2.3 | 2.0 | 1.2 |
| Percentage of Variance | 59.0 | 41.0 | 62.7 | 37.3 |
| Cumulative percentage | 59.0 | 100.0 | 62.7 | 100.0 |
| Canonical Correlation | 0.9 | 0.8 | 0.8 | 0.7 |
| Wilks’ Lambda | 0.1 | 0.3 | 0.2 | 0.5 |
| Chi-square | 30.4 | 13.7 | 23.5 | 9.8 |
| df | 12. | 5 | 12 | 5 |
| Significance | 0.002 | 0.018 | 0.023 | 0.081 |
| Function | C. occidentalis | T. europaea | ||
|---|---|---|---|---|
| DF1 | DF2 | DF1 | DF2 | |
| Eigenvalue | 532.6 | 26.3 | 3978.5 | 15.5 |
| Percentage of Variance | 95.3 | 4.7 | 99.6 | 0.4 |
| Cumulative percentage | 95.3 | 100.0 | 99.6 | 100.0 |
| Canonical Correlation | 1.0 | 0.9 | 1.0 | 0.9 |
| Wilks’ Lambda | 0.0 | 0.04 | 0.0 | 0.1 |
| Chi-square | 33.6 | 11.6 | 38.8 | 9.8 |
| df | 12 | 5 | 12 | 5 |
| Significance | 0.001 | 0.041 | <0.001 | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, V.É.; Tőzsér, D.; Szabó, S.; Tóthmérész, B.; Simon, E. Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (APTI), Dust Amount and Elemental Concentration of Metals. Plants 2020, 9, 1743. https://doi.org/10.3390/plants9121743
Molnár VÉ, Tőzsér D, Szabó S, Tóthmérész B, Simon E. Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (APTI), Dust Amount and Elemental Concentration of Metals. Plants. 2020; 9(12):1743. https://doi.org/10.3390/plants9121743
Chicago/Turabian StyleMolnár, Vanda Éva, Dávid Tőzsér, Szilárd Szabó, Béla Tóthmérész, and Edina Simon. 2020. "Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (APTI), Dust Amount and Elemental Concentration of Metals" Plants 9, no. 12: 1743. https://doi.org/10.3390/plants9121743
APA StyleMolnár, V. É., Tőzsér, D., Szabó, S., Tóthmérész, B., & Simon, E. (2020). Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (APTI), Dust Amount and Elemental Concentration of Metals. Plants, 9(12), 1743. https://doi.org/10.3390/plants9121743








