Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense
Abstract
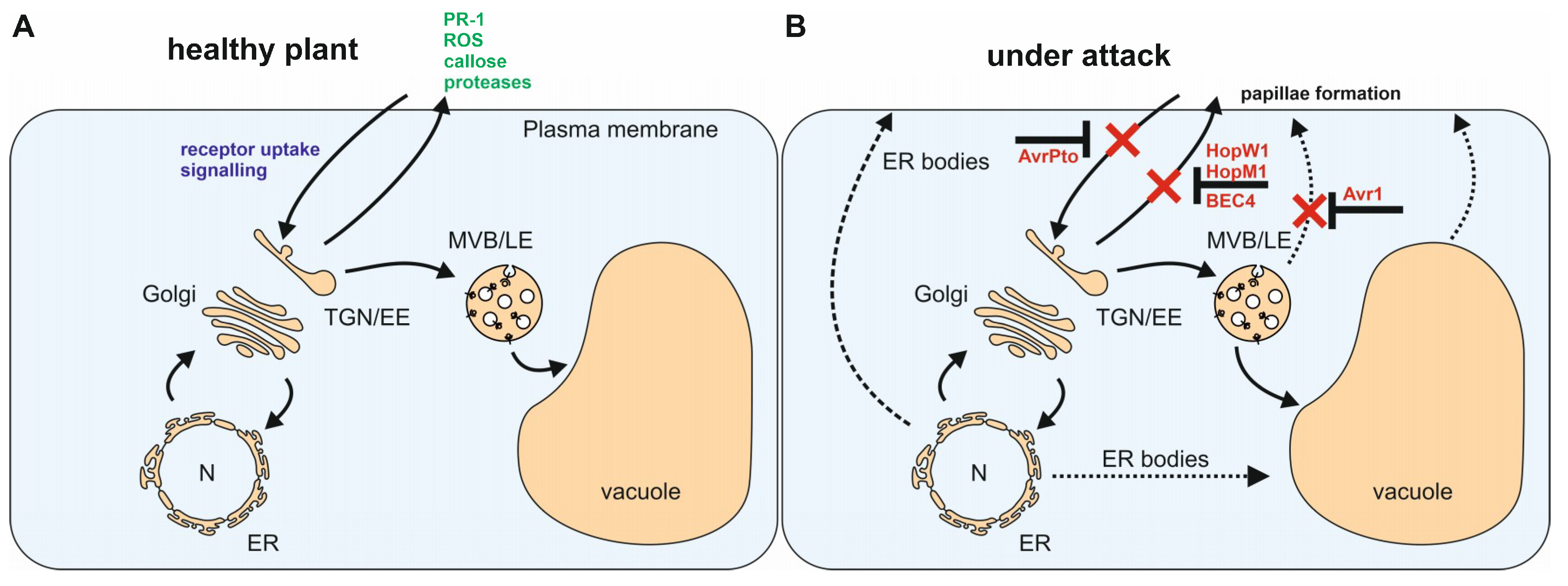
:1. Introduction
2. Endoplasmic Reticulum
3. The Golgi Apparatus and the Trans-Golgi Network/Early Endosome (TGN/EE)
4. The Multivesicular Body/Late Endosome
5. The Vacuole
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Emonet, A.; Dénervaud Tendon, V.; Marhavý, P.; Wu, D.; Lahaye, T.; Geldner, N. Co-incidence of Damage and Microbial Patterns Controls Localized Immune Responses in Roots. Cell 2020, 180, 440–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Panstruga, R. Tete a tete inside a plant cell: Establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol. 2006, 171, 699–718. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Thomma, B.P.; Nurnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Costa, G.G.; Thomazella, D.P.; Teixeira, P.J.; Carazzolle, M.F.; Schuster, S.C.; Carlson, J.E.; Guiltinan, M.J.; Mieczkowski, P.; Farmer, A.; et al. Genome and secretome analysis of the hemibiotrophic fungal pathogen, Moniliophthora roreri, which causes frosty pod rot disease of cacao: Mechanisms of the biotrophic and necrotrophic phases. BMC Genom. 2014, 15, 164. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, A.P.; Vega-Arreguín, J.C.; Fei, Z.; Ponnala, L.; Lee, S.J.; Matas, A.J.; Patev, S.; Fry, W.E.; Rose, J.K. Transcriptional dynamics of Phytophthora infestans during sequential stages of hemibiotrophic infection of tomato. Mol. Plant Pathol. 2016, 17, 29–41. [Google Scholar] [CrossRef]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O'Connell, R.J.; Narusaka, Y.; Takano, Y.; Kubo, Y.; Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef]
- Rudd, J.J.; Kanyuka, K.; Hassani-Pak, K.; Derbyshire, M.; Andongabo, A.; Devonshire, J.; Lysenko, A.; Saqi, M.; Desai, N.M.; Powers, S.J.; et al. Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle definition. Plant Physiol. 2015, 167, 1158–1185. [Google Scholar] [PubMed] [Green Version]
- Foresti, O.; Denecke, J. Intermediate organelles of the plant secretory pathway: Identity and function. Traffic 2008, 9, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef]
- Tang, D.; Wang, G.; Zhou, J.M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, J.M. Plant immunity triggered by microbial molecular signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao-Hui, C.; Batoux, M.; Nekrasov, V.; Roux, M.; Chinchilla, D.; Zipfel, C.; Jones, J.D. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc. Natl. Acad. Sci. USA 2009, 106, 15973–15978. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Ding, Y.; Gao, C.; Rojo, E.; Jiang, L. N-linked glycosylation of AtVSR1 is important for vacuolar protein sorting in Arabidopsis. Plant J. 2014, 80, 977–992. [Google Scholar] [CrossRef] [Green Version]
- Nakano, R.T.; Yamada, K.; Bednarek, P.; Nishimura, M.; Hara-Nishimura, I. ER bodies in plants of the Brassicales order: Biogenesis and association with innate immunity. Front. Plant Sci. 2014, 5, 73. [Google Scholar] [CrossRef]
- Watanabe, S.; Shimada, T.L.; Hiruma, K.; Takano, Y. Pathogen infection trial increases the secretion of proteins localized in the endoplasmic reticulum body of Arabidopsis. Plant Physiol. 2013, 163, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Pečenková, T.; Pleskot, R.; Žárský, V. Subcellular Localization of Arabidopsis Pathogenesis-Related 1 (PR1) Protein. Int. J. Mol. Sci. 2017, 18, 825. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.P.; Zeng, Y.; Li, Y.; Ji, C.; Xia, Y.; Jiang, L. Signal motif-dependent ER export of the Qc-SNARE BET12 interacts with MEMB12 and affects PR1 trafficking in Arabidopsis. J. Cell Sci. 2018, 131, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Innes, R.W. The KEEP ON GOING protein of Arabidopsis regulates intracellular protein trafficking and is degraded during fungal infection. Plant Cell 2012, 24, 4717–4730. [Google Scholar] [CrossRef] [Green Version]
- Nakazaki, A.; Yamada, K.; Kunieda, T.; Sugiyama, R.; Hirai, M.Y.; Tamura, K.; Hara-Nishimura, I.; Shimada, T. Leaf Endoplasmic Reticulum Bodies Identified in Arabidopsis Rosette Leaves Are Involved in Defense against Herbivory. Plant Physiol. 2019, 179, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Andréasson, E.; Bolt Jørgensen, L.; Höglund, A.S.; Rask, L.; Meijer, J. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol. 2001, 127, 1750–1763. [Google Scholar] [CrossRef]
- Vorster, B.J.; Cullis, C.A.; Kunert, K.J. Plant Vacuolar Processing Enzymes. Front. Plant Sci. 2019, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Yamada, K.; Shimada, T.; Matsushima, R.; Nishizawa, N.K.; Nishimura, M.; Hara-Nishimura, I. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001, 42, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Hatsugai, N.; Yamada, K.; Goto-Yamada, S.; Hara-Nishimura, I. Vacuolar processing enzyme in plant programmed cell death. Front Plant Sci. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Böhlenius, H.; Mørch, S.M.; Godfrey, D.; Nielsen, M.E.; Thordal-Christensen, H. The multivesicular body-localized GTPase ARFA1b/1c is important for callose deposition and ROR2 syntaxin-dependent preinvasive basal defense in barley. Plant Cell 2010, 22, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Kulich, I.; Vojtíková, Z.; Sabol, P.; Ortmannová, J.; Neděla, V.; Tihlaříková, E.; Žárský, V. Exocyst Subunit EXO70H4 Has a Specific Role in Callose Synthase Secretion and Silica Accumulation. Plant Physiol. 2018, 176, 2040–2051. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Lee, S.E.; Agrawal, G.K.; Rakwal, R.; Park, S.; Wang, Y.; Kim, S.T. Understanding the plant-pathogen interactions in the context of proteomics-generated apoplastic proteins inventory. Front Plant Sci. 2015, 6, 352. [Google Scholar] [CrossRef] [Green Version]
- Underwood, W.; Somerville, S.C. Perception of conserved pathogen elicitors at the plasma membrane leads to relocalization of the Arabidopsis PEN3 transporter. Proc. Natl. Acad. Sci. USA 2013, 110, 12492–12497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukašinović, N.; Žárský, V. Tethering Complexes in the Arabidopsis Endomembrane System. Front. Cell Dev. Biol. 2016, 4, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pečenková, T.; Hála, M.; Kulich, I.; Kocourková, D.; Drdová, E.; Fendrych, M.; Toupalová, H.; Zársky, V. The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J. Exp. Bot. 2011, 62, 2107–2116. [Google Scholar] [CrossRef] [Green Version]
- Stegmann, M.; Anderson, R.G.; Ichimura, K.; Pečenková, T.; Reuter, P.; Žársky, V.; McDowell, J.M.; Shirasu, K.; Trujillo, M. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 2012, 24, 4703–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Rui, L.; Li, J.; Nishimura, M.T.; Vogel, J.P.; Liu, N.; Liu, S.; Zhao, Y.; Dangl, J.L.; Tang, D. A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet. 2015, 11, e1004945. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, N.; Gao, C.; Rui, L.; Tang, D. The Pseudomonas Syringae Effector AvrPtoB Associates With and Ubiquitinates Arabidopsis Exocyst Subunit EXO70B1. Front. Plant Sci. 2019, 10, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Mpina, M.H.; Birch, P.R.; Bouwmeester, K.; Govers, F. Phytophthora infestans RXLR Effector AVR1 Interacts with Exocyst Component Sec5 to Manipulate Plant Immunity. Plant Physiol. 2015, 169, 1975–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Overdijk, E.J.R.; Berg, J.A.; Govers, F.; Bouwmeester, K. Solanaceous exocyst subunits are involved in immunity to diverse plant pathogens. J. Exp. Bot. 2018, 69, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Brown, M.Q.; van de Ven, W.; Zhang, Z.M.; Wu, B.; Young, M.C.; Synek, L.; Borchardt, D.; Harrison, R.; Pan, S.; et al. Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc. Natl. Acad. Sci. USA 2016, 113, E41–E50. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Li, X.; Li, Y.; Yin, X.; Li, Y.; Wu, B.; Mo, H.; Liao, C.J.; Mengiste, T.; Guo, W.; et al. Endosidin2-14 Targets the Exocyst Complex in Plants and Fungal Pathogens to Inhibit Exocytosis. Plant Physiol. 2019, 180, 1756–1770. [Google Scholar] [CrossRef] [Green Version]
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.C.; Thordal-Christensen, H.; Lipka, V.; Bau, S.; Kombrink, E.; Qiu, J.L.; Hückelhoven, R.; Stein, M.; Freialdenhoven, A.; Somerville, S.C.; et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 2003, 425, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, M.; Stammler, J.; Douchkov, D.; Eichmann, R.; Hückelhoven, R. The conserved oligomeric Golgi complex is involved in penetration resistance of barley to the barley powdery mildew fungus. Mol. Plant Pathol. 2013, 14, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lipka, V.; Dittgen, J.; Bednarek, P.; Bhat, R.; Wiermer, M.; Stein, M.; Landtag, J.; Brandt, W.; Rosahl, S.; Scheel, D.; et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 2005, 310, 1180–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, C.; Neu, C.; Pajonk, S.; Yun, H.S.; Lipka, U.; Humphry, M.; Bau, S.; Straus, M.; Kwaaitaal, M.; Rampelt, H.; et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008, 451, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Matern, A.; Böttcher, C.; Eschen-Lippold, L.; Westermann, B.; Smolka, U.; Döll, S.; Trempel, F.; Aryal, B.; Scheel, D.; Geisler, M.; et al. A substrate of the ABC transporter PEN3 stimulates bacterial flagellin (flg22)-induced callose deposition in Arabidopsis thaliana. J. Biol. Chem. 2019, 294, 6857–6870. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.E.; Jürgens, G.; Thordal-Christensen, H. VPS9a Activates the Rab5 GTPase ARA7 to Confer Distinct Pre- and Postinvasive Plant Innate Immunity. Plant Cell 2017, 29, 1927–1937. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Aryal, B.; Langenecker, T.; Hagmann, J.; Geisler, M.; Grebe, M. Arabidopsis BTB/POZ protein-dependent PENETRATION3 trafficking and disease susceptibility. Nat. Plants 2017, 3, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.M.; Kuhn, H.; Micali, C.; Liller, C.; Kwaaitaal, M.; Panstruga, R. Interaction of a Blumeria graminis f. sp. hordei effector candidate with a barley ARF-GAP suggests that host vesicle trafficking is a fungal pathogenicity target. Mol. Plant Pathol. 2014, 15, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Stefano, G.; Renna, L.; Rossi, M.; Azzarello, E.; Pollastri, S.; Brandizzi, F.; Baluska, F.; Mancuso, S. AGD5 is a GTPase-activating protein at the trans-Golgi network. Plant J. 2010, 64, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Delgadillo, M.O.; Zouhar, J.; Reynolds, G.D.; Pennington, J.G.; Jiang, L.; Liljegren, S.J.; Stierhof, Y.D.; De Jaeger, G.; Otegui, M.S.; et al. MTV1 and MTV4 encode plant-specific ENTH and ARF GAP proteins that mediate clathrin-dependent trafficking of vacuolar cargo from the trans-Golgi network. Plant Cell 2013, 25, 2217–2235. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Nakamura, M.; Viotti, C.; Grebe, M. A Framework for Lateral Membrane Trafficking and Polar Tethering of the PEN3 ATP-Binding Cassette Transporter. Plant Physiol. 2016, 172, 2245–2260. [Google Scholar] [CrossRef] [Green Version]
- Sassmann, S.; Rodrigues, C.; Milne, S.W.; Nenninger, A.; Allwood, E.; Littlejohn, G.R.; Talbot, N.J.; Soeller, C.; Davies, B.; Hussey, P.J.; et al. An Immune-Responsive Cytoskeletal-Plasma Membrane Feedback Loop in Plants. Curr. Biol. 2018, 28, 2136–2144. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Xu, J.; Wang, X.; He, X.; Wang, Y.; Zhou, J.; Zhang, S.; Meng, X. The Arabidopsis Pleiotropic Drug Resistance Transporters PEN3 and PDR12 Mediate Camalexin Secretion for Resistance to Botrytis cinerea. Plant Cell 2019, 31, 2209–2222. [Google Scholar] [CrossRef]
- Xin, X.F.; Nomura, K.; Underwood, W.; He, S.Y. Induction and suppression of PEN3 focal accumulation during Pseudomonas syringae pv. tomato DC3000 infection of Arabidopsis. Mol. Plant Microbe Interact. 2013, 26, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Jelenska, J.; Cecchini, N.M.; Li, Y.; Lee, M.W.; Kovar, D.R.; Greenberg, J.T. HopW1 from Pseudomonas syringae disrupts the actin cytoskeleton to promote virulence in Arabidopsis. PLoS Pathog. 2014, 10, e1004232. [Google Scholar] [CrossRef]
- Shimono, M.; Lu, Y.J.; Porter, K.; Kvitko, B.H.; Henty-Ridilla, J.; Creason, A.; He, S.Y.; Chang, J.H.; Staiger, C.J.; Day, B. The Pseudomonas syringae Type III Effector HopG1 Induces Actin Remodeling to Promote Symptom Development and Susceptibility during Infection. Plant Physiol. 2016, 171, 2239–2255. [Google Scholar] [CrossRef] [Green Version]
- Leontovyčová, H.; Kalachova, T.; Trdá, L.; Pospíchalová, R.; Lamparová, L.; Dobrev, P.I.; Malínská, K.; Burketová, L.; Valentová, O.; Janda, M. Actin depolymerization is able to increase plant resistance against pathogens via activation of salicylic acid signalling pathway. Sci. Rep. 2019, 9, 10397. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Nakano, R.T.; Takagi, J.; Wang, Y.; Kramer, K.; Finkemeier, I.; Nakagami, H.; Tsuda, K.; Ueda, T.; Schulze-Lefert, P.; et al. A Golgi-Released Subpopulation of the Trans-Golgi Network Mediates Protein Secretion in Arabidopsis. Plant Physiol. 2019, 179, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.; Moore, I.; Kirchhelle, C. Spatio-temporal control of post-Golgi exocytic trafficking in plants. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Ebine, K.; Fujimoto, M.; Okatani, Y.; Nishiyama, T.; Goh, T.; Ito, E.; Dainobu, T.; Nishitani, A.; Uemura, T.; Sato, M.H.; et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011, 13, 853–859. [Google Scholar] [CrossRef]
- Yang, X.; Liao, C.Y.; Tang, J.; Bassham, D.C. Overexpression of trans-Golgi network t-SNAREs rescues vacuolar trafficking and TGN morphology defects in a putative tethering factor mutant. Plant J. 2019, 99, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Noirot, E.; Der, C.; Lherminier, J.; Robert, F.; Moricova, P.; Kiêu, K.; Leborgne-Castel, N.; Simon-Plas, F.; Bouhidel, K. Dynamic changes in the subcellular distribution of the tobacco ROS-producing enzyme RBOHD in response to the oomycete elicitor cryptogein. J. Exp. Bot. 2014, 65, 5011–5022. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar]
- Lu, D.; Wu, S.; Gao, X.; Zhang, Y.; Shan, L.; He, P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, W.; Xiang, T.; Liu, Z.; Laluk, K.; Ding, X.; Zou, Y.; Gao, M.; Zhang, X.; Chen, S.; et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 2010, 7, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Greenshields, D.L.; Sammynaiken, R.; Hirji, R.N.; Selvaraj, G.; Wei, Y. Targeted alterations in iron homeostasis underlie plant defense responses. J. Cell Sci. 2007, 120 Pt 4, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Krüger, F.; Beckmann, H.; Brumm, S.; Vermeer, J.E.M.; Munnik, T.; Mayer, U.; Stierhof, Y.D.; Grefen, C.; Schumacher, K.; et al. Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr. Biol. 2014, 24, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Munnik, T.; Sato, M.H. Phosphatidylinositol 3-Phosphate 5-Kinase, FAB1/PIKfyve Kinase Mediates Endosome Maturation to Establish Endosome-Cortical Microtubule Interaction in Arabidopsis. Plant Physiol. 2015, 169, 1961–1974. [Google Scholar] [CrossRef] [Green Version]
- Geldner, N.; Hyman, D.L.; Wang, X.; Schumacher, K.; Chory, J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007, 21, 1598–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuring, D.; Künzl, F.; Viotti, C.; Yan, M.S.; Jiang, L.; Schellmann, S.; Robinson, D.G.; Pimpl, P. Ubiquitin initiates sorting of Golgi and plasma membrane proteins into the vacuolar degradation pathway. BMC Plant Biol. 2012, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, E450–E458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, V.; Hauser, M.T. Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 2006, 11, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Haas, T.J.; Sliwinski, M.K.; Martínez, D.E.; Preuss, M.; Ebine, K.; Ueda, T.; Nielsen, E.; Odorizzi, G.; Otegui, M.S. The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 2007, 19, 1295–1312. [Google Scholar] [CrossRef] [Green Version]
- Jia, T.; Gao, C.; Cui, Y.; Wang, J.; Ding, Y.; Cai, Y.; Ueda, T.; Nakano, A.; Jiang, L. ARA7(Q69L) expression in transgenic Arabidopsis cells induces the formation of enlarged multivesicular bodies. J. Exp. Bot. 2013, 64, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Luo, M.; Zhao, Q.; Yang, R.; Cui, Y.; Zeng, Y.; Xia, J.; Jiang, L. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 2014, 24, 2556–2563. [Google Scholar] [CrossRef] [Green Version]
- Thordal-Christensen, H. Fresh insights into processes of nonhost resistance. Curr. Opin. Plant Biol. 2003, 6, 351–357. [Google Scholar] [CrossRef]
- Hansen, L.L.; Nielsen, M.E. Plant exosomes: Using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2017, 69, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Assaad, F.F.; Qiu, J.L.; Youngs, H.; Ehrhardt, D.; Zimmerli, L.; Kalde, M.; Wanner, G.; Peck, S.C.; Edwards, H.; Ramonell, K.; et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell 2004, 15, 5118–5129. [Google Scholar] [CrossRef] [Green Version]
- An, Q.; Hückelhoven, R.; Kogel, K.H.; van Bel, A.J. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006, 8, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, M.; Bourdais, G.; Gervasi, F.; Beck, M.; Zhou, J.; Spallek, T.; Bartels, S.; Boller, T.; Ueda, T.; Kuhn, H.; et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl. Acad. Sci. USA 2016, 113, 11034–11039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Morea, F.A.; Savatin, D.V.; Dejonghe, W.; Kumar, R.; Luo, Y.; Adamowski, M.; Van den Begin, J.; Dressano, K.; Pereira de Oliveira, G.; Zhao, X.; et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl. Acad. Sci. USA 2016, 113, 11028–11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallek, T.; Beck, M.; Ben Khaled, S.; Salomon, S.; Bourdais, G.; Schellmann, S.; Robatzek, S. ESCRT-I mediates FLS2 endosomal sorting and plant immunity. PLoS Genet. 2013, 9, e1004035. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.F.; Nomura, K.; Aung, K.; Velásquez, A.C.; Yao, J.; Boutrot, F.; Chang, J.H.; Zipfel, C.; He, S.Y. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 2016, 539, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, K.; Mecey, C.; Lee, Y.N.; Imboden, L.A.; Chang, J.H.; He, S.Y. Effector-triggered immunity blocks pathogen degradation of an immunity-associated vesicle traffic regulator in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 10774–10779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Kitakura, S.; Rakusová, H.; Uemura, T.; Feraru, M.I.; De Rycke, R.; Robert, S.; Kakimoto, T.; Friml, J. Cell polarity and patterning by PIN trafficking through early endosomal compartments in Arabidopsis thaliana. PLoS Genet. 2013, 9, e1003540. [Google Scholar] [CrossRef] [Green Version]
- Segonzac, C.; Macho, A.P.; Sanmartín, M.; Ntoukakis, V.; Sánchez-Serrano, J.J.; Zipfel, C. Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J. 2014, 33, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Ham, JH.; Hage, R.; Zhao, W.; Soto-Hernández, J.; Lee, S.Y.; Paek, S.M.; Kim, M.G.; Boone, C.; Coplin, D.L.; et al. Direct and Indirect Targeting of PP2A by Conserved Bacterial Type-III Effector Proteins. PLoS Pathog. 2016, 12, e1005609. [Google Scholar] [CrossRef]
- Xin, X.F.; Nomura, K.; Ding, X.; Chen, X.; Wang, K.; Aung, K.; Uribe, F.; Rosa, B.; Yao, J.; Chen, J.; et al. Pseudomonas syringae Effector Avirulence Protein E Localizes to the Host Plasma Membrane and Down-Regulates the Expression of the NONRACE-SPECIFIC DISEASE RESISTANCE1/HARPIN-INDUCED1-LIKE13 Gene Required for Antibacterial Immunity in Arabidopsis. Plant Physiol. 2015, 169, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Shang, Y.; Fan, B.; Yu, J.Q.; Chen, Z. Arabidopsis LIP5, a positive regulator of multivesicular body biogenesis, is a critical target of pathogen-responsive MAPK cascade in plant basal defense. PLoS Pathog. 2014, 10, e1004243. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, J.; Liang, S.; Liang, R.; Zhou, X.; Chen, Z.; Zhao, W.; Wang, J.; Li, W.; He, M.; et al. The Multivesicular Bodies (MVBs)-Localized AAA ATPase LRD6-6 Inhibits Immunity and Cell Death Likely through Regulating MVBs-Mediated Vesicular Trafficking in Rice. PLoS Genet. 2016, 12, e1006311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninoles, R.; Rubio, L.; García-Sánchez, M.J.; Fernández, J.A.; Bueso, E.; Alejandro, S.; Serrano, R. A dominant-negative form of Arabidopsis AP-3 beta-adaptin improves intracellular pH homeostasis. Plant J. 2013, 74, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Paciorek, T.; Feraru, M.I.; Zwiewka, M.; De Groodt, R.; De Rycke, R.; Kleine-Vehn, J.; Friml, J. The AP-3 beta adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell 2010, 22, 2812–2824. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Tejos, R.; Beck, M.; Himschoot, E.; Li, H.; Robatzek, S.; Vanneste, S.; Friml, J. Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc. Natl. Acad. Sci. USA 2013, 110, 7946–7951. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Xu, Z.Y.; Kim, S.Y.; Lee, J.; Choi, B.; Lee, J.; Kim, H.; Sim, H.J.; Hwang, I. Spatial Regulation of ABCG25, an ABA Exporter, Is an Important Component of the Mechanism Controlling Cellular ABA Levels. Plant Cell 2016, 28, 2528–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Y.; Kim, D.H.; Hwang, I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013, 32, 807–813. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Steuer, B.; Stuhlfauth, T.; Fock, H.P. The efficiency of water use in water stressed plants is increased due to ABA induced stomatal closure. Photosynth. Res. 1988, 18, 327–336. [Google Scholar] [CrossRef]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.; Nakamura, Y.; Murata, Y. Guard Cell Salicylic Acid Signaling Is Integrated into Abscisic Acid Signaling via the Ca(2+)/CPK-Dependent Pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwiewka, M.; Bielach, A.; Tamizhselvan, P.; Madhavan, S.; Ryad, E.E.; Tan, S.; Hrtyan, M.N.; Dobrev, P.; Vankovï, R.; Friml, J.; et al. Root Adaptation to H2O2-Induced Oxidative Stress by ARF GEF BEN1- and Cytoskeleton-Mediated PIN2 Trafficking. Plant Cell Physiol. 2019, 60, 255–273. [Google Scholar] [CrossRef]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjärvi, J. Reactive Oxygen Species in the Regulation of Stomatal Movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Shen, J.; Lee, M.H.; Geem, K.R.; Jiang, L.; Hwang, I. AtCAP2 is crucial for lytic vacuole biogenesis during germination by positively regulating vacuolar protein trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E1675–E1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, E.; Fung, N.; Liu, J.; Drakakaki, G.; Coaker, G. Beyond glycolysis: GAPDHs are multi-functional enzymes involved in regulation of ROS, autophagy, and plant immune responses. PLoS Genet. 2015, 11, e1005199. [Google Scholar] [CrossRef]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef]
- Schultz-Larsen, T.; Lenk, A.; Kalinowska, K.; Vestergaard, L.K.; Pedersen, C.; Isono, E.; Thordal-Christensen, H. The AMSH3 ESCRT-III-Associated Deubiquitinase Is Essential for Plant Immunity. Cell Rep. 2018, 25, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Isono, E.; Katsiarimpa, A.; Müller, I.K.; Anzenberger, F.; Stierhof, Y.D.; Geldner, N.; Chory, J.; Schwechheimer, C. The deubiquitinating enzyme AMSH3 is required for intracellular trafficking and vacuole biogenesis in Arabidopsis thaliana. Plant Cell 2010, 22, 1826–1837. [Google Scholar] [CrossRef] [Green Version]
- Katsiarimpa, A.; Muñoz, A.; Kalinowska, K.; Uemura, T.; Rojo, E.; Isono, E. The ESCRT-III-interacting deubiquitinating enzyme AMSH3 is essential for degradation of ubiquitinated membrane proteins in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heard, W.; Sklenář, J.; Tomé, D.F.; Robatzek, S.; Jones, A.M. Identification of Regulatory and Cargo Proteins of Endosomal and Secretory Pathways in Arabidopsis thaliana by Proteomic Dissection. Mol. Cell Proteom. 2015, 14, 1796–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatsugai, N.; Hara-Nishimura, I. Two vacuole-mediated defense strategies in plants. Plant Signal. Behav. 2010, 5, 1568–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatsugai, N.; Kuroyanagi, M.; Nishimura, M.; Hara-Nishimura, I. A cellular suicide strategy of plants: Vacuole-mediated cell death. Apoptosis 2006, 11, 905–911. [Google Scholar] [CrossRef]
- Wang, D.; Weaver, N.D.; Kesarwani, M.; Dong, X. Induction of protein secretory pathway is required for systemic acquired resistance. Science 2005, 308, 1036–1040. [Google Scholar] [CrossRef]
- Rojo, E.; Zouhar, J.; Carter, C.; Kovaleva, V.; Raikhel, N.V. A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 7389–7394. [Google Scholar] [CrossRef] [Green Version]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. Serpin1 and WSCP differentially regulate the activity of the cysteine protease RD21 during plant development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 2212–2217. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.X.; Zhang, F.C.; Zhang, W.Z.; Song, L.F.; Wu, W.H.; Chen, Y.F. Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Mol. Plant 2013, 6, 1487–1502. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bao, H.; Wang, Z.; Wang, M.; Fan, B.; Zhu, C.; Chen, Z. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004, 305, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Yamada, K.; Hatsugai, N.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J. Biol. Chem. 2005, 280, 32914–32920. [Google Scholar] [CrossRef] [Green Version]
- Sugano, S.; Hayashi, N.; Kawagoe, Y.; Mochizuki, S.; Inoue, H.; Mori, M.; Nishizawa, Y.; Jiang, C.J.; Matsui, M.; Takatsuji, H. Rice OsVAMP714, a membrane-trafficking protein localized to the chloroplast and vacuolar membrane, is involved in resistance to rice blast disease. Plant Mol. Biol. 2016, 91, 81–95. [Google Scholar] [CrossRef]
- Zheng, J.; Han, S.W.; Rodriguez-Welsh, M.F.; Rojas-Pierce, M. Homotypic vacuole fusion requires VTI11 and is regulated by phosphoinositides. Mol. Plant 2014, 7, 1026–1040. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, D.G.; Jiang, J.; Movahed, N.; Germain, H.; Yamaji, Y.; Zheng, H.; Laliberté, J.F. Turnip Mosaic Virus Uses the SNARE Protein VTI11 in an Unconventional Route for Replication Vesicle Trafficking. Plant Cell 2018, 30, 2594–2615. [Google Scholar] [CrossRef] [Green Version]
- Movahed, N.; Cabanillas, D.G.; Wan, J.; Vali, H.; Laliberté, J.F.; Zheng, H. Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves. Plant Physiol. 2019, 180, 1375–1388. [Google Scholar] [CrossRef]
- Inada, N.; Betsuyaku, S.; Shimada, T.L.; Ebine, K.; Ito, E.; Kutsuna, N.; Hasezawa, S.; Takano, Y.; Fukuda, H.; Nakano, A.; et al. Modulation of Plant RAB GTPase-Mediated Membrane Trafficking Pathway at the Interface Between Plants and Obligate Biotrophic Pathogens. Plant Cell Physiol. 2016, 57, 1854–1864. [Google Scholar] [CrossRef] [Green Version]
- Hatsugai, N.; Iwasaki, S.; Tamura, K.; Kondo, M.; Fuji, K.; Ogasawara, K.; Nishimura, M.; Hara-Nishimura, I. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 2009, 23, 2496–2506. [Google Scholar] [CrossRef] [Green Version]
- Kleijnen, M.F.; Kirkpatrick, D.S.; Gygi, S.P. The ubiquitin-proteasome system regulates membrane fusion of yeast vacuoles. Embo J. 2007, 26, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.I.; Cho, H.J.; Jung, J.H.; Yoshimoto, K.; Shirasu, K.; Park, O.K. The Rab GTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. Plant J. 2010, 64, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.I.; Cho, H.J.; Kim, S.R.; Park, O.K. The Rab GTPase RabG3b positively regulates autophagy and immunity-associated hypersensitive cell death in Arabidopsis. Plant Physiol. 2013, 161, 1722–1736. [Google Scholar] [CrossRef] [Green Version]
- Hatsugai, N.; Nakatsuji, A.; Unten, O.; Ogasawara, K.; Kondo, M.; Nishimura, M.; Shimada, T.; Katagiri, F.; Hara-Nishimura, I. Involvement of Adapter Protein Complex 4 in Hypersensitive Cell Death Induced by Avirulent Bacteria. Plant Physiol. 2018, 176, 1824–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, R.T.; Piślewska-Bednarek, M.; Yamada, K.; Edger, P.P.; Miyahara, M.; Kondo, M.; Böttcher, C.; Mori, M.; Nishimura, M.; Schulze-Lefert, P.; et al. PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana. Plant J. 2017, 89, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Osbourn, A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Hara-Nishimura, I. Specialized Vacuoles of Myrosin Cells: Chemical Defense Strategy in Brassicales Plants. Plant Cell Physiol. 2018, 59, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Ueda, H.; Shimada, T.; Kohchi, T.; Hara-Nishimura, I. Myrosin cell development is regulated by endocytosis machinery and PIN1 polarity in leaf primordia of Arabidopsis thaliana. Plant Cell 2014, 26, 4448–4461. [Google Scholar] [CrossRef] [Green Version]
- Andrés, Z.; Pérez-Hormaeche, J.; Leidi, E.O.; Schlücking, K.; Steinhorst, L.; McLachlan, D.H.; Schumacher, K.; Hetherington, A.M.; Kudla, J.; Cubero, B.; et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 2014, 111, E1806–E1814. [Google Scholar] [CrossRef] [Green Version]
- Scheuring, D.; Löfke, C.; Krüger, F.; Kittelmann, M.; Eisa, A.; Hughes, L.; Smith, R.S.; Hawes, C.; Schumacher, K.; Kleine-Vehn, J. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl. Acad. Sci. USA 2016, 113, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Löfke, C.; Scheuring, D.; Dünser, K.; Schöller, M.; Luschnig, C.; Kleine-Vehn, J. Auxin regulates SNARE-dependent vacuolar morphology restricting cell size. Elife 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Bourdais, G.; McLachlan, D.H.; Rickett, L.M.; Zhou, J.; Siwoszek, A.; Häweker, H.; Hartley, M.; Kuhn, H.; Morris, R.J.; MacLean, D.; et al. The use of quantitative imaging to investigate regulators of membrane trafficking in Arabidopsis stomatal closure. Traffic 2019, 20, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Dunser, K.; Gupta, S.; Herger, A.; Feraru, M.I.; Ringli, C.; Kleine-Vehn, J. Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Masachis, S.; Segorbe, D.; Turrà, D.; Leon-Ruiz, M.; Fürst, U.; El Ghalid, M.; Leonard, G.; López-Berges, M.S.; Richards, T.A.; Felix, G.; et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Bourdais, G.; Pan, H.; Robatzek, S.; Tang, D. Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc. Natl. Acad. Sci. USA 2017, 114, 5749–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofius, D.; Li, L.; Hafrén, A.; Coll, N.S. Autophagy as an emerging arena for plant-pathogen interactions. Curr. Opin. Plant Biol. 2017, 38, 117–123. [Google Scholar] [CrossRef]
- Leary, A.Y.; Sanguankiattichai, N.; Duggan, C.; Tumtas, Y.; Pandey, P.; Segretin, M.E.; Salguero Linares, J.; Savage, Z.D.; Yow, R.J.; Bozkurt, T.O. Modulation of plant autophagy during pathogen attack. J. Exp. Bot. 2018, 69, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruano, G.; Scheuring, D. Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense. Plants 2020, 9, 389. https://doi.org/10.3390/plants9030389
Ruano G, Scheuring D. Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense. Plants. 2020; 9(3):389. https://doi.org/10.3390/plants9030389
Chicago/Turabian StyleRuano, Guillermo, and David Scheuring. 2020. "Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense" Plants 9, no. 3: 389. https://doi.org/10.3390/plants9030389
APA StyleRuano, G., & Scheuring, D. (2020). Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense. Plants, 9(3), 389. https://doi.org/10.3390/plants9030389




