Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms
Abstract
:1. Introduction
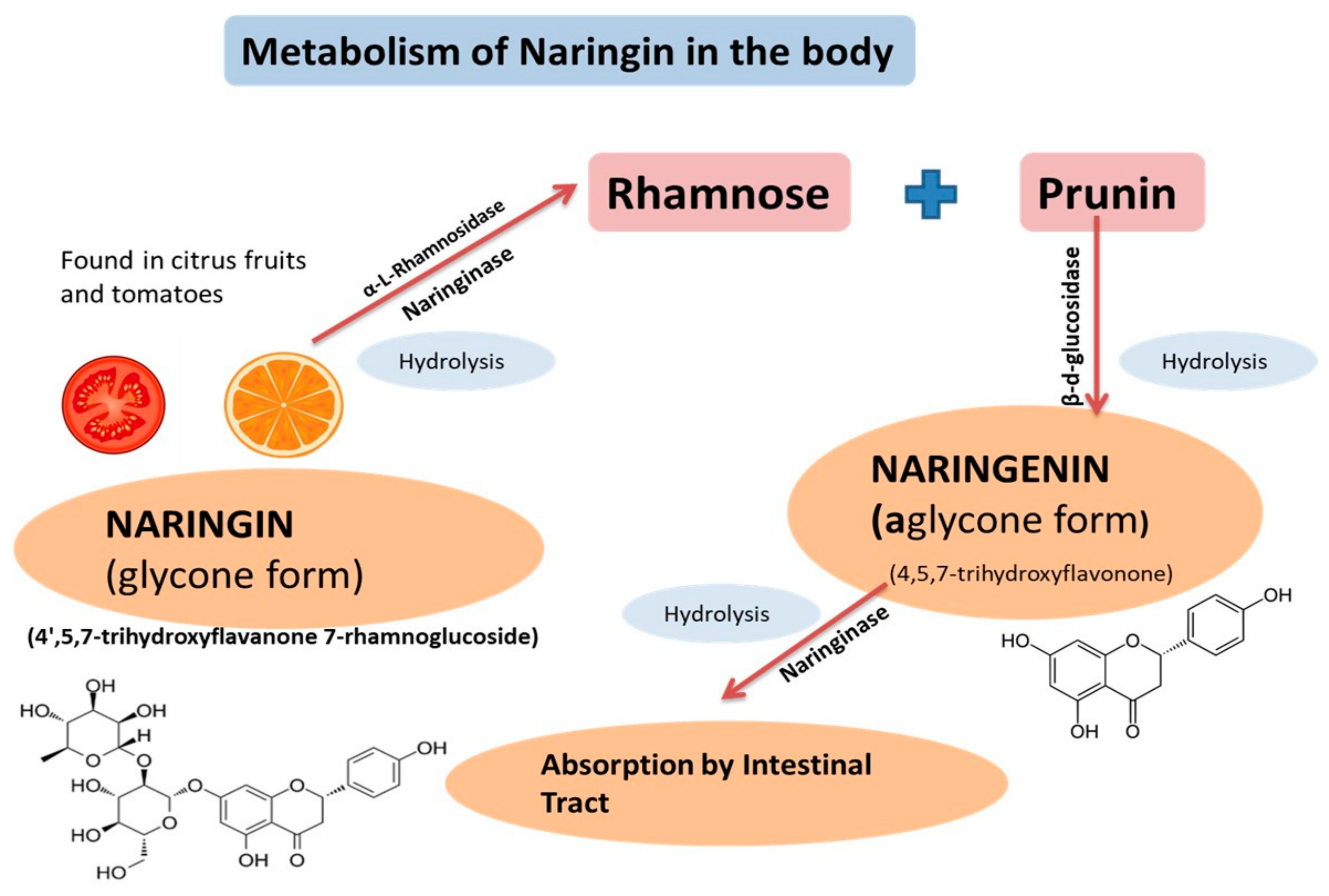
2. Physicochemical Characteristics, Pharmacokinetics, and Pharmacodynamics of Naringenin
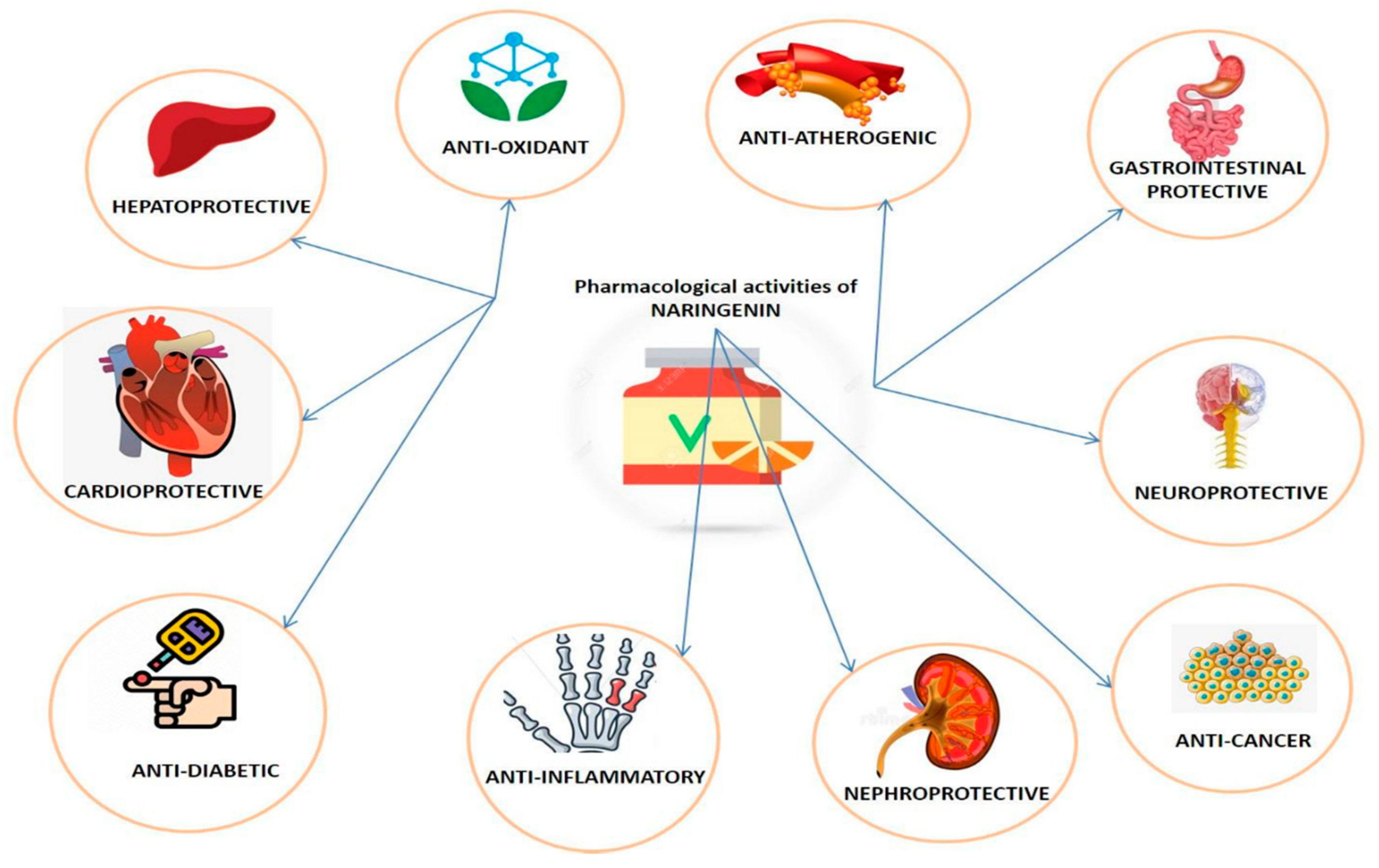
3. Pharmacological Properties of Naringenin
3.1. Anticancer Properties
3.1.1. Carcinogen Inactivation
3.1.2. Anti-Proliferative Action
3.1.3. Cell Cycle Arrest
3.1.4. Induction of Apoptosis
3.1.5. Inhibition of Sustained Angiogenesis
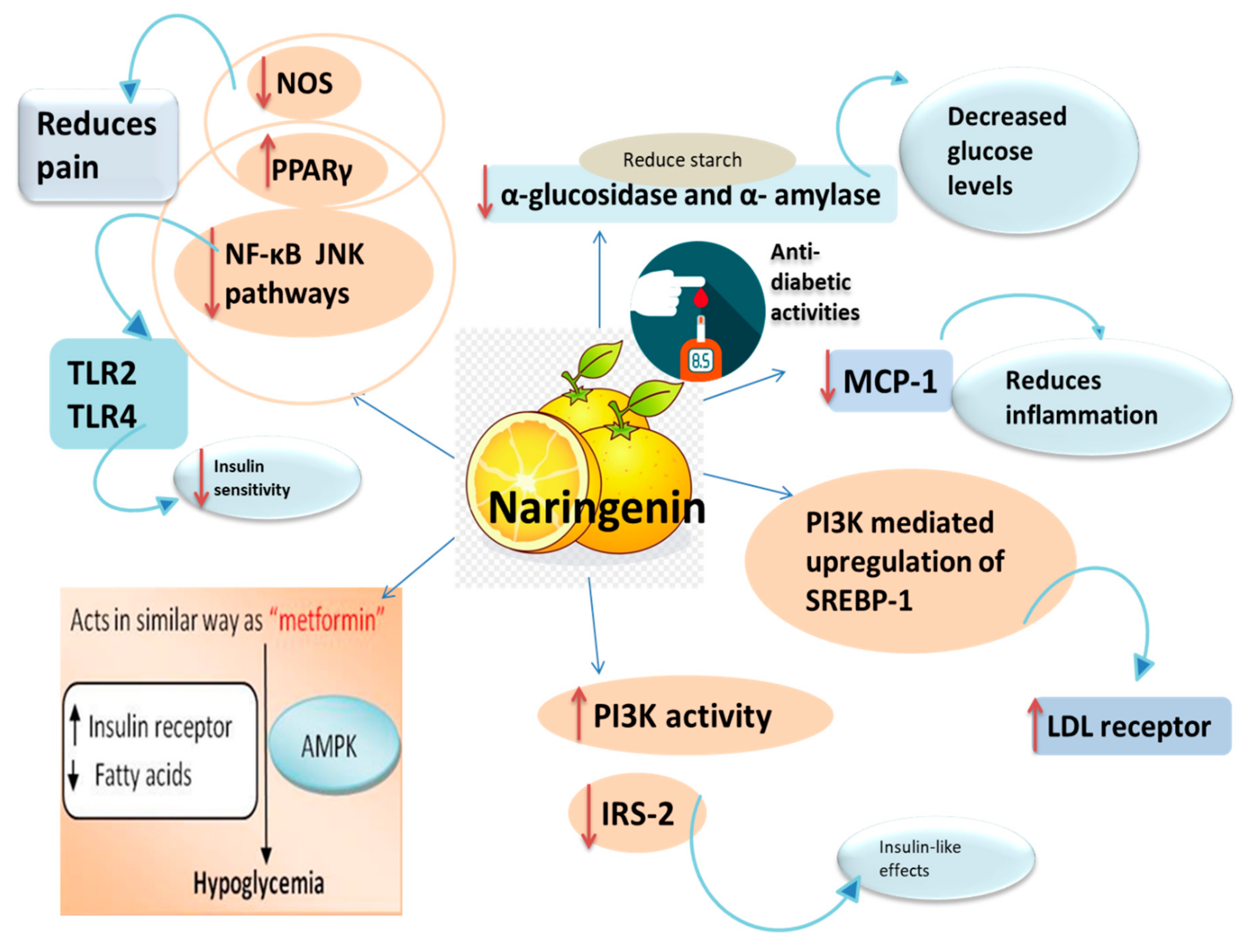
3.2. Anti-Diabetic Activity
3.3. Hepatoprotective Action
3.4. Neuroprotective Action
3.5. Cardioprotective Action
3.6. Nephroprotective Action
3.7. Gastroprotective Action
3.8. Role of Naringenin in Pulmonary Diseases
3.9. Antimicrobial Action
4. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin. J. Integr. Med. 2014, 24, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.; Rehman, M.U.; Tabassum, N.; Amin, U. Effect of naringenin (A naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharm. Mag. 2017, 13, 154. [Google Scholar] [CrossRef]
- Mir, I.A.; Tiku, A.B. Chemopreventive and therapeutic potential of “naringenin,” a flavanone presents in citrus fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, S. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, T.; Zhou, J. Fermentation and metabolic pathway optimization to de novo synthesize (2S)-naringenin in Escherichia coli. J. Microbiol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 62750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.M.; Saleh, M.C.; Connell, B.J.; Song, Y.H. A co-drug conjugate of naringenin and lipoic acid mediates neuroprotection in a rat model of oxidative stress. Clin. Exp. Pharmacol. Physiol. 2017, 46, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dong, T.; Zhang, A.; Yang, J.; Yan, G.; Sakurai, T.; Wang, X. Pharmacokinetics of hesperetin and naringenin in the Zhi Zhu Wan, a traditional Chinese medicinal formulae, and its pharmacodynamics study. Phytother. Res. 2013, 27, 1345–1351. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; Tolosa de Talamoni, N. New Perspectives in the Pharmacological Potential of Naringin in Medicine. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.; Sánchez-Salgado, J.; Navarrete-Vázquez, G.; Webster, S.; Binnie, M.; García-Jiménez, S.; Estrada-Soto, S. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes. Metab. 2008, 10, 1097–1104. [Google Scholar] [CrossRef]
- Fuhr, U.; Klittich, K.; Staib, A.H. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br. J. Clin. Pharmacol. 1994, 35, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Fuhr, U.; Kummert, A.L. The fate of naringin in humans: A key to grapefruit juice-drug interactions? Clin. Pharmacol. Ther. 1995, 58, 365–373. [Google Scholar] [CrossRef]
- Katavic, P.L.; Lamb, K.; Navarro, H.; Prisinzano, T.E. Flavonoids as Opioid Receptor Ligands: Identification and Preliminary Structure− Activity Relationships. J. Nat. Prod. 2007, 70, 1278–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Shetty, T.; Bhattacharya, R. Modulating effect of plant flavonoids on the mutagenicity of N-methyl- N′-nitro-N-nitrosoguanidine. Carcinogenesis 1989, 10, 1953–1955. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, G.; Rajendran, P.; Magesh, V.; Sakthisekaran, D. Naringenin reduces tumor size and weight lost in N-methyl-N′-nitro-N-nitrosoguanidine–induced gastric carcinogenesis in rats. Nutr. Res. 2008, 28, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.F.R.; Oliveira, T.T.D.; Nagem, T.J.; Pinto, A.D.S.; Oliveira, M.G. Hypolipidaemic effects of naringenin, rutin, nicotinic acid and their associations. Pharm. Res. 1999, 40, 493–496. [Google Scholar] [CrossRef]
- Rayidi, S.; Pari, L. Effect of naringenin on carbohydrate metabolism in streptozotocin-nicotinamide induced diabetic rats. Biomirror 2011, 2, 12–19. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, 12399. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.H.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Varghese, F.; Kabasakal, B.V.; Cotton, C.; Schumacher, J.; Rutherford, A.W.; Fantuzzi, A.; Murray, J.W. A low-potential terminal oxidase associated with the iron-only nitrogenase from the nitrogen-fixing bacterium Azotobacter vinelandii. J. Biol. Chem. 2019, 294, 9367–9376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesen, C.; Herr, I.; Krammer, P.H.; Debatin, K.M. Involvement of the CD95 (APO–1/Fas) receptor/ligand system in drug–induced apoptosis in leukemia cells. Nat. Med. 1996, 2, 574. [Google Scholar] [CrossRef] [PubMed]
- Durgo, K.; Koncar, M.; Komes, D.; Belscak-Cvitanovic, A.; Franekic, J.; Jakopovich, I.; Jakopovich, B. Cytotoxicity of blended versus single medicinal mushroom extracts on human cancer cell lines: Contribution of polyphenol and polysaccharide content. Int. J. Med. Mushrooms 2013, 15, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Stefani, E.D.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, N.D. Role of flavonoids in DNA damage and carcinogenesis prevention. J. Carcinog. Mutagen. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Brueggemeier, R.W. Aromatase, aromatase inhibitors, and breast cancer. Am. J. Ther. 2001, 8, 333–344. [Google Scholar] [CrossRef]
- Pouget, C.; Fagnere, C.; Basly, J.P.; Besson, A.E.; Champavier, Y.; Habrioux, G.; Chulia, A.J. Synthesis and aromatase inhibitory activity of flavanones. Pharm. Res. 2002, 19, 286–291. [Google Scholar] [CrossRef]
- Wilcox, L.J.; Borradaile, N.M.; Huff, M.W. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc. Ther. 1999, 17, 160–178. [Google Scholar] [CrossRef]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Komatsu, H.; Enya, T.; Matsushima-Hibiya, Y.; Wakabayashi, K. Suppression by Flavonoids of Cyclooxygenase-2 Promoter-dependent Transcriptional Activity in Colon Cancer Cells: Structure-Activity Relationship. Cancer Sci. 2000, 91, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Rahman, M.U.; Farooq, A.; Rashid, S.M.; Ahmad, B.; Muzamil, S. Naringenin (4,5,7-trihydroxyflavanone) suppresses the development of precancerous lesions via controlling hyperproliferation and inflammation in the colon of Wistar rats. Environ. Toxicol. 2018, 33, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci. Biotechnol. Biochem. 1999, 63, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Lauthier, F.; Simon, A.; Fagnere, C.; Basly, J.P.; Delage, C.; Chulia, A.J. Flavonoids: Structural requirements for antiproliferative activity on breast cancer cells. Bioorg. Med. Chem. Lett. 2001, 11, 3095–3097. [Google Scholar] [CrossRef]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar] [PubMed]
- Iwashita, K.; Kobori, M.; Yamaki, K.; Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawabata, K.; Kakumoto, M.; Makita, H.; Hara, A.; Mori, H.; Kuki, W. Citrus auraptene inhibits chemically induced colonic aberrant crypt foci in male F344 rats. Carcinogenesis 1997, 18, 2155–2161. [Google Scholar] [CrossRef] [Green Version]
- Chandrika, B.B.; Steephan, M.; Kumar, T.R.S.; Sabu, A.; Haridas, M. Hesperetin and Naringenin sensitize HER2 positive cancer cells to death by serving as HER2 Tyrosine Kinase inhibitors. Life Sci. 2016, 1, 47–56. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef]
- Lin, J.K.; Chen, Y.C.; Huang, Y.T.; Lin-Shiau, S.Y. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J. Cell. Biochem. 1997, 28, 39–48. [Google Scholar] [CrossRef]
- Weber, G.; Shen, F.; Prajda, N.; Yang, H.; Li, W.; Yeh, A.; Look, K.Y. Regulation of the signal transduction program by drugs. Adv. Enzym. Regul. 1995, 37, 35–55. [Google Scholar] [CrossRef]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37 Pt 3, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2010, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Shen, S.C.; Lin, H.U. Rutinoside at C7 attenuates the apoptosis-inducing activity of flavonoids. Biochem. Pharmacol. 2003, 66, 1139–1150. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor α-palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef] [Green Version]
- Totta, P.; Acconcia, F.; Leone, S.; Cardillo, I.; Marino, M. Mechanisms of Naringenin-induced Apoptotic Cascade in Cancer Cells: Involvement of Estrogen Receptor a and ß Signalling. IUBMB 2004, 56, 491–499. [Google Scholar] [CrossRef]
- Bulzomi, P.; Bolli, Z.Z.; Galluzzo, P.; Leone, S.; Acconcia, F.; Marino, M. Naringenin and 17β-estradiol coadministration prevents hormone-induced human cancer cell growth. IUBMB 2010, 62, 51–60. [Google Scholar] [CrossRef]
- Morikawa, K.; Nonaka, M.; Mochizuki, H.; Handa, K.; Hanada, H.; Hirota, K. Naringenin and hesperetin induce growth arrest, apoptosis, and cytoplasmic fat deposit in human preadipocytes. J. Agric. Food Chem. 2008, 56, 11030–11037. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.-C.; Zavodnik, I.B. Flavonoids modulate liposomal membrane structure, regulate mitochondrial membrane permeability and prevent erythrocyte oxidative damage. Biochim. Biophys. Acta 2020, 1862, 183442. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Alitalo, K. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 1999, 5, 1359. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, F.; Ferrari, N.; De Flora, S.; Albini, A. Angioprevention’: Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Sarkar, A.; Kumar, A.; Ambasta, P.K.; Kumar, P. Combinatorial antitumor effect of naringenin and curcumin elicit angioinhibitory activities in vivo. Nutr. Cancer 2012, 64, 714–724. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, F.; Yang, M.; Zhao, J.; Zeng, W.; Fang, X.; Liang, W. Naringenin decreases invasiveness and metastasis by inhibiting TGF-β-induced epithelial to mesenchymal transition in pancreatic cancer cells. PLoS ONE 2012, 7, 50956. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, P.; Kumar, A.; Doble, M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. -Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.; Ortiz-Andrade, R.; Aguirre-Crespo, F.; Vergara-Galicia, J.; León-Rivera, I.; Montes, S.; Estrada-Soto, S. Hypoglycemic, vasorelaxant and hepatoprotective effects of Cochlospermum vitifolium (Willd.) Sprengel: A potential agent for the treatment of metabolic syndrome. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, S.; Anuradha, C.V. Naringenin enhances insulin-stimulated tyrosine phosphorylation and improves the cellular actions of insulin in a dietary model of metabolic syndrome. Eur. J. Nutr. 2010, 49, 101–109. [Google Scholar] [CrossRef]
- Mulvihill, E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Huff, M.H. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor–null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushotham, A.; Tian, M.; Belury, M. The citrus fruit flavonoid naringenin suppresses hepatic glucose production from Fao hepatoma cells. Mol. Nutr. Food Res. 2009, 53, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Silva, N.; Khanolkar, M.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. 2007, 292, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, H.C.; Kim, M.J.; Lee, J.M.; Choi, S.J.; Cho, H.Y.; Hong, B.; Shin, D.H. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement. Geriatr. Cogn. Disord. 2004, 17, 151–157. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 11, 3015–3025. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, W.; Oomagari, H.; Tsuruta, E.; Shida, M.; Kurokawa, M. Citrus flavonoid naringenin inhibits TLR2 expression in adipocytes. J. Nutr. Biochem. 2013, 24, 1276–1284. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, H.; Ishida, A.; Watanabe, W.; Narumi, K.; Atsumi, T.; Kurokawa, M. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochem. Biophys. Res. Commun. 2014, 454, 95–101. [Google Scholar] [CrossRef]
- Ghofrani, S.; Joghataei, M.T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borradaile, N.M.; de Dreu, L.E.; Huff, M.W. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 2003, 52, 2554–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, D.; Fonseca, V. From guideline to patient: A review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J. Diabetes Complicat. 2015, 29, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S. Advances in the management of diabetic peripheral neuropathy. Curr. Opin. Supportive Palliat. Care 2009, 3, 136–143. [Google Scholar] [CrossRef]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes/Metab. Res. Rev. 2012, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Fuseli, F. Role of naringenin in protection against diabetic hyperalgesia and tactile allodynia in male Wistar rats. J. Physiol. Biochem. 2014, 70, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jaggi, A.S.; Singh, N. Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol. Res. 2009, 59, 385–392. [Google Scholar] [CrossRef]
- Freshwater, J.D.; Svensson, C.I.; Malmberg, A.B.; Calcutt, N.A. Elevated spinal cyclooxygenase and prostaglandin release during hyperalgesia in diabetic rats. Diabetes 2002, 51, 2249–2255. [Google Scholar] [CrossRef]
- Rehman, K.; Khan, I.I.; Akash, M.; Jabeen, K.; Haider, K. Naringenin downregulates inflammation-mediated nitric oxide overproduction and potentiates endogenous antioxidant status during hyperglycemia. J. Food Biochem. 2020, e13422, advance online publication. [Google Scholar] [CrossRef]
- Chao, C.L.; Weng, C.S.; Chang, N.C.; Lin, J.S.; Kao, S.T.; Ho, F.M. Naringenin more effectively inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in macrophages than in microglia. Nutr. Res. 2010, 30, 858–864. [Google Scholar] [CrossRef]
- Gill, R.Q.; Sterling, R.K. Acute liver failure. J. Clin. Gastroenterol. 2001, 33, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Golli-Bennour, E.; Timoumi, R.; Annaibi, E.; Mokni, M.; Omezzine, A.; Bacha, H.; Abid-Essefi, S. Protective effects of kefir against deltamethrin-induced hepatotoxicity in rats. Envrion. Sci. Pollut. Res. Int. 2019, 26, 18856–18865. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Wu, M.Y.; Lin, H.C. Yin-Chen-Hao-Tang ameliorates obstruction-induced hepatic apoptosis in rats. J. Pharm. Pharmacol. 2007, 59, 583–590. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [Green Version]
- Hermenean, A.; Ardelean, A.; Stan, M.; Herman, H.; Mihali, C.V.; Costache, M.; Dinischiotu, A. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem. Biol. Interact. 2013, 205, 138–147. [Google Scholar] [CrossRef]
- Mershiba, S.D.; Dassprakash, M.V.; Saraswathy, S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 2013, 40, 3681–3691. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Rashmi, R.; Magesh, S.B.; Mohanram, R.K.; Suryanarayanan, S.; Venkata SubbaRao, M. Antioxidant Potential of Naringenin Helps to Protect Liver Tissue from Streptozotocin-Induced Damage. Rep. Biochem. Mol. Biol. 2018, 7, 76–84. [Google Scholar]
- Tauchen, J.; Huml, L.; Rimpelova, S.; Jurášek, M. Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work? Molecules 2020, 25, 3846. [Google Scholar] [CrossRef] [PubMed]
- Chetia, P.P.; Bala, A.; Khandelwal, B.; Haldar, P. Comparative in vitro free radical scavenging property of â-carotene and naringenin with respect to vitamin C and N-acetyl cysteine. Pharmacologia 2012, 3, 724–728. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Aquino, E.; Zarco, N.; Casas-Grajales, S.; Ramos-Tovar, E.; Flores-Beltrán, R.E.; Arauz, J.; Shibayama, M.; Favari, L.; Tsutsumi, V.; Segovia, J.; et al. Naringenin prevents experimental liver fibrosis by blocking TGFβ-Smad3 and JNK-Smad3 pathways. World J. Gastroenterol. 2017, 23, 4354–4368. [Google Scholar] [CrossRef]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Takamatsu, H.; Liu, S.; Kataoka, K.; Huh, N.H.; Sakaguchi, M. NRF2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE 2015, 10, e0142438. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharm. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Hermenean, A.; Ardelean, A.; Stan, M.; Hadaruga, N.; Mihali, C.V.; Costache, M.; Dinischiotu, A. Antioxidant and hepatoprotective effects of naringenin and its β-cyclodextrin formulation in mice intoxicated with carbon tetrachloride: A comparative study. J. Med. Food 2014, 17, 670–677. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Cham, T.M.; Lin, C.C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm. Res. 2007, 26, 893–902. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Li, Y.; Li, X. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: Promising phytomedicines for the treatment of liver diseases. Chin. Med. 2020, 15, 89. [Google Scholar] [CrossRef]
- Wu, J.; Kuncio, G.S.; Zern, M.A. Liver Growth Repair: From Basic Science to Clinical Practice; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Rajappa, R.; Sireesh, D.; Salai, M.B.; Ramkumar, K.M.; Sarvajayakesavulu, S.; Madhunapantula, S.V. Treatment with naringenin elevates the activity of transcription factor nrf2 to protect pancreatic β-cells from streptozotocin-induced diabetes in vitro and in vivo. Front. Pharm. 2019, 9, 1562. [Google Scholar] [CrossRef] [Green Version]
- Kegel, V.; Pfeiffer, E.; Burkhardt, B.; Liu, J.L.; Zeilinger, K.; Nüssler, A.K.; Seehofer, D.; Damm, G. Subtoxic Concentrations of Hepatotoxic Drugs Lead to Kupffer Cell Activation in a Human in Vitro Liver Model: An Approach to Study DILI. Mediat. Inflamm. 2015, 2015, 640631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, R.G. Cellular sources of extracellular matrix in hepatic fibrosis. Clin. Liver Dis. 2008, 2, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arriazu, E.; Ruiz de Galarreta, M.; Cubero, F.J.; Varela-Rey, M.; Pérez de Obanos, M.P.; Leung, T.M.; Lopategi, A.; Benedicto, A.; Abraham-Enachescu, I.; Nieto, N. Extracellular matrix and liver disease. Antioxid. Redox Signal. 2014, 21, 1078–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Chtourou, Y.; Fetoui, H.; Jemai, R.; Slima, A.B.; Makni, M.; Gdoura, R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur. J. Pharmacol. 2015, 746, 96–105. [Google Scholar] [CrossRef]
- Annadurai, T.; Thomas, P.; Geraldine, P. Ameliorative effect of naringenin on hyperglycemia-mediated inflammation in hepatic and pancreatic tissues of Wistar rats with streptozotocin-nicotinamide-induced experimental diabetes mellitus. Free Radic. Res. 2013, 47, 793–803. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar] [CrossRef]
- Drey, M.; Hasmann, S.E.; Krenovsky, J.P.; Hobert, M.A.; Straub, S.; Elshehabi, M.; Suenkel, U. Associations between Early Markers of Parkinson’s Disease and Sarcopenia. Front. Aging Neurosci. 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef]
- Tsai, T.H. Determination of naringin in rat blood, brain, liver, and bile using microdialysis and its interaction with cyclosporin a, a p-glycoprotein modulator. J. Agric. Food Chem. 2002, 50, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeline, M.S.; Sarkar, A.; Anand, K.; Ambasta, R.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef]
- Yang, W.; Ma, J.; Liu, Z.; Lu, Y.; Hu, B.; Yu, H. Effect of naringenin on brain insulin signaling and cognitive functions in ICV-STZ induced dementia model of rats. Neurol. Sci. 2014, 35, 741–751. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal SIRT1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Raza, S.; Khan, M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.; Safhi, M. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Chen, L.; Zhang, J.; Zhang, L.; Zhao, X.; Zhao, Y. Protective effect of naringenin in experimental ischemic stroke: Down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem. Res. 2014, 39, 1405–1415. [Google Scholar] [CrossRef]
- Chtourou, Y.; Fetoui, H.; Gdoura, R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol. Trace Elem. Res. 2014, 158, 376–383. [Google Scholar] [CrossRef]
- Khan, M.B.; Khan, M.M.; Khan, A.; Ahmed, M.E.; Ishrat, T.; Tabassum, R.; Islam, F. Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem. Int. 2012, 61, 1081–1093. [Google Scholar] [CrossRef]
- Liaquat, L.; Batool, Z.; Sadir, S.; Rafiq, S.; Shahzad, S.; Perveen, T.; Haider, S. Naringenin-induced enhanced antioxidant defence system meliorates cholinergic neurotransmission and consolidates memory in male rats. Life Sci. 2017, 194, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Bala, K.; Wani, A.; Makhdoomi, U.; Malik, F.; Sharma, A. Arginase purified from endophytic Pseudomonas aeruginosa IH2: Induce apoptosis through both cell cycle arrest and MMP loss in human leukemic HL-60 cells. Chem. Biol. Interact. 2017, 274, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.; Husain, I.; Sharma, A. Arginine deaminase from Pseudomonas aeruginosa PS2: Purification, biochemical characterization and in vitro evaluation of anticancer activity. 3 Biotech 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Nabavi, S.; Daglia, M.C.; Tenore, G.; Mansouri, K.; Nabavi, S. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Arafa, H.M.; Abd-Ellah, M.F.; Hafez, H.F. Abatement by naringenin of doxorubicin-induced cardiac toxicity in rats. J. Egypt Natl. Canc. Inst. 2015, 17, 291–300. [Google Scholar]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, F.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef]
- Xu, V.C.; Chen, J.; Zhang, J.; Hu, X.; Zhou, X.; Lu, Z.H. Naringenin inhibits angiotensin II-induced vascular smooth muscle cells proliferation and migration and decreases neointimal hyperplasia in balloon injured rat carotid arteries through suppressing oxidative stress. Biol. Pharm. Bull. 2013, 36, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.M.; Ma, H.J.; Guo, H.; Kong, Q.Q.; Zhang, Y. The cardioprotective effect of naringenin against ischemia–reperfusion injury through activation of ATP-sensitive potassium channel in rat. Can. J. Physiol. Pharmacol. 2016, 94, 973–978. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Marino, A.; Antongiovanni, V.; Ciregia, F.; Giusti, L.; Calderone, V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem. Pharmacol. 2013, 85, 1634–1643. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Matera, S.; Magnanti, M.; Pasquazzi, E.; Schifano, E.; Paroli, M. Effects of pink grapefruit juice on QT variability in patients with dilated or hypertensive cardiomyopathy and in healthy subjects. Transl. Res. 2008, 151, 267–272. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Yuan, Y.; Li, F.; Liu, Y.; Ma, Z.; Zhou, H. Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp. Ther. Med. 2015, 10, 2206–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Wu, K.; You, Q.; Huang, R.; Li, S. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int. J. Mol. Med. 2013, 32, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojžišová, G.; Šarišský, M.; Mirossay, L.; Martinka, P.; Mojžis, J. Effect of Flavonoids on Daunorubicin-induced Toxicity in H9c2 Cardiomyoblasts. Phytother. Res. 2009, 23, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Assini, S.B.G.; DiMattia, A.S.; Khami, M.; Koppes, J.B.; Huff, M.W. Naringenin decreases progression of atherosclerosis by improving dyslipidemia in highfatfed lowdensity lipoprotein receptornull mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetikno, V.; Arozal, W.; Louisa, M.; Setiabudy, R. New insight into the molecular drug target of diabetic nephropathy. Int. J. Endocrinol. 2014, 2014, 968681. [Google Scholar] [CrossRef] [PubMed]
- Arozal, W.; Watanabe, K.; Veeraveedu, P.T.; Thandavarayan, R.A.; Sukumaran, V.; Aizawa, Y. Telmisartan prevents the progression of renal injury in daunorubicin rats with the alteration of angiotensin II and endothelin-1 receptor expression associated with its PPAR-γ agonist actions. Toxicology 2011, 279, 91–99. [Google Scholar] [CrossRef]
- Esteban, V.; Lorenzo, O.; Rupérez, M.; Suzuki, Y.; Mezzano, S.; Blanco, J.; Ruiz-Ortega, M. Angiotensin II, via AT1 and AT2 receptors and NF-κB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2004, 15, 1514–1529. [Google Scholar] [CrossRef] [Green Version]
- Ozbek, E.; Ilbey, Y.O.; Ozbek, M.; Simsek, A.; Cekmen, M.; Somay, A. Melatonin attenuates unilateral ureteral obstruction–induced renal injury by reducing oxidative stress, iNOS, MAPK, and NF-kB expression. J. Endourol. 2009, 23, 1165–1173. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Nakamura, M. Naringenin ameliorates daunorubicin induced nephrotoxicity by mitigating AT1R, ERK1/2-NFκB p65 mediated inflammation. Int. Immunopharmacol. 2015, 28, 154–159. [Google Scholar] [CrossRef]
- Fouad, A.A.; Albuali, W.H.; Zahran, A.; Gomaa, W. Protective effect of naringenin against gentamicin-induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2014, 38, 420–429. [Google Scholar] [CrossRef]
- Badary, O.A.; Abdel-Maksoud, S.; Ahmed, W.A.; Owieda, G.H. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 2005, 76, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.H.A.; Huang, C.C.; Tsai, F.Y.; Lee, J.Y.C.; Cheng, S.M.; Chang, Y.C.; Chang, J.Y. Survivin–biology and potential as a therapeutic target in oncology. OncoTargets Ther. 2013, 6, 1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. Survivin knockdown increased anti-cancer effects of (−)-epigallocatechin-3-gallate in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Exp. Cell Res. 2012, 318, 1597–1610. [Google Scholar] [CrossRef] [Green Version]
- Orrego-Lagarón, N.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. High gastrointestinal permeability and local metabolism of naringenin: Influence of antibiotic treatment on absorption and metabolism. Br. J. Nutr. 2015, 114, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, Q.; Zhou, X.; Kolosov, V.; Perelman, J. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol. Cell. Biochem. 2011, 351, 29–40. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Manabe, N.; Wong, B.S.; Camilleri, M. New-generation 5-HT4 receptor agonists: Potential for treatment of gastrointestinal motility disorders. Expert Opin. Investig. Drugs 2010, 19, 765–775. [Google Scholar] [CrossRef]
- Peeters, T.L. Potential of ghrelin as a therapeutic approach for gastrointestinal motility disorders. Curr. Opin. Pharmacol. 2006, 6, 553–558. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.W.; Oh, J.; Hong, G.S.; Seo, E.K.; Oh, U.; Shim, W.S. Ghrelin receptor is activated by naringin and naringenin, constituents of a prokinetic agent Poncirus fructus. J. Ethnopharmacol. 2013, 148, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, F.; Guo, H.; Li, Y.; Tan, B.; Zhang, W.; Peng, Y. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumor Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, X.; Zhang, X.; Shang, D.; Zhou, Y.; Zhang, C. Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Exp. Ther. Med. 2016, 11, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.M.; Paudel, K.R.; Kim, D.W. Eriobotrya japonica leaf extract attenuates airway inflammation in ovalbumin-induced mice model of asthma. J. Ethnopharmacol. 2020, 253, 112082. [Google Scholar] [CrossRef]
- Gorniak, I.; Bartoszewski, R.; Kroliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Synthesis and Biological Activity of Novel O-Alkyl Derivatives of Naringenin and Their Oximes. Molecules 2017, 22, 1485. [Google Scholar] [CrossRef] [Green Version]
- Duda-Madej, A.; Kozłowska, J.; Krzyżek, P.; Anioł, M.; Seniuk, A.; Jermakow, K.; Dworniczek, E. Antimicrobial O-Alkyl Derivatives of Naringenin and Their Oximes Against Multidrug-Resistant Bacteria. Molecules 2020, 25, 3642. [Google Scholar] [CrossRef]
- Tran Trung, H.; Truong Thi Huynh, H.; Nguyen Thi Thuy, L.; Nguyen Van Minh, H.; Thi Nguyen, M.N.; Luong Thi, M.N. Growth-Inhibiting, Bactericidal, Antibiofilm, and Urease Inhibitory Activities of Hibiscus rosa sinensis L. Flower Constituents toward Antibiotic Sensitive- and Resistant-Strains of Helicobacter pylori. ACS Omega 2020, 5, 20080–20089. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. https://doi.org/10.3390/plants9121784
Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W, Khan R, Ahmad A, Aga SS, Alqahtani S, et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants. 2020; 9(12):1784. https://doi.org/10.3390/plants9121784
Chicago/Turabian StyleArafah, Azher, Muneeb U. Rehman, Tahir Maqbool Mir, Adil Farooq Wali, Rayeesa Ali, Wajhul Qamar, Rehan Khan, Ajaz Ahmad, Syed Sameer Aga, Saeed Alqahtani, and et al. 2020. "Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms" Plants 9, no. 12: 1784. https://doi.org/10.3390/plants9121784





