Grain Yields and Nitrogen Use Efficiencies in Different Types of Stay-Green Maize in Response to Nitrogen Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
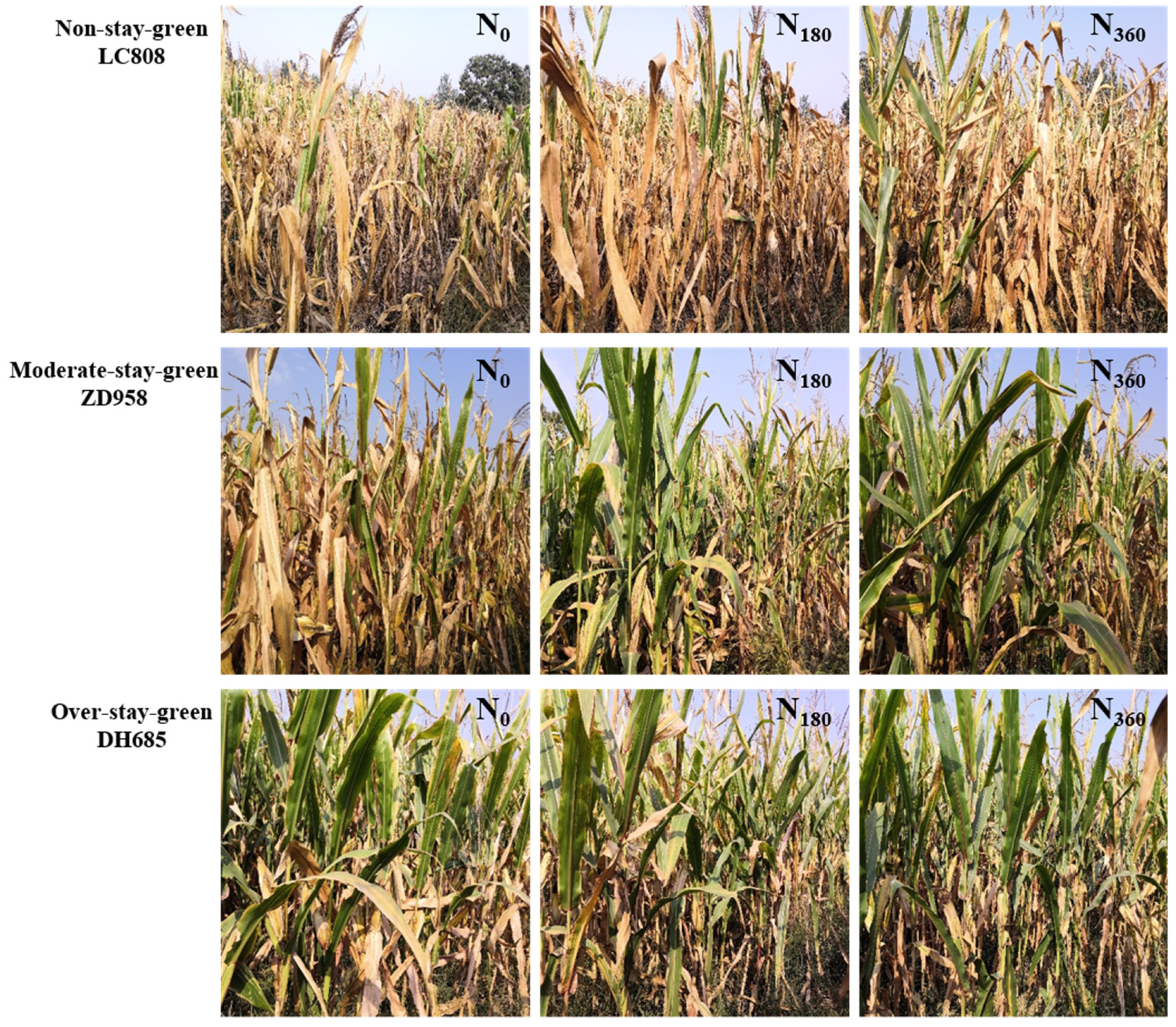
2.1. Plant Material and Experimental Design
2.2. SPAD Values Measurements
2.3. Relative Leaf Conductivity
2.4. Chlorophyll Content
2.5. Plant Sampling and Determination of Total N Concentration
2.6. Statistical Analyses
- TNA [kg hm−2] = plant N concentration [kg kg−1] × plant dry matter [kg hm−2];
- NRE [%] = (TNA of N applied − TNA of N omission) [kg hm−2]/N applied [kg hm−2] × 100 [%];
- N-PFP [kg kg−1] = Grain yield [kg hm−2]/N applied [kg hm−2];
- NHI [%] = grain N accumulation [kg hm−2]/TNA [kg hm−2] × 100 [%]
3. Results
3.1. Relationship between △SPAD and Grain Yield
3.2. SPAD Value
3.3. Chlorophyll Content
3.4. Relative Electrical Conductivity (EC)
3.5. Grain Yield
3.6. N Accumulation and N Use Efficiency
4. Discussion
4.1. Stay-Green Improves Crop Yields
4.2. Effects of N Application on the Duration of Greenness for the Different Types of Stay-Green Maize
4.3. Optimal N Rate for the Different Types of Stay-Green Maize
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Peter, B.K.; Xu, M.L. Quantitative disease resistance: Dissection and adoption in maize. Mol. Plant 2017, 10, 402–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Agriculture Organization of the United Nations. Special Report FAO/WFP Crop and Food Security Assessment Mission to Mozambique; Food Agriculture Organization of the United Nations: Rome Italy, 2010. [Google Scholar]
- Wang, N.; Wu, X.; Ku, L.; Chen, Y.; Wang, W. Evaluation of Three Protein-Extraction Methods for Proteome Analysis of Maize Leaf Midrib, a Compound Tissue Rich in Sclerenchyma Cells. Front. Plant Sci. 2016, 7, 1–856. [Google Scholar] [CrossRef] [Green Version]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Lawal, I.A.; Gbashi, S.; Njobeh, P.B. Decontamination of T-2 Toxin in Maize by Modified Montmorillonite Clay. Toxins 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Shao, X.; Pei, Y.; Guo, X.; Li, J.; Song, X.; Zhao, M. Genetic Diversity and Genome-Wide Association Study of Major Ear Quantitative Traits Using High-Density SNPs in Maize. Front. Plant Sci. 2018, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, T.; Peng, H.; Luo, S.; Tan, J.; Jiang, K.; Heng, Y.; Zhang, X.; Guo, X.; Zheng, J.; et al. Rice Premature Leaf Senescence 2, Encoding a Glycosyltransferase (GT), Is Involved in Leaf Senescence. Front. Plant Sci. 2018, 9, 560. [Google Scholar] [CrossRef]
- Willman, M.R.; Below, F.R.; Lambert, R.J.; Howey, A.E.; Mies, D.W. Plant traits related to productivity of maize. II. Development of multiple trait models1. Crop Sci. 1987, 27, 1122–1126. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Kamal, N.M.; Gorafi, Y.S.A.; Abdelrahman, M.; Abdellatef, E.; Tsujimoto, H. Stay-green trait: A prospective approach for yield potential, and drought and heat stress adaptation in globally important cereals. Int. J. Mol. Sci. 2019, 20, 5837. [Google Scholar] [CrossRef] [Green Version]
- Hortensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Wu, D.; Xia, T.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Zhang, F.; Cui, Z.; Liu, X. Nitrogen Uptake and Remobilization in Maize Hybrids Differing in Leaf Senescence. J. Plant Nutr. 2003, 26, 237–247. [Google Scholar] [CrossRef]
- Kosgey, J.R.; Moot, D.J.; Fletcher, A.L.; Mckenzie, B.A. Dry matter accumulation and post-silking N economy of ‘stay-green’ maize (Zea mays L.) hybrids. Eur. J. Agron. 2013, 51, 43–52. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.N.; Zhang, W.Z.; Gao, J.P.; Zhao, Y.; Peng, C.C.; Zhao, C. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Differential Regulation of Carbon and Nitrogen Metabolism in Response to Nitrogen Availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Tian, Q.Y.; Li, L.H.; Zhang, W.H. Nitric Oxide is Involved in Nitrate-induced Inhibition of Root Elongation in Zea mays. Ann. Bot. 2007, 100, 497–503. [Google Scholar] [CrossRef]
- Jiang, C.Q.; Lu, D.J.; Zu, C.L.; Shen, J.; Wang, S.J.; Guo, Z.B.; Zhou, J.M.; Wang, H.Y. One-time root. zone N fertilization increases maize yield, NUE and reduces soil N losses in lime concretion black soil. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Nazir, M.; Pandey, R.; Siddiqi, T.O.; Ibrahim, M.M.; Qureshi, M.I.; Abraham, G.; Vengavasi, K.; Ahmad, A. Nitrogen-deficiency stress induces protein expression differentially in low-N tolerant and low-N sensitive Maize genotypes. Front. Plant Sci. 2016, 7, 298. [Google Scholar] [CrossRef] [Green Version]
- Gully, K.; Hander, T.; Boller, T.; Bartels, S. Perception of Arabidopsis AtPep peptides, but not bacterial elicitors, accelerates starvation-induced senescence. Front. Plant Sci. 2015, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Guo, Z.; Huang, C.; Wang, K.; Jiang, N.; Feng, H.; Chen, G.; Liu, Q.; Xiong, L. Genome-wide association study of rice (Oryza sativa L.) leaf traits with a high-throughput leaf scorer. J. Exp. Bot. 2015, 66, 5605–5615. [Google Scholar] [CrossRef] [Green Version]
- Thornley, J.H.M. Acclimation of Photosynthesis to Light and Canopy Nitrogen Distribution: An Interpretation. Ann. Bot. 2004, 93, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Crafts-Brandner, S.J.; Below, F.E.; Wittenbach, V.A.; Harper, J.E.; Hageman, R.H. Differential Senescence of Maize Hybrids following Ear Removal. Plant Physiol. 1984, 74, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.T.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Duvick, D.N.; Smith, J.S.C.; Cooper, M. Long-term selection in a commercial hybrid maize breeding program. Plant Breed. Rev. 2004, 24, 109–152. [Google Scholar]
- Sakuraba, Y.; Lee, S.H.; Kim, Y.S.; Park, O.K.; Hörtensteiner, S.; Paek, N.C. Delayed degradation of chlorophylls and photosynthetic proteins in Arabidopsis autophagy mutants during stress-induced leaf yellowing. J. Exp. Bot. 2014, 65, 3915–3925. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.A.; Tollenaar, M. Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 2007, 47, S-202–S-205. [Google Scholar] [CrossRef]
- Thomas, H.; Smart, C.M. Crops that stay green. Ann. Appl. Biol. 1993, 123, 193–219. [Google Scholar] [CrossRef]
- Erley, G.S.; Begum, N.; Worku, M.; Bänziger, M.; Horst, W.J. Leaf senescence induced by nitrogen deficiency as indicator of genotypic differences in nitrogen efficiency in tropical maize. J. Plant Nutr. Soil Sci. 2007, 170, 106–114. [Google Scholar] [CrossRef]
- Robson, P.R.H.; Farrar, K.; Gay, A.P.; Jensen, E.F.; Clifton-Brown, J.C.; Donnison, L.S. Variation in canopy duration in the perennial biofuel crop Miscanthus reveals complex associations with yield. J. Exp. Bot. 2013, 64, 2373–2383. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.W.; Pan, X.F.; Guo, X.D.; Fan, K.; Lin, W.X. Physiological and Transcriptome Analyses of Early Leaf senescence for ospls1 mutant rice (Oryza sativa L.) during the grain-filling stage. Int. J. Mol. Sci. 2019, 20, 1098. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, Y.; Li, J.; Ren, Y.; Wang, Z.; Xin, Z.; Lin, T. Identification of Two Novel Wheat Drought Tolerance-Related Proteins by Comparative Proteomic Analysis Combined with Virus-Induced Gene Silencing. Int. J. Mol. Sci. 2018, 19, 4020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.Y.; Ma, X.Z.; Liao, S.Y.; Shi, H.; Liu, C.Y.; Shen, Y.; Lv, X.; Yuan, M.T.; Fan, G.J.; Huang, J.; et al. Cellulose nanocrystal surface cationization: A new fungicide with high activity against phycomycetes capsic. Molecules 2019, 24, 2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Liu, Z.Q.; Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, K.K.; Liu, W.; Nie, G.; He, L.W. Regulation of heat shock factor pathways by γ-aminobutyric acid (GABA) associated with thermotolerance of creeping bentgrass. Int. J. Mol. Sci. 2019, 20, 4713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi. J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; Camara, C.A.; Cabral, F.F.; Oliveiar, J.F.; Carvalho, L.W.T.; Santos, J.M.; Filho, B.G.S. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L.) seedlings subjected to salt stress conditions. Ind. Crop Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Biswas, A.K.; Choudhuri, M.A. Mechanism of monocarpic senescence in rice. Plant Physiol. 1980, 65, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Sivasankar, A.; Lakkineni, K.C.; Jain, V.; Abrol, Y.P. Differential response of two wheat genotypes to nitrogen supply. I. Ontogenic changes in laminae growth and photosynthesis. J. Agron. Crop Sci. 1998, 181, 21–27. [Google Scholar] [CrossRef]
- Vos, J.; Putten, P.E.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crop. Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Taylor, L.; Nunes-Nesi, A.; Parsley, K.; Leiss, A.; Leach, G.; Coates, S.; Wingler, A.; Fernie, A.R.; Hibberd, J.M. Cytosolic pyruvate, orthophosphate dikinase functions in nitrogen remobilization during leaf senescence and limits individual seed growth and nitrogen content. Plant J. 2010, 62, 641–652. [Google Scholar] [CrossRef]
- Pommel, B.; Gallais, A.; Coque, M.; Quilleré, I.; Hirel, B.; Prioul, J.K.; Andrieu, B.; Floriot, M. Carbon and nitrogen allocation and grain filling in three maize hybrids differing in leaf senescence. Eur. J. Agron. 2006, 24, 203–211. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Wu, D.; Xia, T.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Physiological and genetic mechanisms for nitrogen-use efficiency in maize. J. Crop Sci. Biotechnol. 2007, 10, 57–63. [Google Scholar]
- Zhang, W.; Dou, Z.; He, P.; Ju, X.; Powlson, D.; Chadwick, D.; Norse, D.; Liu, Y.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Han, W.; Tang, O.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Mi, G.; Chen, F.; Li, F.; Chen, X.; Li, J.; Shi, L. Interaction between genotypic difference and nitrogen management strategy in determining nitrogen use efficiency of summer maize. Plant Soil 2009, 317, 267–276. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, G.; Yue, S.; Wu, L.; Zhang, W.; Zhang, F.; Chen, X. Closing the N-Use efficiency gap to achieve food and environmental security. Environ. Sci. Technol. 2014, 48, 5780–5787. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef]
- Matile, P.; Hortensteiner, S.; Thomas, H. Chlorophyll Degradation. Annu. Rev. Plant Biol. 2006, 50, 67–95. [Google Scholar] [CrossRef] [Green Version]






| No. | Cultivar | No. | Cultivar |
|---|---|---|---|
| 1 | Zhengdan958 | 11 | Denghai939 |
| 2 | Xianyu048 | 12 | Fengdecunyu10 |
| 3 | Lianchuang808 | 13 | Lianchuang839 |
| 4 | Cunyu10 | 14 | Lianchuang825 |
| 5 | Dika653 | 15 | Denghai605 |
| 6 | DikaJ1652 | 16 | Denghai533 |
| 7 | D4111 | 17 | Denghai685 |
| 8 | Dedan5 | 18 | Xianyu1466 |
| 9 | Denghai618 | 19 | Xianyu1366 |
| 10 | D4117 | 20 | Xianyu335 |
| Cultivar | N Level | N Recovery Efficiency (%) | N PFP (kg kg−1) | N Harvest Index (%) |
|---|---|---|---|---|
| 2018 | ||||
| LC808 | N0 | - | - | 65.2a |
| N120 | 33.5a | 57.0a | 60.3a | |
| N180 | 34.0a | 38.6b | 65.7a | |
| N240 | 37.1a | 26.0c | 62.6a | |
| N360 | 22.3b | 16.8d | 62.1a | |
| ZD958 | N0 | - | - | 73.2a |
| N120 | 38.6a | 63.2a | 76.4a | |
| N180 | 35.0a | 44.8b | 72.1a | |
| N240 | 34.7a | 33.8c | 70.0a | |
| N360 | 28.3a | 21.5d | 63.1b | |
| DH685 | N0 | - | - | 58.5b |
| N120 | 47.9a | 70.2a | 66.6a | |
| N180 | 48.8a | 51.4b | 58.8b | |
| N240 | 47.3a | 37.6c | 58.2b | |
| N360 | 31.9b | 23.6d | 54.7b | |
| 2019 | ||||
| LC808 | N0 | - | - | 62.8a |
| N120 | 48.6a | 59.1a | 63.3a | |
| N180 | 48.7a | 45.2b | 57.7b | |
| N240 | 41.4a | 37.0c | 58.8b | |
| N360 | 33.9b | 24.1d | 52.6b | |
| ZD958 | N0 | - | - | 64.2a |
| N120 | 54.2a | 76.7a | 62.0ab | |
| N180 | 51.9a | 53.8b | 63.9a | |
| N240 | 46.1ab | 39.6c | 57.6b | |
| N360 | 33.6b | 25.4d | 60.3ab | |
| DH685 | N0 | - | - | 61.0a |
| N120 | 55.5a | 67.8a | 60.8a | |
| N180 | 45.6ab | 49.9b | 54.8ab | |
| N240 | 38.0ab | 38.9c | 56.3ab | |
| N360 | 28.3b | 26.2d | 51.3b | |
| ANOVA | ||||
| N rate (N) | 14.01 ** | 546.17 ** | 0.47 | |
| Cultivar (C) | 2.54 | 46.42 ** | 14.71 ** | |
| Year (Y) | 14.48 ** | 43.24 ** | 18.25 ** | |
| N×C | 0.34 | 2.99 * | 2.58 * | |
| N×Y | 1.67 | 0.19 | 2.78* | |
| C×Y | 6.05 ** | 1.36 | 2.75 | |
| N×C×Y | 0.49 | 1.49 | 1.17 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Wang, Y.; Ye, Y.; Zhen, S.; Zhou, B.; Wang, Y.; Hu, Y.; Zhao, Y.; Huang, Y. Grain Yields and Nitrogen Use Efficiencies in Different Types of Stay-Green Maize in Response to Nitrogen Fertilizer. Plants 2020, 9, 474. https://doi.org/10.3390/plants9040474
Fu W, Wang Y, Ye Y, Zhen S, Zhou B, Wang Y, Hu Y, Zhao Y, Huang Y. Grain Yields and Nitrogen Use Efficiencies in Different Types of Stay-Green Maize in Response to Nitrogen Fertilizer. Plants. 2020; 9(4):474. https://doi.org/10.3390/plants9040474
Chicago/Turabian StyleFu, Wen, Yang Wang, Youliang Ye, Shuai Zhen, Binghui Zhou, Yin Wang, Yujie Hu, Yanan Zhao, and Yufang Huang. 2020. "Grain Yields and Nitrogen Use Efficiencies in Different Types of Stay-Green Maize in Response to Nitrogen Fertilizer" Plants 9, no. 4: 474. https://doi.org/10.3390/plants9040474




