Allocation of Resources to Cyanogenic Glucosides Does Not Incur a Growth Sacrifice in Sorghum bicolor (L.) Moench
Abstract
:1. Introduction
2. Results and Discussion
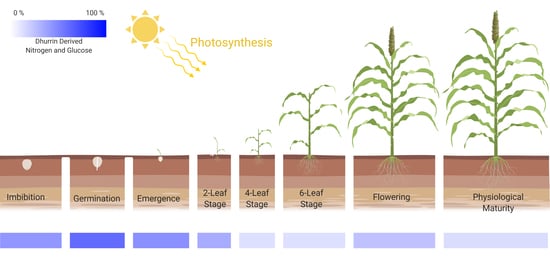
2.1. Role of Dhurrin in Seedling Development: Emergence, Plant Stage and Height
2.2. Effect of Gibberellic Acid (GA3) on Germination and Early Plant Growth
2.3. Role of Phytohormones in Dhurrin Biosynthesis
2.4. Role of Dhurrin in Reproductive Fitness
3. Implications for Function of Cyanogenesis in Defence and Growth
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. The Role of Dhurrin in Early Growth and Development of Sorghum
4.3. Role of Dhurrin on Sorghum Reproductive Fitness
4.4. Effect of GibberellicAacid (GA3) on Germination
4.5. Role of Hormones in the Biosynthesis of Dhurrin
4.6. Measurement of Cyanogenic Glucoside Concentrations
4.7. Data Measurements and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Böttger, A.; Vothknecht, U.; Bolle, C.; Wolf, A. Plant secondary metabolites and their general function in plants. In Lessons on Caffeine, Cannabis & Co-Plant-Derived Drugs and Their Interaction with Human Receptors; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. [Google Scholar]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Krothapalli, K.; Buescher, E.M.; Li, X.; Brown, E.; Chapple, C.; Dilkes, B.P.; Tuinstra, M.R. Forward genetics by genome sequencing reveals that rapid cyanide release deters insect herbivory of Sorghum bicolor. Genetics 2013, 195, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pičmanová, M.; Neilson, E.H.; Motawia, M.S.; Olsen, C.E.; Agerbirk, N.; Gray, C.J.; Flitsch, S.; Meier, S.; Silvestro, D.; Jørgensen, K. A recycling pathway for cyanogenic glycosides evidenced by the comparative metabolic profiling in three cyanogenic plant species. Biochem. J. 2015, 469, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Bjarnholt, N.; Neilson, E.H.J.; Crocoll, C.; Jorgensen, K.; Motawia, M.S.; Olsen, C.E.; Dixon, D.P.; Edwards, R.; Møller, B.L. Glutathione transferases catalyze recycling of auto-toxic cyanogenic glucosides in sorghum. Plant J. 2018, 94, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Busk, P.K.; Møller, B.L. Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol. 2002, 129, 1222–1231. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.E.; Gleadow, R.M.; Woodrow, I.E. Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia 2002, 133, 288–294. [Google Scholar] [CrossRef]
- Blomstedt, C.K.; O’Donnell, N.H.; Bjarnholt, N.; Neale, A.D.; Hamill, J.D.; Møller, B.L.; Gleadow, R.M. Metabolic consequences of knocking out UGT85B1, the gene encoding the glucosyltransferase required for synthesis of dhurrin in Sorghum bicolor (L. Moench). Plant Cell Physiol. 2016, 57, 373–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.E.; Gleadow, R.M.; Cavagnaro, T.R. Age versus stage: Does ontogeny modify the effect of phosphorus and arbuscular mycorrhizas on above-and below-ground defence in forage sorghum? Plant Cell Environ. 2014, 37, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Jenrich, R.; Trompetter, I.; Bak, S.; Olsen, C.E.; Møller, B.L.; Piotrowski, M. Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18848–18853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akazawa, T.; Miljanich, P.; Conn, E.E. Studies on cyanogenic glycoside of Sorghum vulgare. Plant Physiol. 1960, 35, 535. [Google Scholar] [CrossRef] [PubMed]
- McKey, D. The distribution of secondary compounds within plants. In Herbivores-Their Interaction with Secondary Plant Metabolites; Rosenthal, G.A., Berenbaum, M., Eds.; Academic Press: New York, NY, USA, 1979; pp. 55–134. [Google Scholar]
- Halkier, B.A.; Møller, B.L. Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol. 1989, 90, 1552–1559. [Google Scholar] [CrossRef] [Green Version]
- Adewusi, S.R. Turnover of dhurrin in green sorghum seedlings. Plant Physiol. 1990, 94, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.J.; Stuart, P.; Pičmanová, M.; Rasmussen, S.; Olsen, C.E.; Harholt, J.; Møller, B.L.; Bjarnholt, N. Dhurrin metabolism in the developing grain of Sorghum bicolor (L.) Moench investigated by metabolite profiling and novel clustering analyses of time-resolved transcriptomic data. BMC Genom. 2016, 17, 1021. [Google Scholar] [CrossRef] [Green Version]
- Montini, L.; Crocoll, C.; Gleadow, R.M.; Motawia, M.S.; Janfelt, C.; Bjarnholt, N. Matrix-assisted laser desorption/ionization-mass spectrometry imaging of metabolites during sorghum germination. Plant Physiol. 2020, 183, 925–942. [Google Scholar] [CrossRef]
- Jørgensen, K.; Bak, S.; Busk, P.K.; Sørensen, C.; Olsen, C.E.; Puonti-Kaerlas, J.; Møller, B.L. Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol. 2005, 139, 363–374. [Google Scholar]
- Blomstedt, C.K.; Gleadow, R.M.; O’Donnell, N.; Naur, P.; Jensen, K.; Laursen, T.; Olsen, C.E.; Stuart, P.; Hamill, J.D.; Møller, B.L. A combined biochemical screen and TILLING approach identifies mutations in Sorghum bicolor (L.) Moench resulting in acyanogenic forage production. Plant Biotechnol. J. 2012, 10, 54–66. [Google Scholar] [CrossRef]
- Jia, L.; Wu, Q.; Ye, N.; Liu, R.; Shi, L.; Xu, W.; Zhi, H.; Rahman, A.R.B.; Xia, Y.; Zhang, J. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana. J. Integr. Plant Biol. 2012, 54, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-G.; Sheng, Z.-W.; Xu, W.-F.; Li, Y.-X.; Liu, Y.-G.; Xia, Y.-J.; Zhang, J.-H. Modulation of anti-oxidation ability by proanthocyanidins during germination of Arabidopsis thaliana seeds. Mol. Plant 2012, 5, 472–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.-j.; Deng, X.-g.; Zou, L.-j.; Wu, J.-q.; Zhang, D.-w.; Lin, H.-h. Proanthocyanidins accelerate the germination of cucumber (Cucumis sativus L.) seeds. J. Plant Biol. 2016, 59, 143–151. [Google Scholar] [CrossRef]
- Yip, W.-K.; Yang, S.F. Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol. 1988, 88, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, S.; Taylorson, R. Promotion of seed germination by nitrates and cyanides. Nature 1972, 237, 169–170. [Google Scholar] [CrossRef]
- Major, W.; Roberts, E. Dormancy in cereal seeds: I. The effects of oxygen and respiratory inhibitors. J. Exp. Bot. 1968, 19, 77–89. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Bogatek, R.; Corbineau, F.; Bailly, C. Release of sunflower seed dormancy by cyanide: Cross-talk with ethylene signalling pathway. J. Exp. Bot. 2008, 59, 2241–2251. [Google Scholar] [CrossRef] [Green Version]
- Bethke, P.C.; Libourel, I.G.; Reinöhl, V.; Jones, R.L. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 2006, 223, 805–812. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [Green Version]
- Blomstedt, C.K.; Rosati, V.C.; Møller, B.L.; Gleadow, R. Counting the costs: Nitrogen partitioning in Sorghum mutants. Funct. Plant Biol. 2018, 45, 705–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, L.; Iwasaki, H. p-Hydroxyphenylacetic acid and other phenolic compounds as growth stimulators of the red alga Porphyra tenera. Plant Sci. Lett. 1976, 6, 299–307. [Google Scholar] [CrossRef]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012, 71, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A. The role of auxin in shaping shoot architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef] [Green Version]
- Ordonio, R.L.; Ito, Y.; Hatakeyama, A.; Ohmae-Shinohara, K.; Kasuga, S.; Tokunaga, T.; Mizuno, H.; Kitano, H.; Matsuoka, M.; Sazuka, T. Gibberellin deficiency pleiotropically induces culm bending in sorghum: An insight into sorghum semi-dwarf breeding. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: The role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Rosati, V.C.; Quinn, A.A.; Fromhold, S.M.; Gleadow, R.; Blomstedt, C.K. Investigation into the role of DNA methylation in cyanogenesis in sorghum (Sorghum bicolor (L.) Moench). Plant Growth Regul. 2019, 88, 73–85. [Google Scholar] [CrossRef]
- Gleadow, R.; Ottman, M.; Kimball, B. Drought–induced changes in nitrogen partitioning in sorghum are not moderated by elevated CO2 in FACE studies. Field Crop. Res. 2016, 185, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Shehab, A.; Yao, L.; Wei, L.; Wang, D.; Li, Y.; Zhang, X.; Guo, Y. The increased hydrocyanic acid in drought-stressed sorghums could be alleviated by plant growth regulators. Crop Pasture Sci. 2020, 71, 459–468. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, N.H.; Møller, B.L.; Neale, A.D.; Hamill, J.D.; Blomstedt, C.K.; Gleadow, R.M. Effects of PEG-induced osmotic stress on growth and dhurrin levels of forage sorghum. Plant Physiol. Biochem. 2013, 73, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, K.; Chen, C.; Wu, Y.; Tang, Y.; Georgiev, M.I.; Zhang, X.; Lin, M.; Zhou, M. Biosynthesis and regulation of cyanogenic glycoside production in forage plants. Appl. Microbiol. Biotechnol. 2018, 102, 9–16. [Google Scholar] [CrossRef]
- Kautz, S.; Trisel, J.A.; Ballhorn, D.J. Jasmonic acid enhances plant cyanogenesis and resistance to herbivory in lima bean. J. Chem. Ecol. 2014, 40, 1186–1196. [Google Scholar] [CrossRef]
- Burke, J.J.; Chen, J.; Burow, G.; Mechref, Y.; Rosenow, D.; Payton, P.; Xin, Z.; Hayes, C.M. Leaf dhurrin content is a quantitative measure of the level of pre-and postflowering drought tolerance in sorghum. Crop Sci. 2013, 53, 1056–1065. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Aravind, J.; Vimala, D.; Radharani, J.; Jacob, S.; Srinivasa, K. The Germinationmetrics Package: A Brief Introduction; ICAR-National Bureau of Plant Genetic Resources: New Delhi, India, 2019. [Google Scholar]
- Gleadow, R.; Bjarnholt, N.; Jørgensen, K.; Fox, J.; Miller, R. Detection, identification and quantitative measurement of cyanogenic glycosides. Chapter 12. In Research Methods in Plant Science: Soil Allelochemicals; Narwal, S.S., Szajdak, L., Sampietro, D.A., Eds.; International Allelopathy Foundation: Rohtak, India; Studium Press: Houston, TX, USA, 2012; Volume 1, pp. 283–310. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Statistics, I.S. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp: New York, NY, USA, 2017. [Google Scholar]






| Genotype | Seed Mass (mg) | Embryo Mass (mg) | Flowering (DAP) | Leaf Number at Flowering | |
|---|---|---|---|---|---|
| Elite | 18.0 ± 0.3 a | 0.26 | 91 ± 1 a | 12.4 ± 0.1 b | |
| tcd1 | 19.8 ± 1.3 b | 0.31 | 103 ± 1 c | 12.4 ± 0.3 b | |
| TCD1 | 20.1 ± 0.9 c | 0.42 | 97 ± 2 b | 11.9 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, M.N.; Blomstedt, C.K.; Gleadow, R.M. Allocation of Resources to Cyanogenic Glucosides Does Not Incur a Growth Sacrifice in Sorghum bicolor (L.) Moench. Plants 2020, 9, 1791. https://doi.org/10.3390/plants9121791
Sohail MN, Blomstedt CK, Gleadow RM. Allocation of Resources to Cyanogenic Glucosides Does Not Incur a Growth Sacrifice in Sorghum bicolor (L.) Moench. Plants. 2020; 9(12):1791. https://doi.org/10.3390/plants9121791
Chicago/Turabian StyleSohail, Muhammad N., Cecilia K. Blomstedt, and Roslyn M. Gleadow. 2020. "Allocation of Resources to Cyanogenic Glucosides Does Not Incur a Growth Sacrifice in Sorghum bicolor (L.) Moench" Plants 9, no. 12: 1791. https://doi.org/10.3390/plants9121791