Formation of Annual Ring Eccentricity in Coarse Roots within the Root Cage of Pinus ponderosa Growing on Slopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Tree Sampling
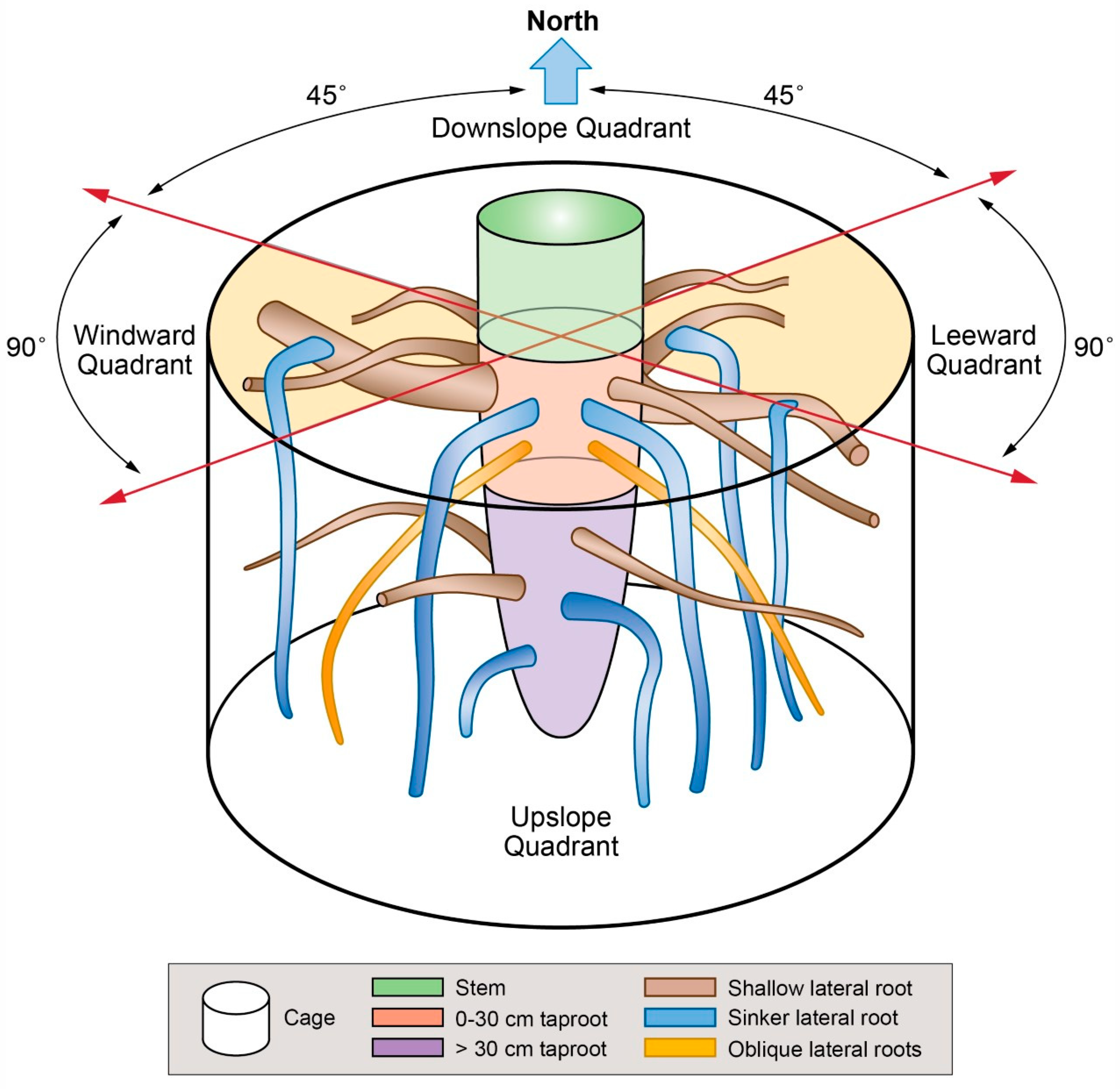
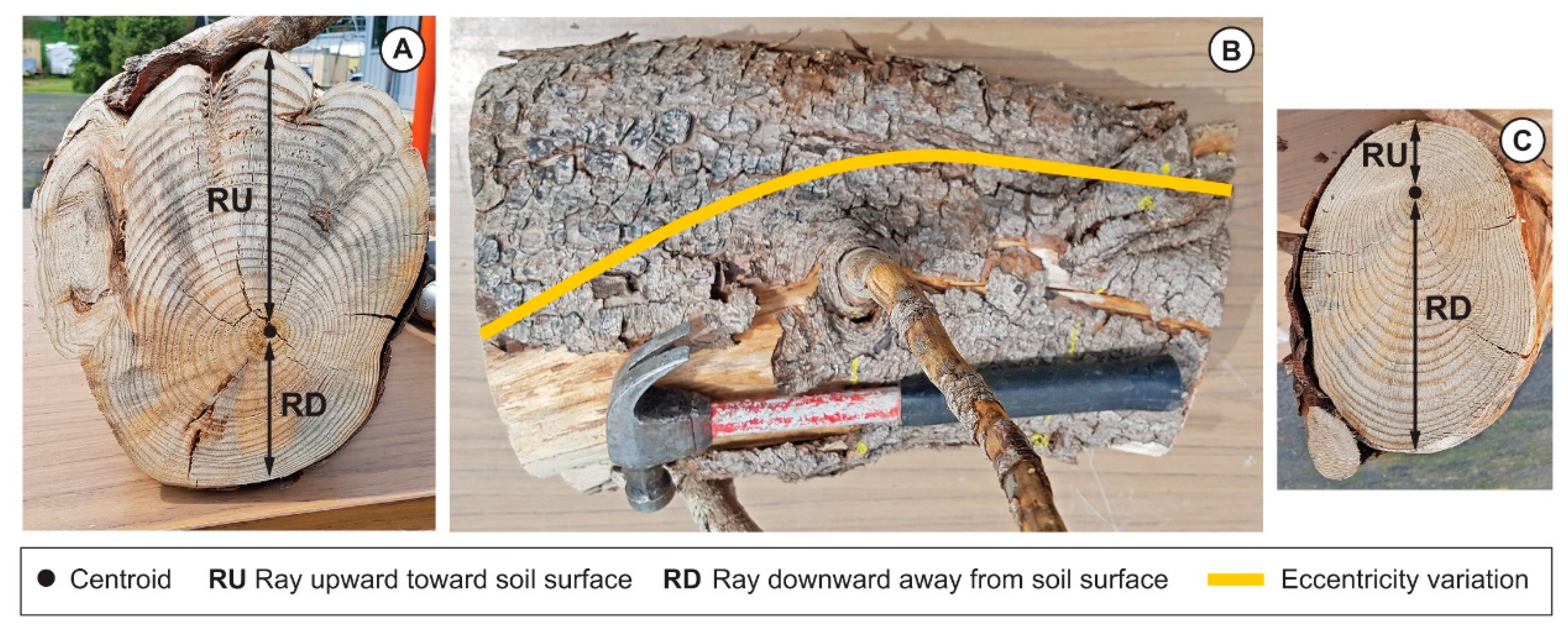
2.2. 3-Dimensional Analysis and Root Cage Definition
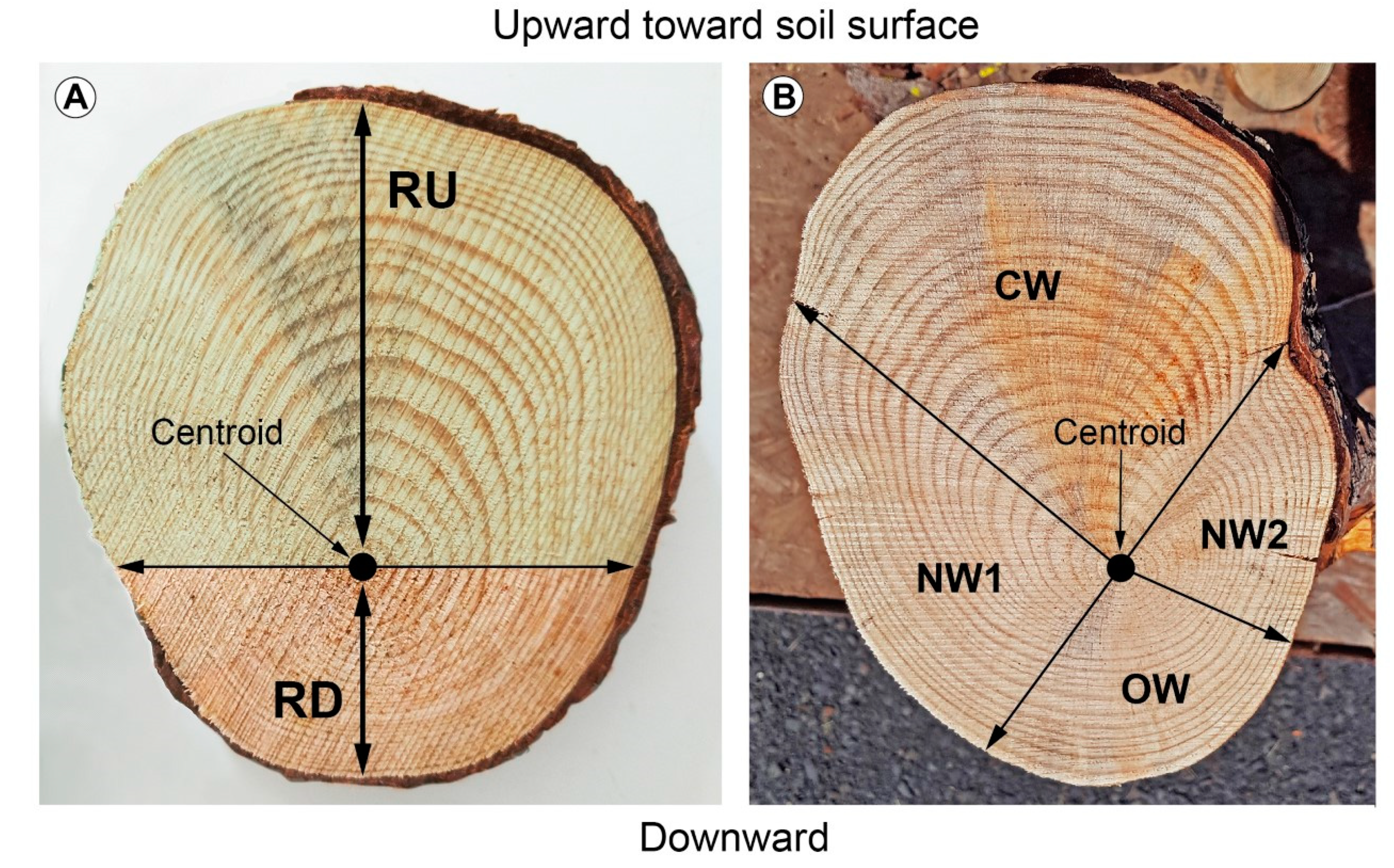
2.3. Root Volume, Radius Length, and Cross-Sectional Area (CSA) Measurements
2.4. Modelling
2.5. Statistical Analysis
3. Results
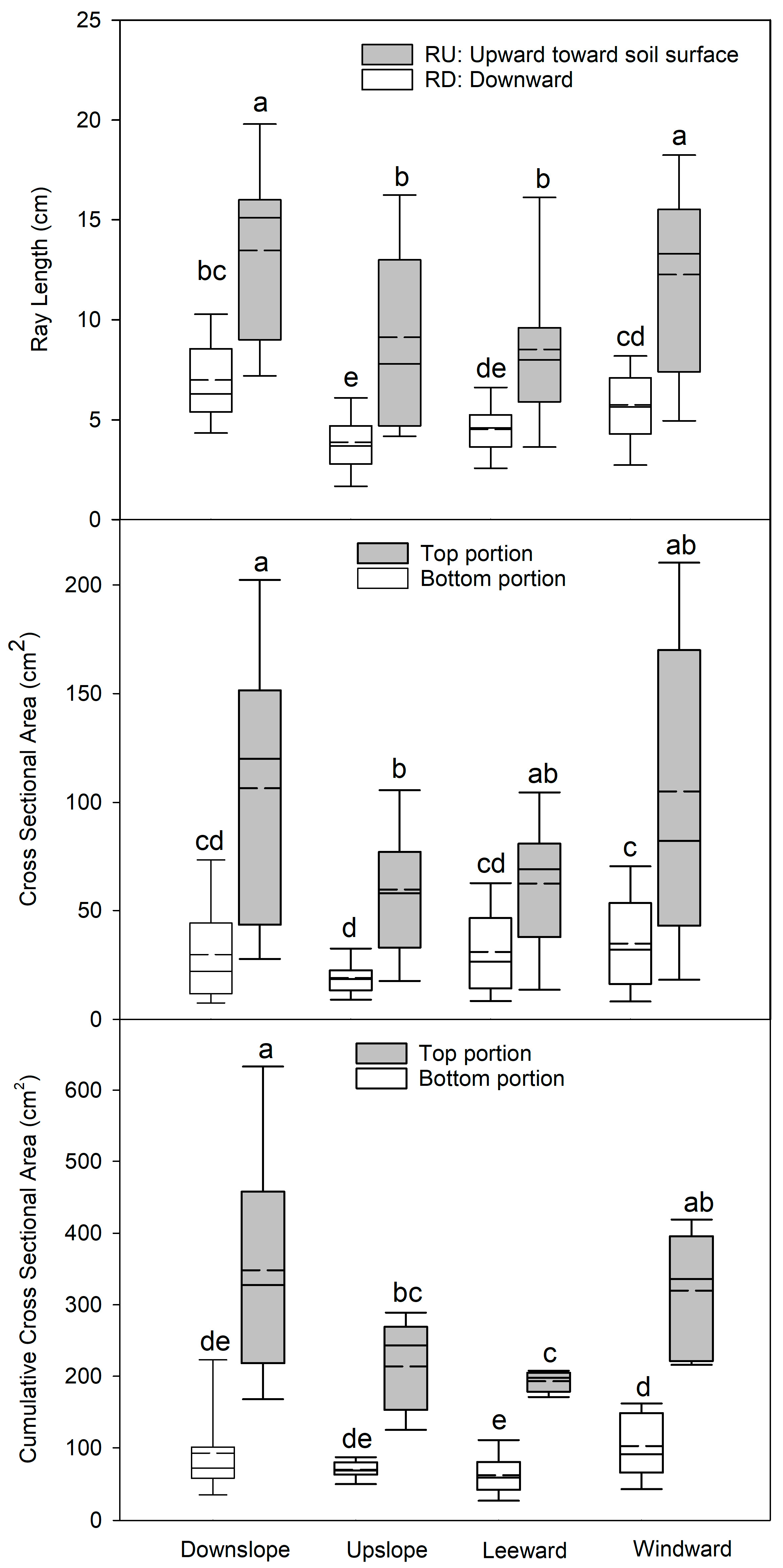
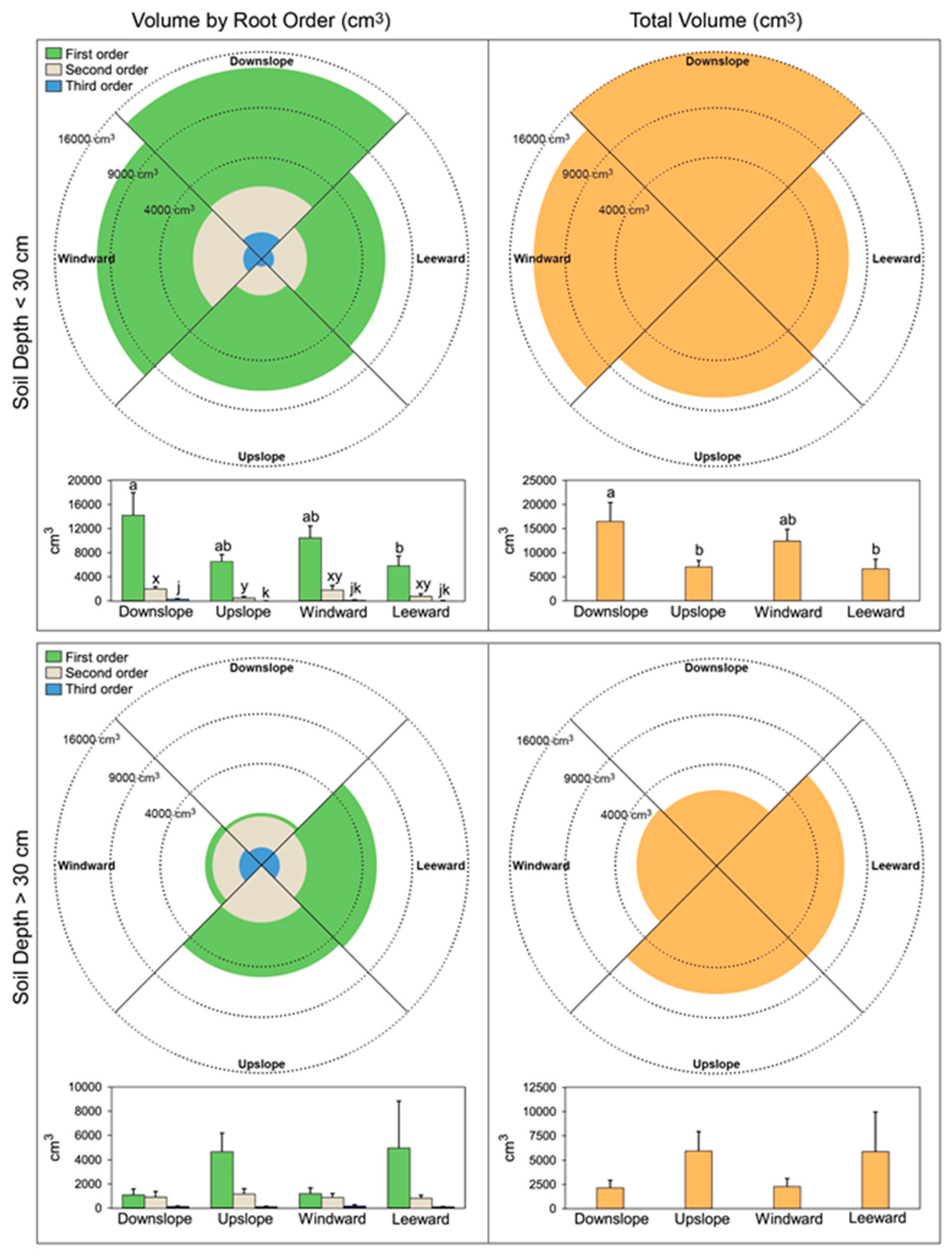
3.1. Radius Length, CSA, and Lateral Root Volume
3.2. Variation of the Eccentricity of the Annual Rings
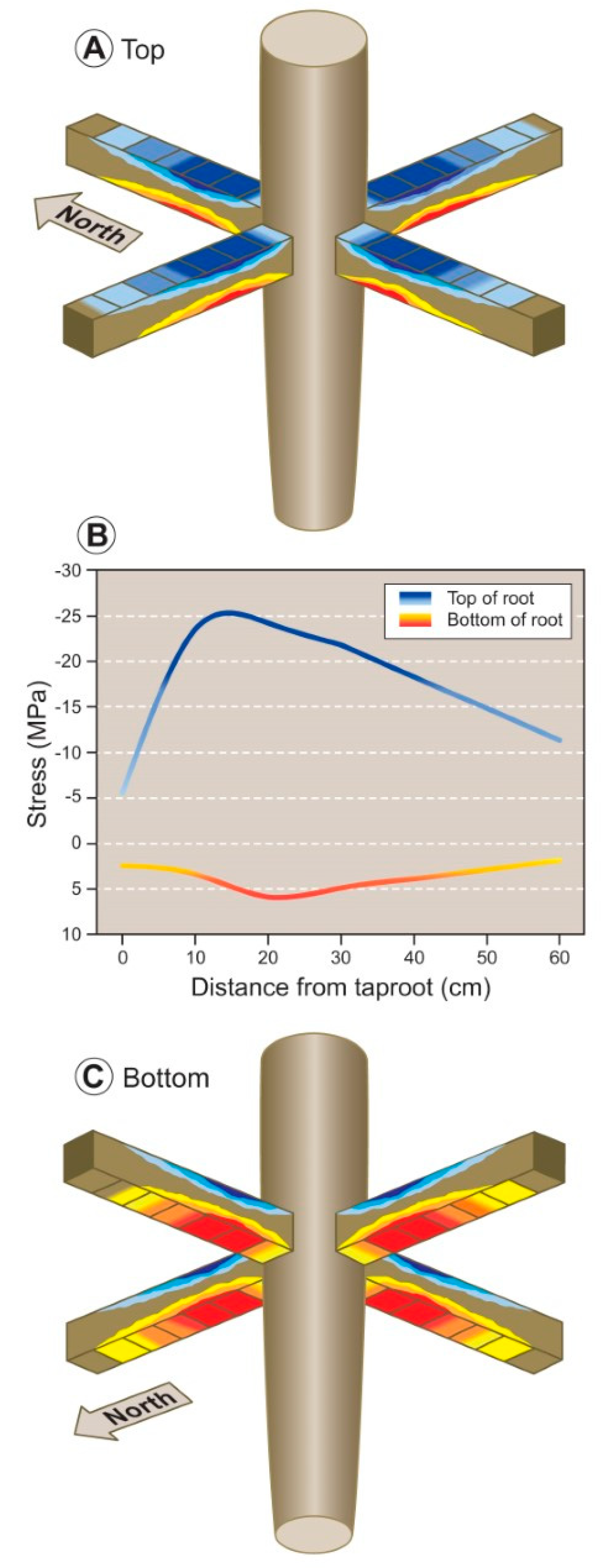
3.3. Modelling Mechanical Forces
4. Discussion
4.1. Root System Architecture and Root Cage
4.2. Mechanical Forces and Annual Ring Eccentricity
4.3. Modeling of Mechanical Forces within the Root Cage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seidl, R.; Dominik, T.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduces species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Nabuurs, G.J.; Masera, O.; Andrasko, K.; Benitez-Ponce, P.; Boer, R.; Dutschke, M.; Elsiddig, E.; Ford-Robertson, J.; Frumhoff, P.; Karjalainen, T.; et al. Forestry. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Masiero, M.; Pettenella, D.; Boscolo, M.; Barua, S.K.; Animon, I.; Matta, J.R. Valuing Forest Ecosystem Services: A Training Manual for Planners and Project Developers; Forestry Working Paper No. 11; FAO: Rome, Italy, 2019; p. 216. [Google Scholar]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Pinto, J.R.; Fulgaro, N.; Scippa, G.S.; Chiatante, D. Tree seedling response to LED spectra: Implications for forest restoration. Plant Biosyst. 2018, 3, 515–523. [Google Scholar] [CrossRef]
- Telewski, F.W.; Moore, J.R. Trait selection to improve windfirmness in trees. CAB Rev. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Brown, P.; Pröbstl-Haider, U.; Koch, N.E. Social and political aspects of sustainable forestry. In Reference Module in Earth Systems and Environmental Sciences; Elsevier Inc.: Cambridge, MA, USA, 2016. [Google Scholar]
- Cameron, A.D. Importance of early selective thinning in the development of long-term stand stability and improved log quality: A review. Forestry 2002, 75, 25–35. [Google Scholar] [CrossRef]
- Lindgren, D. The role of tree breeding in reforestation. Reforesta 2016, 1, 221–237. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Lasserre, B.; Montagnoli, A. Functional traits of Pinus ponderosa coarse-roots in response to slope conditions. Front. Plant Sci. 2019, 10, 947. [Google Scholar] [CrossRef]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Onelli, E.; Scippa, G.S.; Chiatante, D. Seasonality of fine root dynamics and activity of root and shoot vascular cambium in a Quercus ilex L. forest (Italy). For. Ecol. Manag. 2019, 431, 26–34. [Google Scholar] [CrossRef]
- Terzaghi, M.; Di Iorio, A.; Montagnoli, A.; Baesso, A.; Scippa, G.S.; Chiatante, D. Forest canopy reduction stimulates xylem production and lowers carbon concentration in fine roots of European beech. For. Ecol. Manag. 2016, 379, 81–90. [Google Scholar] [CrossRef]
- Dupuy, L.; Gregory, P.J.; Bengough, G. Root growth models: Towards a new generation of continuous approaches. J. Exp. Bot. 2010, 61, 2131–2143. [Google Scholar] [CrossRef]
- Stokes, A.; Mattheck, C. Variation of wood strength in tree roots. J. Exp. Bot. 1996, 47, 693–699. [Google Scholar] [CrossRef]
- Ennos, A.R.; Fitter, A.H. Comparative functional morphology of the anchorage systems of annual dicots. Funct. Ecol. 1992, 6, 71–78. [Google Scholar] [CrossRef]
- Ennos, A.R. The mechanics of root anchorage. Adv. Bot. Res. 2000, 33, 133–157. [Google Scholar]
- Mattheck, C. Trees. In The Mechanical Design; Springer: Berlin/Heidelberg, Germany, 1991; p. 121. [Google Scholar]
- Busgen, M.; Munch, E.; Thomson, T. The Structure and Life of Forest Trees; Chapman and Hall: London, UK, 1929. [Google Scholar]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root phenotyping for drought tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Canales, F.J.; Nagel, K.A.; Müller, C.; Rispail, N.; Prats, E. Deciphering root architectural traits involved to cope with water deficit in Oat. Front. Plant Sci. 2019, 10, 1558. [Google Scholar] [CrossRef]
- Fromm, H. Root plasticity in the pursuit of water. Plants 2019, 8, 236. [Google Scholar] [CrossRef]
- Di Iorio, A.; Lasserre, B.; Scippa, G.S.; Chiatante, D. Root system of Quercus pubescens trees growing on different sloping conditions. Ann. Bot. 2005, 95, 351–361. [Google Scholar] [CrossRef]
- Mairhofer, S.; Zappala, S.; Tracy, S.; Sturrock, C.; Bennett, M.; Mooney, S.J.; Pridmore, T. RooTrak: Automated recovery of three-dimensional plant root architecture in soil from X-ray microcomputed tomography using visual tracking. Plant Physiol. 2011, 158, 561–569. [Google Scholar] [CrossRef]
- Yang, M.; Défossez, P.; Danjon, F.; Fourcaud, T. Tree stability under wind: Simulating uprooting with root breakage using a finite element method. Ann. Bot. 2014, 114, 695–709. [Google Scholar] [CrossRef]
- Yang, M.; Défossez, P.; Danjon, F.; Dupont, S.; Fourcaud, T. Which root architectural elements contribute the best to anchorage of Pinus species? Insights from in silico experiments. Plant Soil 2016, 411, 275–291. [Google Scholar] [CrossRef]
- Danjon, F.; Fourcaud, T.; Bert, D. Root architecture and wind-firmness of mature Pinus pinaster. New Phytol. 2005, 168, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. B 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Front. Funct. Plant Ecol. 2013, 4, 344–355. [Google Scholar]
- Felten, J.; Sundberg, B. Biology, chemistry and structure of tension wood. In Cellular Aspects of Wood Formation; Fromm, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 203–224. [Google Scholar]
- Groover, A. Gravitropisms and reaction woods of forest trees—Evolution, functions and mechanisms. New Phytol. 2016, 211, 790–802. [Google Scholar] [CrossRef]
- Scurfield, G. Reaction wood: Its structure and function. Science 1973, 179, 647–655. [Google Scholar]
- Westing, A.H. Formation and function of compression wood in Gymnosperms. Bot. Rev. 1968, 34, 51–78. [Google Scholar] [CrossRef]
- Timell, T.E. Compression Wood in Gymnosperms; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Fayle, D.C.F. Extension and longitudinal growth during the development of red pine root systems. Can. J. For. Res. 1975, 5, 109–121. [Google Scholar] [CrossRef]
- Fayle, D.C.F. Distribution of radial growth during the development of red pine root systems. Can. J. For. Res. 1975, 5, 608–625. [Google Scholar] [CrossRef]
- Fayle, D.C.F. Stem sway affects ring width and compression wood formation in exposed root bases. For. Sci. 1976, 22, 193–194. [Google Scholar]
- Fayle, D.C.F. Radial growth in tree roots. In Faculty of Forestry; Technical Report No. 9; University: Toronto, ON, Canada, 1968; p. 183. [Google Scholar]
- Nicoll, B.C.; Dunn, A.J. The effects of wind speed and direction on radial growth of structural roots. In The Supporting Roots of Trees and Woody Plants: Form, Function and Physiology; Stokes, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 219–225. [Google Scholar]
- Creber, G.T.; Chaloner, W.G. Influence of environmental factors on the wood structure of living and fossil trees. Bot. Rev. 1984, 50, 357–448. [Google Scholar] [CrossRef]
- Donaldson, L.; Singh, A.P. Reaction wood. In Secondary Xylem Biology; Kim, Y.S., Ed.; Elsevier: Oxford, UK, 2016; pp. 93–110. [Google Scholar]
- Fischer, U.; Kucukoglu, M.; Heeliarutta, Y.; Bhalerao, R.P. The dynamics of cambial stem cell activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Moulia, B.; Coutand, C.; Lenne, C. Posture control and skeletal mechanical acclimation in terrestrial plants: Implications for mechanical modeling of plant architecture. Am. J. Bot. 2006, 93, 1477–1489. [Google Scholar] [CrossRef]
- Moulia, B.; Fournier, M. The power and control of gravitropic movements in plants: A biomechanical and system biology view. J. Exp. Bot. 2009, 60, 461–486. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Lasserre, B.; Dumroese, R.K. Ongoing modifications to root system architecture of Pinus ponderosa growing on a sloped site revealed by tree-ring analysis. Dendrochronologia 2019, 58, 125650. [Google Scholar] [CrossRef]
- Soil Survey Staff, 2013. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/V/VASSAR.html (accessed on 24 January 2019).
- Western Regional Climate Center, 2019. Available online: https://wrcc.dri.edu (accessed on 1 October 2019).
- Godin, C.; Caraglio, Y. A multiscale model of plant topological structures. J. Theor. Biol. 1998, 191, 1–46. [Google Scholar] [CrossRef]
- Danjon, F.; Khuder, H.; Stokes, A. Deep phenotyping of coarse root architecture in R. pseudoacacia reveals that tree root system plasticity is confined within its architectural model. PLoS ONE 2013, 8, e83548. [Google Scholar] [CrossRef]
- Zobel, R.W.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- Godin, C.; Costes, E.; Caraglio, Y. Exploring plant topological structure with the AMAPmod software: An outline. Silva Fenn. 1997, 31, 357–368. [Google Scholar] [CrossRef][Green Version]
- Bräuning, A.; De Ridder, M.; Zafirov, N.; García-González, I.; Dimitrov, D.P.; Gartner, H. Tree-ring features: Indicators of extreme event impacts. IAWA J. 2016, 37, 216–231. [Google Scholar] [CrossRef]
- Mecway. 2014 Manual. Mecway Finite Element Analysis. Available online: https://www.scribd.com/document/368043994/MecWay-Tutorials-pdf (accessed on 20 January 2020).
- Fourcaud, T.; Ji, J.N.; Zhang, Z.Q.; Stokes, A. Understanding the impact of root morphology on overturning mechanisms: A modelling approach. Ann. Bot. 2008, 101, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy Prealps: Possible implications of coppice conversion to high forest. Plant Biosyst. 2012, 146, 1012–1022. [Google Scholar] [CrossRef]
- Montagnoli, A.; Di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.S.; Chiatante, D. Influence of soil temperature and water content on fine root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef]
- Chiatante, D.; Scippa, G.S.; Di Iorio, A.; Sarnataro, M. The influence of steep slopes on root system development. J. Plant Growth Regul. 2003, 21, 247–260. [Google Scholar] [CrossRef]
- Di Zio, E.; Trupiano, D.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Grosso, A.; Marra, M.; Scaloni, A.; Scippa, G.S. Poplar woody taproot under bending stress: The asymmetric response of the convex and concave sides. Ann. Bot. 2016, 118, 865–883. [Google Scholar] [CrossRef]
- Chiatante, D.; Sarnataro, M.; Fusco, S.; Di Iorio, A.; Scippa, G.S. Modification of root morphological parameters and root architecture in seedlings of Fraxinus ornus L. and Spartium junceum L. growing on slopes. Plant Biosyst. 2003, 137, 47–56. [Google Scholar] [CrossRef]
- Lombardi, F.; Scippa, G.S.; Lasserre, B.; Montagnoli, B.; Tognetti, R.; Marchetti, M.; Chiatante, D. The infuence of slope on Spartium junceum root system: Morphological, anatomical and biomechanical adaptation. J. Plant Res. 2017, 130, 515–525. [Google Scholar] [CrossRef]
- Danjon, F.; Reubens, B. Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil 2008, 303, 1–34. [Google Scholar] [CrossRef]
- Coutts, M.P. Root architecture and tree stability. Plant Soil 1983, 71, 171–188. [Google Scholar] [CrossRef]
- Wu, T.H. Investigation of landslide on Prince of Wales Island Alaska. Department of Civil Engineering, Ohio State University, Columbus. Geotech. Eng. Rpt. 1976, N5, 93. [Google Scholar]
- Ennos, A.R. The scaling of root anchorage. J. Theor. Biol. 1993, 161, 61–75. [Google Scholar] [CrossRef]
- Bodoque, J.M.; Diez-Herreo, A.; Martin-Duque, J.F.; Rubiales, J.M.; Godfrey, A.; Pedraza, J.; Carrasco, R.M.; Sanz, M.A. Sheet erosion determined by using dendrogeomorphological analysis of exposed tree root: Two examples from central Spain. Catena 2005, 64, 81–102. [Google Scholar] [CrossRef]
- Cannell, M.; Courts, M.P. Growing in the wind. New Sci. 1988, 21, 42–46. [Google Scholar]
- Mattheck, C.; Breloer, H. Der wurzelquerschnitt als protokoll der lastgeschichte. (Root cross-sections tell the load history). Allg. Forst. Jagdztg. 1992, 163, 142–145. [Google Scholar]
- Stokes, A. Responses of Young Trees to Wind: Effects on Root Architecture and Anchorage Strength. Ph.D. Thesis, University of York, Heslington, UK, 1994. [Google Scholar]
- Mattheck, C.; Breloer, H. The Body Language of Trees—A Handbook of Failure Analysis; HMSO: London, UK, 1996. [Google Scholar]
- Duncker, P.; Spiecker, H. Cross-sectional compression wood distribution and its relation to eccentric radial growth in Picea abies [L.] Karst. Dendrochronologia 2008, 26, 195–202. [Google Scholar] [CrossRef]
- Duncker, P.; Spiecker, H. Detection and classification of Norway spruce compression wood in reflected light by means of hyperspectral image analysis. IAWA J. 2009, 30, 59–70. [Google Scholar] [CrossRef]
- Yumoto, M.; Ishida, S.; Fukazawa, K. Studies on the formation and structure of the compression wood cells induced by artificial inclination in young trees of Picea glauca. IV. Gradation of the severity of compression wood tracheids. Res. Bull. Coll. Exp. For. Hokkaido Univ. 1983, 40, 409–454. [Google Scholar]
- Kim, Y.S.; Funada, R.; Adya, P. Secondary Xylem Biology: Origins, Functions, and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Telewski, F.W. Intra-annual spiral compression wood: A record of low frequency gravitropic circumnutational movement in trees. IAWA Bull. 1988, 9, 269–274. [Google Scholar] [CrossRef]
- Low, A.J. Compression wood in conifers. A review of literature. For. Abstr. 1964, 25, 35–43. [Google Scholar]

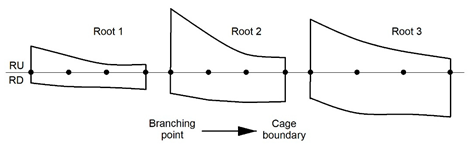 | ||||||
| Location | Root 1 | Root 2 | Root 3 | |||
| RD | RU | RD | RU | RD | RU | |
| Branching point | 3.8 | 9.4 | 7.5 | 22.8 | 9.2 | 19.0 |
| Intermediate 1 | 5.1 | 5.9 | 9.9 | 13.6 | 14.6 | 11.7 |
| Intermediate 2 | 5.4 | 2.9 | 10.8 | 6.5 | 15.0 | 7.5 |
| Cage boundary | 6.1 | 2.8 | 10.5 | 5.3 | 16.0 | 4.9 |
| Quadrant | ||||
|---|---|---|---|---|
| Upslope | Downslope | Windward | Leeward | |
| Top: Toward soil surface | −17.08 ± 6.92 | −17.10 ± 6.92 | −17.09 ± 6.92 | −17.09 ± 6.92 |
| >0.0001 | >0.0001 | >0.0001 | ||
| >0.0001 | >0.0001 | |||
| 0.0714 | ||||
| Bottom: Away from soil surface | 3.60 ± 1.41 | 3.60 ± 1.41 | 3.60 ± 1.42 | 3.60 ± 1.41 |
| 0.1655 | 0.0045 | 0.0005 | ||
| 0.0001 | 0.0046 | |||
| 0.0004 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagnoli, A.; Lasserre, B.; Sferra, G.; Chiatante, D.; Scippa, G.S.; Terzaghi, M.; Dumroese, R.K. Formation of Annual Ring Eccentricity in Coarse Roots within the Root Cage of Pinus ponderosa Growing on Slopes. Plants 2020, 9, 181. https://doi.org/10.3390/plants9020181
Montagnoli A, Lasserre B, Sferra G, Chiatante D, Scippa GS, Terzaghi M, Dumroese RK. Formation of Annual Ring Eccentricity in Coarse Roots within the Root Cage of Pinus ponderosa Growing on Slopes. Plants. 2020; 9(2):181. https://doi.org/10.3390/plants9020181
Chicago/Turabian StyleMontagnoli, Antonio, Bruno Lasserre, Gabriella Sferra, Donato Chiatante, Gabriella Stefania Scippa, Mattia Terzaghi, and R. Kasten Dumroese. 2020. "Formation of Annual Ring Eccentricity in Coarse Roots within the Root Cage of Pinus ponderosa Growing on Slopes" Plants 9, no. 2: 181. https://doi.org/10.3390/plants9020181
APA StyleMontagnoli, A., Lasserre, B., Sferra, G., Chiatante, D., Scippa, G. S., Terzaghi, M., & Dumroese, R. K. (2020). Formation of Annual Ring Eccentricity in Coarse Roots within the Root Cage of Pinus ponderosa Growing on Slopes. Plants, 9(2), 181. https://doi.org/10.3390/plants9020181








