Patatin-Related Phospholipase AtpPLAIIIα Affects Lignification of Xylem in Arabidopsis and Hybrid Poplars
Abstract
:1. Introduction
2. Results
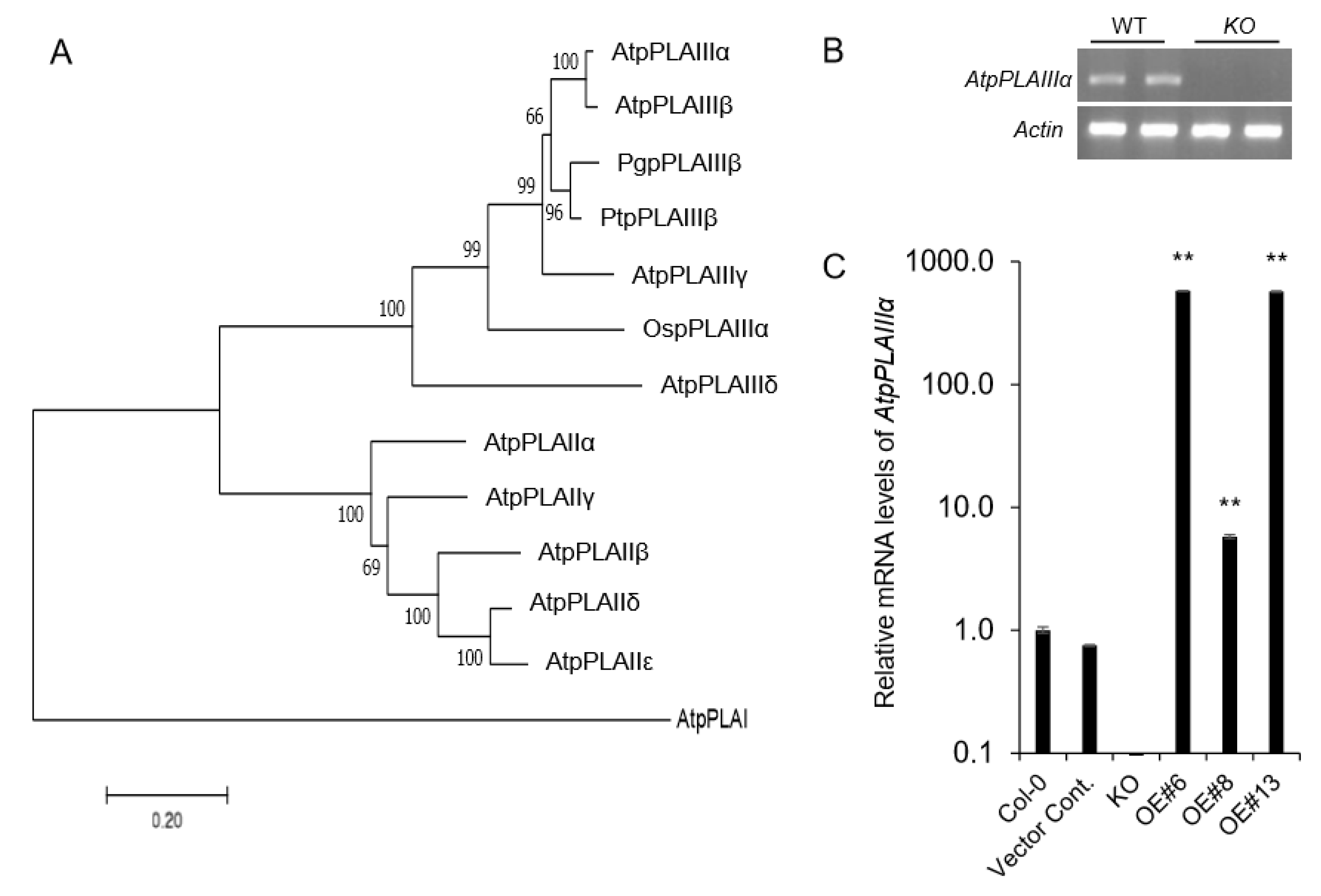
2.1. Isolation of Knockout Mutant and Overexpression Lines of AtpPLAIIIα
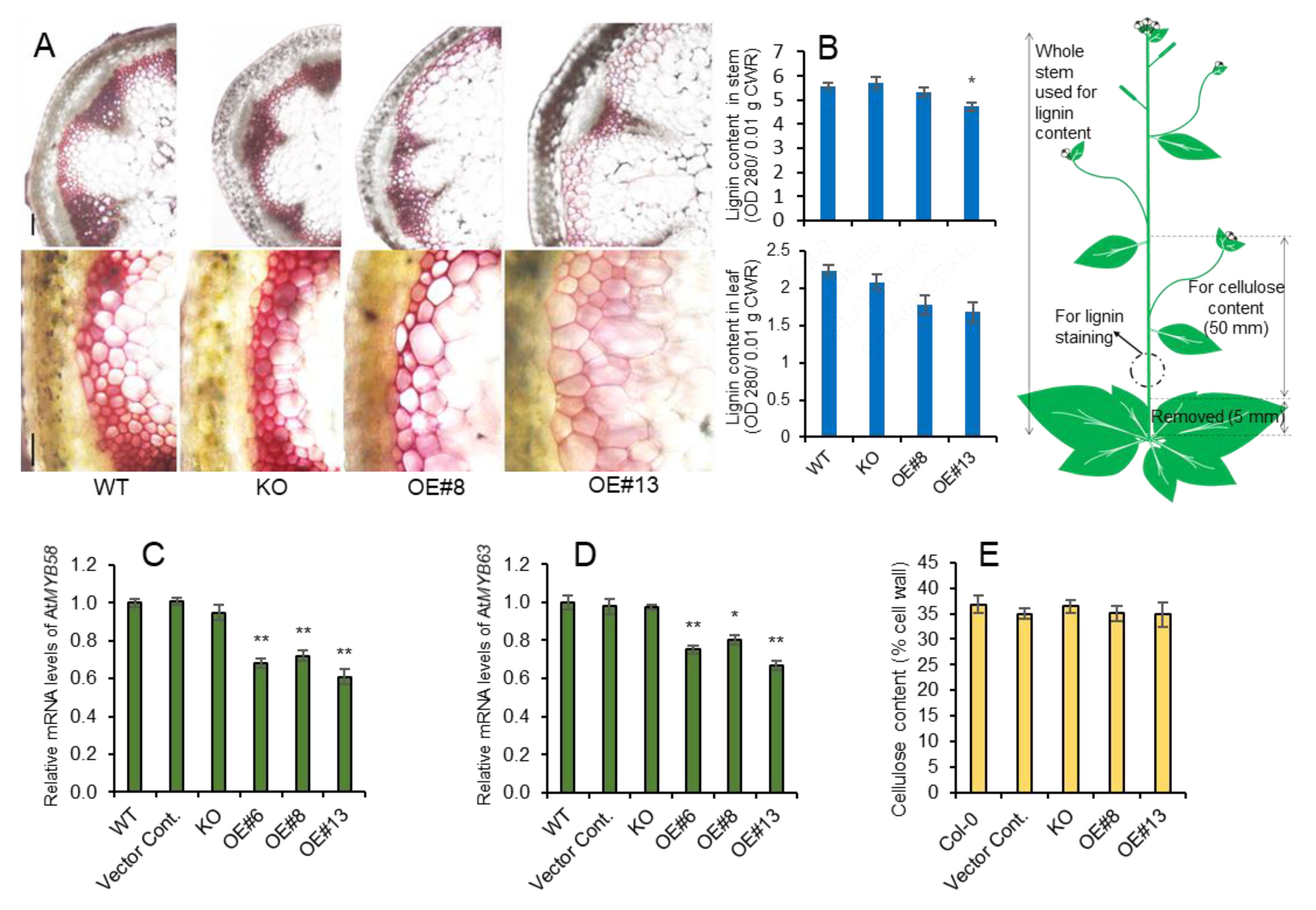
2.2. Overexpression of AtpPLAIIIα Reduced Lignin Content in Arabidopsis
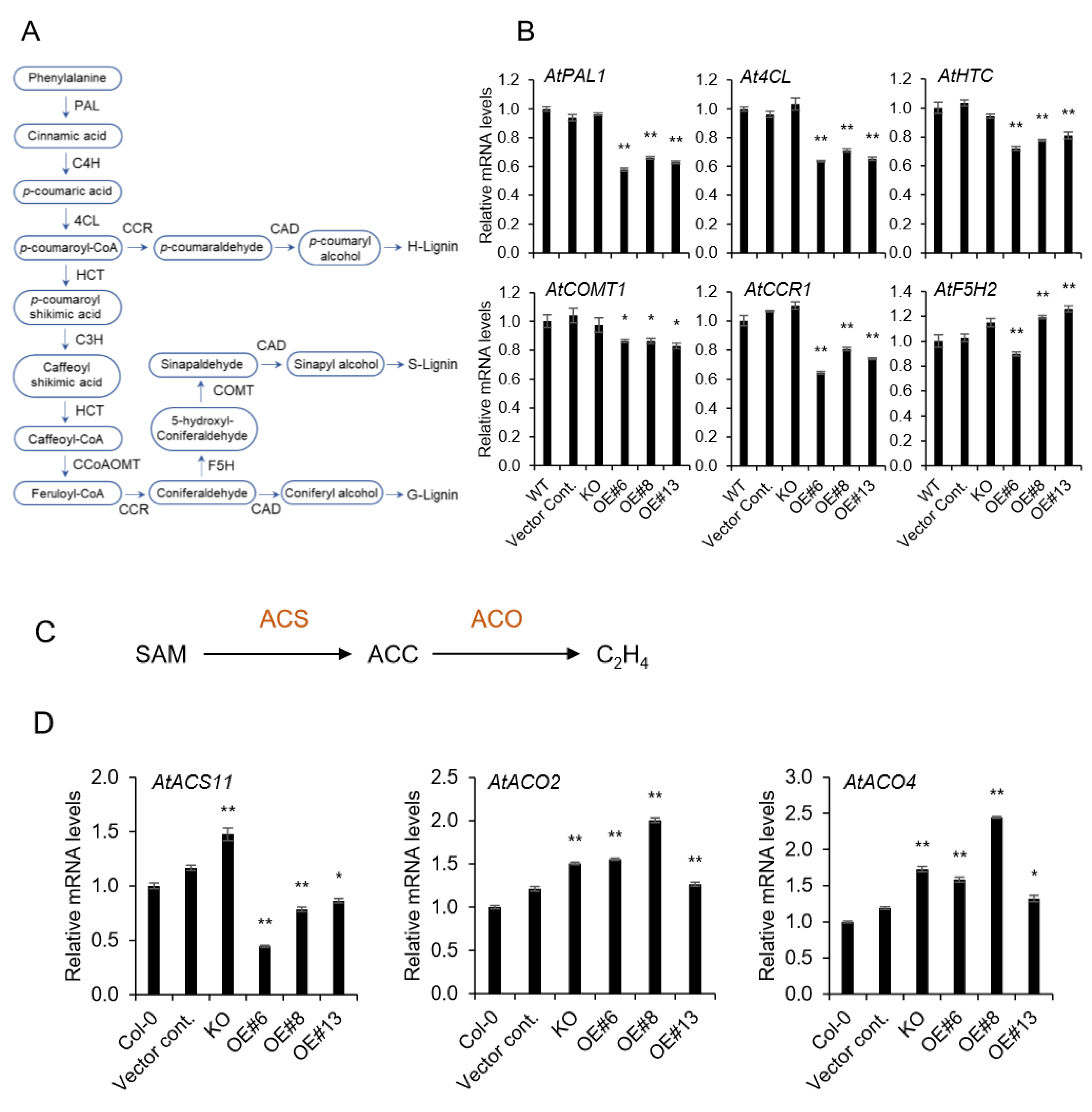
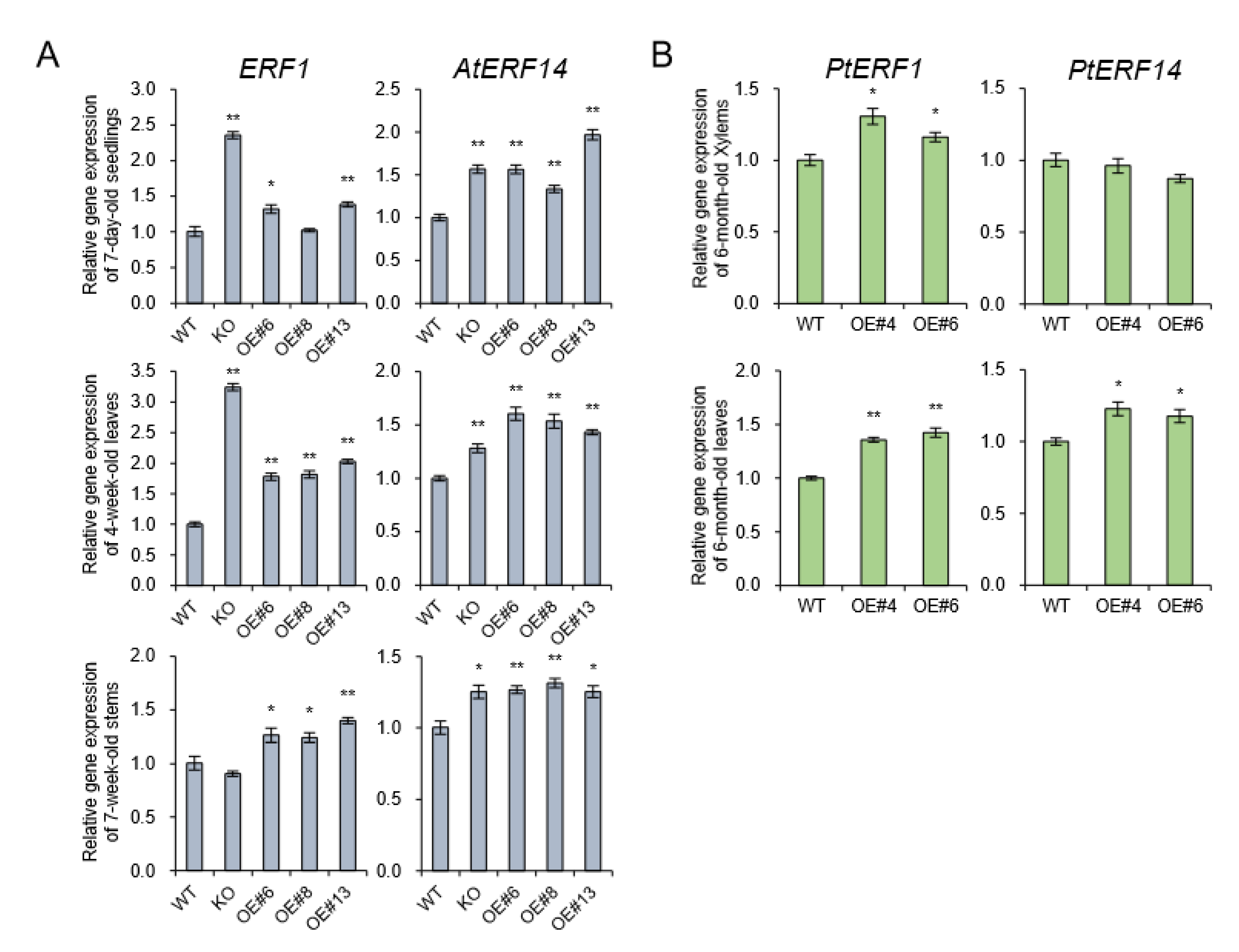
2.3. Lignin Biosynthesis Genes are Decreased in AtpPLAIIIα-OE, and It is Related to Ethylene Biosynthesis
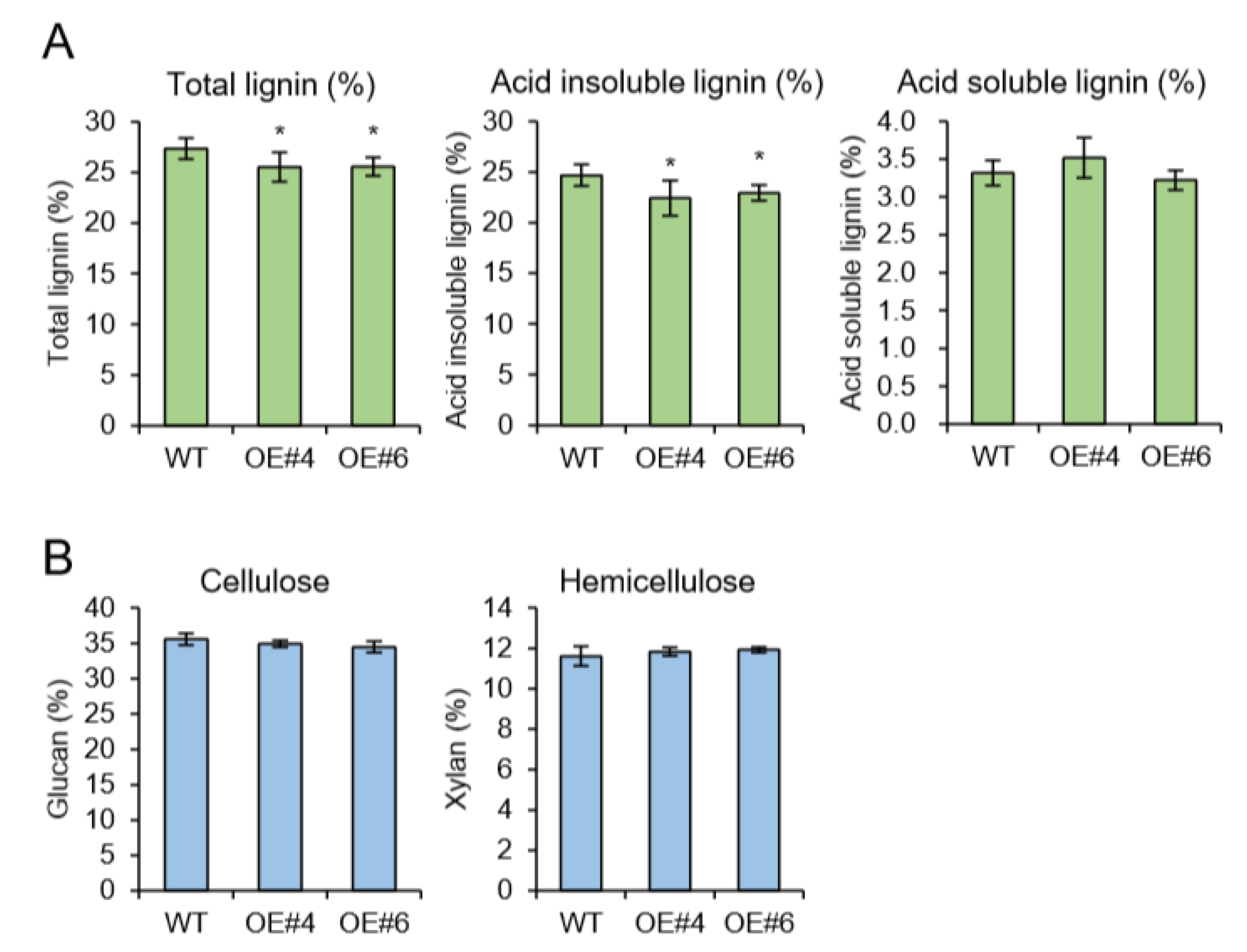
2.4. Overexpression of AtpPLAIIIα in Poplar Reduced the Plant Height and Lignification in Xylem
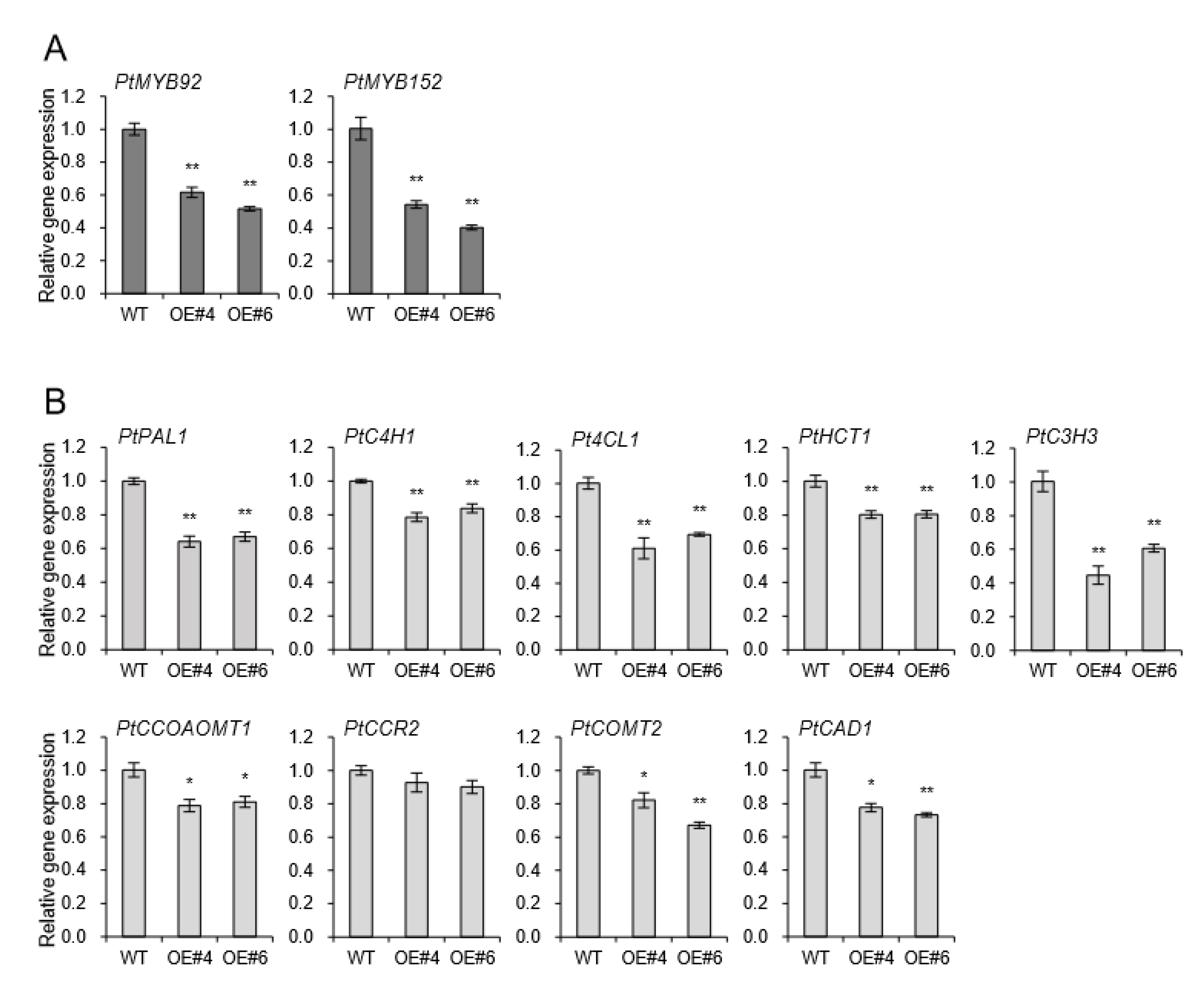
2.5. MYB Transcription Factors and Lignin Biosynthesis Genes are All Downregulated in AtpPLAIIIα-OE of Poplar
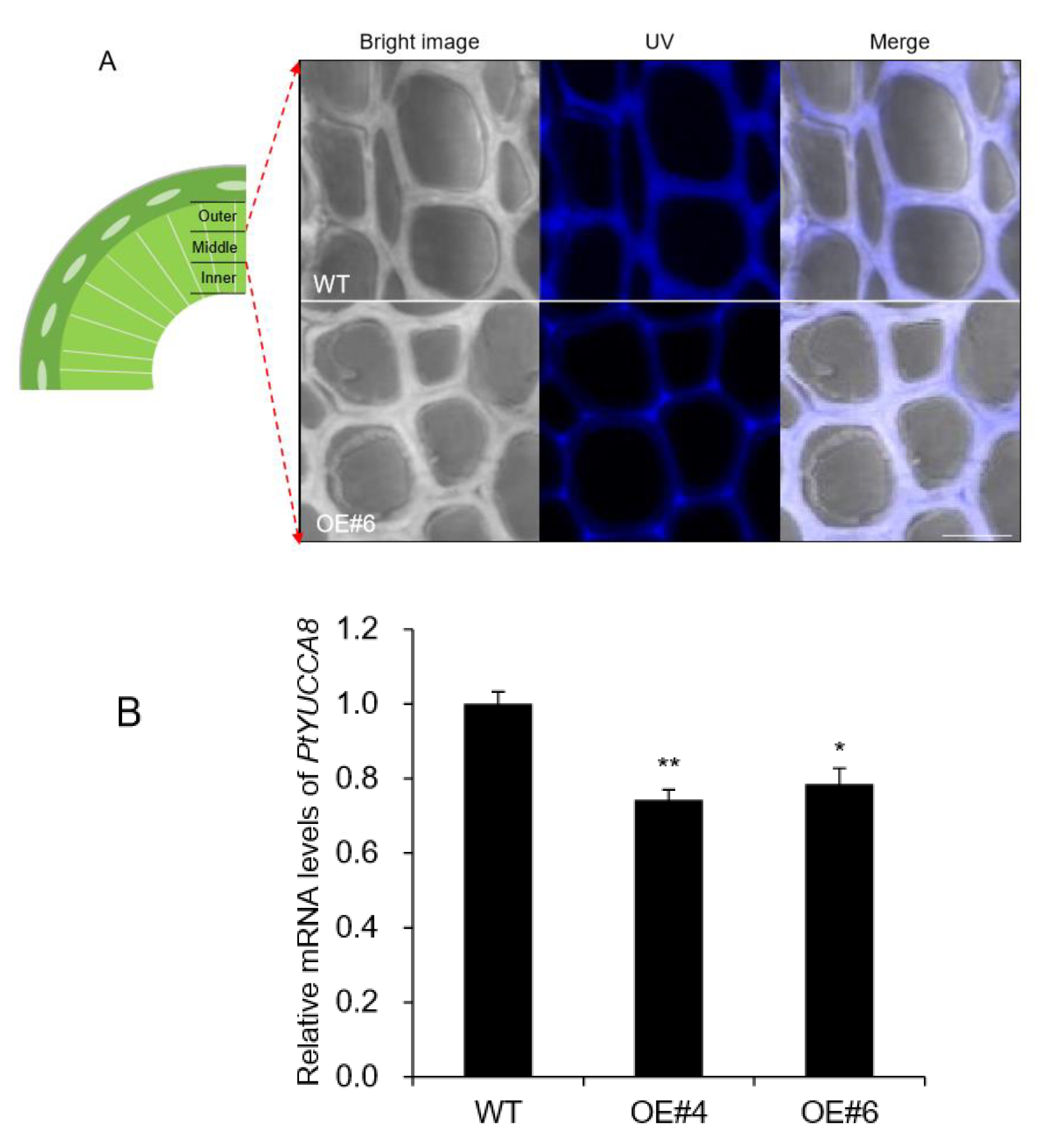
2.6. Lignin Autofluorescence and Expression of YUCCA8 and ERF Genes in AtpPLAIIIα-OE
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transgenic Construct and in planta Transformation
4.3. Total RNA Isolation and PCR Amplification
4.4. Acetyl Bromide Soluble Lignin Assay for Total Lignin Quantification
4.5. Analysis of Carbohydrate Profiles
4.6. Detection of Lignin Autofluorescence
Accession Numbers
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, O.R.; Kim, S.J.; Kim, H.J.; Hong, J.K.; Ryu, S.B.; Lee, S.H.; Ganguly, A.; Cho, H.T. Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 2010, 22, 1812–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, G.F.E.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010, 15, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Plant phospholipases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Holk, A.; Rietz, S.; Zahn, M.; Quader, H.; Scherer, G.F.E. Molecular identification of cytosolic, patatin-related phospholipases A from Arabidopsis with potential functions in plant signal transduction. Plant Physiol. 2002, 130, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Bahn, S.C.; Guo, L.; Musgrave, W.; Berg, H.; Welti, R.; Wang, X. Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell 2011, 23, 1107–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wei, F.; Tawfall, A.; Tang, M.; Saettele, A.; Wang, X. Overexpression of patatin-related phospholipase AIIIδ altered plant growth and increased seed oil content in camelina. Plant Biotechnol. J. 2015, 13, 766–778. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, K.; Ai, J.; Deng, X.; Hong, Y.; Wang, X. Patatin-related phospholipase A, pPLAIIIα, modulates the longitudinal growth of vegetative tissues and seeds in rice. J. Exp. Bot. 2015, 66, 6945–6955. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Bae, E.K.; Choi, Y.I.; Lee, O.R. Ginseng-derived patatin-related phospholipase PgpPLAIIIβ alters plant growth and lignification of xylem in hybrid poplars. Plant Sci. 2019, 288, 110224. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, O.R. Overexpression of ginseng patatin-related phospholipase pPLAIIIβ alters the polarity of cell growth and decreases lignin content in Arabidopsis. J. Ginseng Res. 2020, 44, 321–331. [Google Scholar] [CrossRef]
- Li, M.; Bahn, S.C.; Fan, C.; Li, J.; Phan, T.; Ortiz, M.; Roth, M.R.; Welti, R.; Jaworski, J.; Wang, X. Patatin-related phospholipase pPLAIIIδ increases seed oil content with long-chain fatty acids in Arabidopsis. Plant Physiol. 2013, 162, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Labusch, C.; Shishova, M.; Effendi, Y.; Li, M.; Wang, X.; Scherer, G.F.E. Patterns and timing in expression of early auxin-induced genes imply involvement of phospholipases A (pPLAs) in the regulation of auxin responses. Mol. Plant 2013, 6, 1473–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Mitra, P.P.; Loque, D. Histochemical staining of Arabidopsis thaliana secondary cell wall elements. JoVE J. Vis. Exp. 2014, 87, e51381. [Google Scholar]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.M.; Choi, H.B.; An, G.H. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [Green Version]
- Jansson, S.; Douglas, C.J. Populus: A model system for plant biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef]
- Choi, Y.I.; Noh, E.W.; Lee, H.S.; Han, M.S.; Lee, J.S.; Choi, K.S. An efficient and novel plant selectable marker based on organomercurial resistance. J. Plant Biol. 2005, 48, 351–355. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.H. Complexity of the transcriptional network controlling secondary wall biosynthesis. Plant Sci. 2014, 229, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Ye, Z.H. The poplar PtrWNDs are transcriptional activators of secondary cell wall biosynthesis. Plant Signal. Behav. 2010, 5, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, R.L.; Zhong, R.; Ye, Z.H. MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, X.; Ran, L.; Tian, Q.; Fan, D.; Luo, K. PtoMYB92 is a transcriptional activator of the lignin biosynthetic pathway during secondary cell wall formation in Populus tomentosa. Plant Cell Physiol. 2015, 56, 2436–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, E.; Porth, I.; Chen, J.G.; Mansfield, S.D.; Douglas, C.J. Regulation of secondary cell wall biosynthesis by poplar R2R3 MYB transcription factor PtrMYB152 in Arabidopsis. Sci. Rep. 2014, 4, 5054. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, L.; Williams, N. Imaging and spectroscopy of natural fluorophores in pine needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Albinsson, B.; Li, S.; Lundquist, K.; Stomberg, R. The origin of lignin fluorescence. J. Mol. Struct. 1999, 508, 19–27. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, H.T.; Hwang, I.; Han, K.H. Tissue-type-specific transcriptome analysis identifies developing xylem-specific promoters in poplar. Plant Biotechnol. J. 2012, 10, 587–596. [Google Scholar] [CrossRef]
- Hentrich, M.; Sánchez-Parra, B.; Pérez Alonso, M.M.; Carrasco Loba, V.; Carrillo, L.; Vicente-Carbajosa, J.; Medina, J.; Pollmann, S. YUCCA8 and YUCCA9 overexpression reveals a link between auxin signaling and lignification through the induction of ethylene biosynthesis. Plant Signal. Behav. 2013, 8, e26363. [Google Scholar] [CrossRef] [Green Version]
- Onate-Sanchez, L.; Singh, K.B. Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol. 2002, 128, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Blakeslee, J.J.; Bandyopadhyay, J.; Lee, O.R.; Mravec, J.; Titapiwatanakun, B.; Sauer, M.; Makam, S.N.; Cheng, Y.; Bouchard, R.; Adamec, J.; et al. Interactions among PIN-FORMED and P-Glycoprotein Auxin Transporters in Arabidopsis. Plant Cell 2007, 19, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzyme in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Smith, R.; Ellis, B. Xylem tissue specification, patterning, and differentiation mechanisms. J. Exp. Bot. 2013, 64, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Sachs, T. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–162. [Google Scholar]
- Dettmer, J.; Elo, A.; Helariutta, Y. Hormone interactions during vascular development. Plant Mol. Biol. 2009, 69, 347–360. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- De Perez Souza, L.; Garbowicz, K.; Brotman, Y.; Tohge, T.; Fernie, A.R. The Acetate Pathway Supports Flavonoid and Lipid Biosynthesis in Arabidopsis. Plant Physiol. 2020, 182, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bechtold, N.; Pelletier, G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 1998, 82, 259–266. [Google Scholar]
- Yan, L.; Xu, C.; Kang, Y.; Gu, T.; Wang, D.; Zhao, S.; Xia, G. The heterologous expression in Arabidopsis thaliana of sorghum transcription factor SbbHLH1 downregulates lignin synthesis. J. Exp. Bot. 2013, 64, 3021–3032. [Google Scholar] [CrossRef] [Green Version]
- Dence, C.W. The determination of lignin. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.H.; Lee, O.R. Patatin-Related Phospholipase AtpPLAIIIα Affects Lignification of Xylem in Arabidopsis and Hybrid Poplars. Plants 2020, 9, 451. https://doi.org/10.3390/plants9040451
Jang JH, Lee OR. Patatin-Related Phospholipase AtpPLAIIIα Affects Lignification of Xylem in Arabidopsis and Hybrid Poplars. Plants. 2020; 9(4):451. https://doi.org/10.3390/plants9040451
Chicago/Turabian StyleJang, Jin Hoon, and Ok Ran Lee. 2020. "Patatin-Related Phospholipase AtpPLAIIIα Affects Lignification of Xylem in Arabidopsis and Hybrid Poplars" Plants 9, no. 4: 451. https://doi.org/10.3390/plants9040451
APA StyleJang, J. H., & Lee, O. R. (2020). Patatin-Related Phospholipase AtpPLAIIIα Affects Lignification of Xylem in Arabidopsis and Hybrid Poplars. Plants, 9(4), 451. https://doi.org/10.3390/plants9040451




