Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Makeup of Saline Waters
2.2. Experimental Setup and Statistical Analysis
2.3. Application of Salinity Treatments
2.4. Plant and Soil Collection for Analysis
3. Results
3.1. Salinity Effects on Soil Na, Cl, K, and NO3−
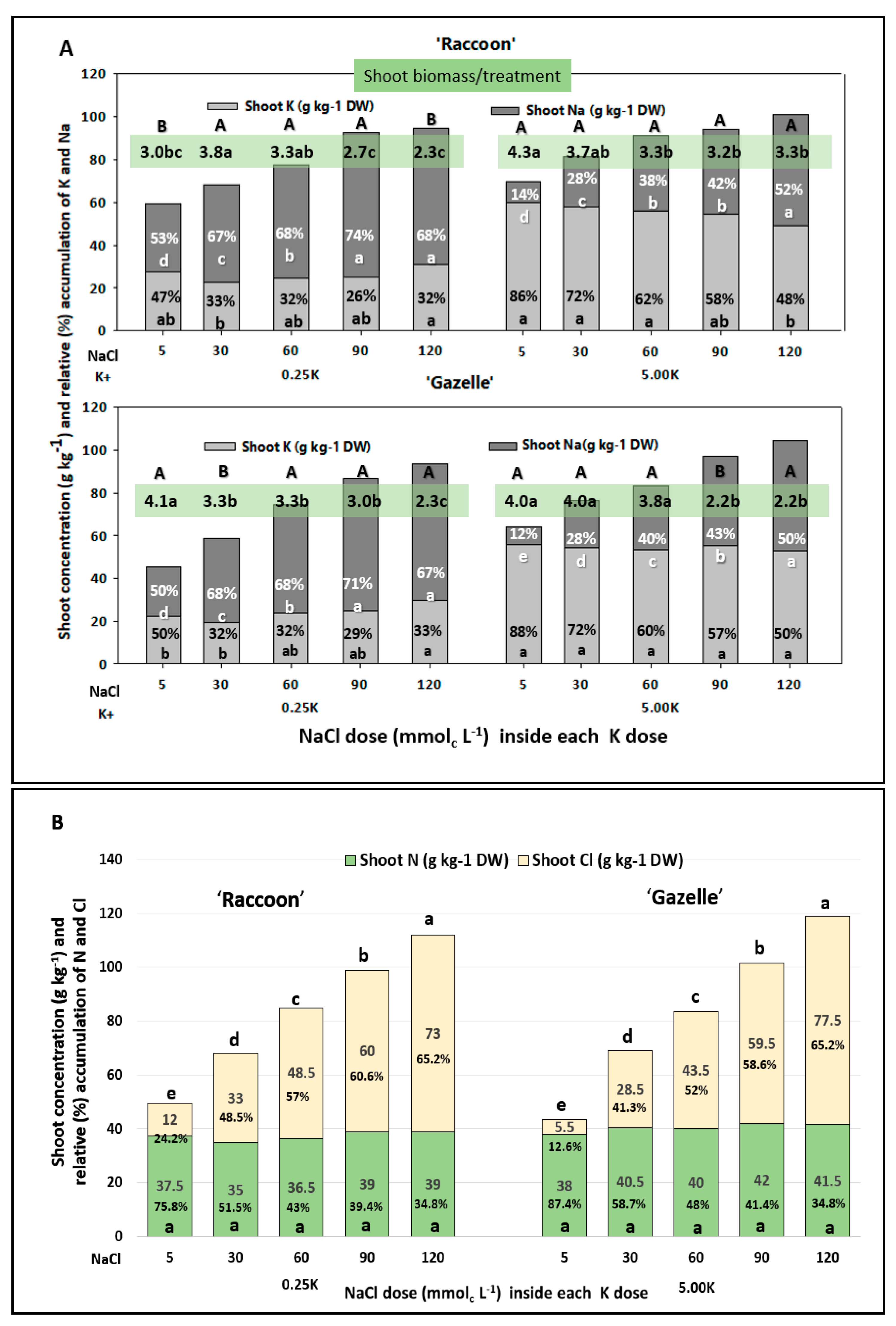
3.2. Shoot Na and Cl Concentrations and Their Relation to K
3.3. Mineral Leaf Composition in Response to NaCl and K Doses
3.4. Interaction among Potassium, Salinity, and Micronutrients
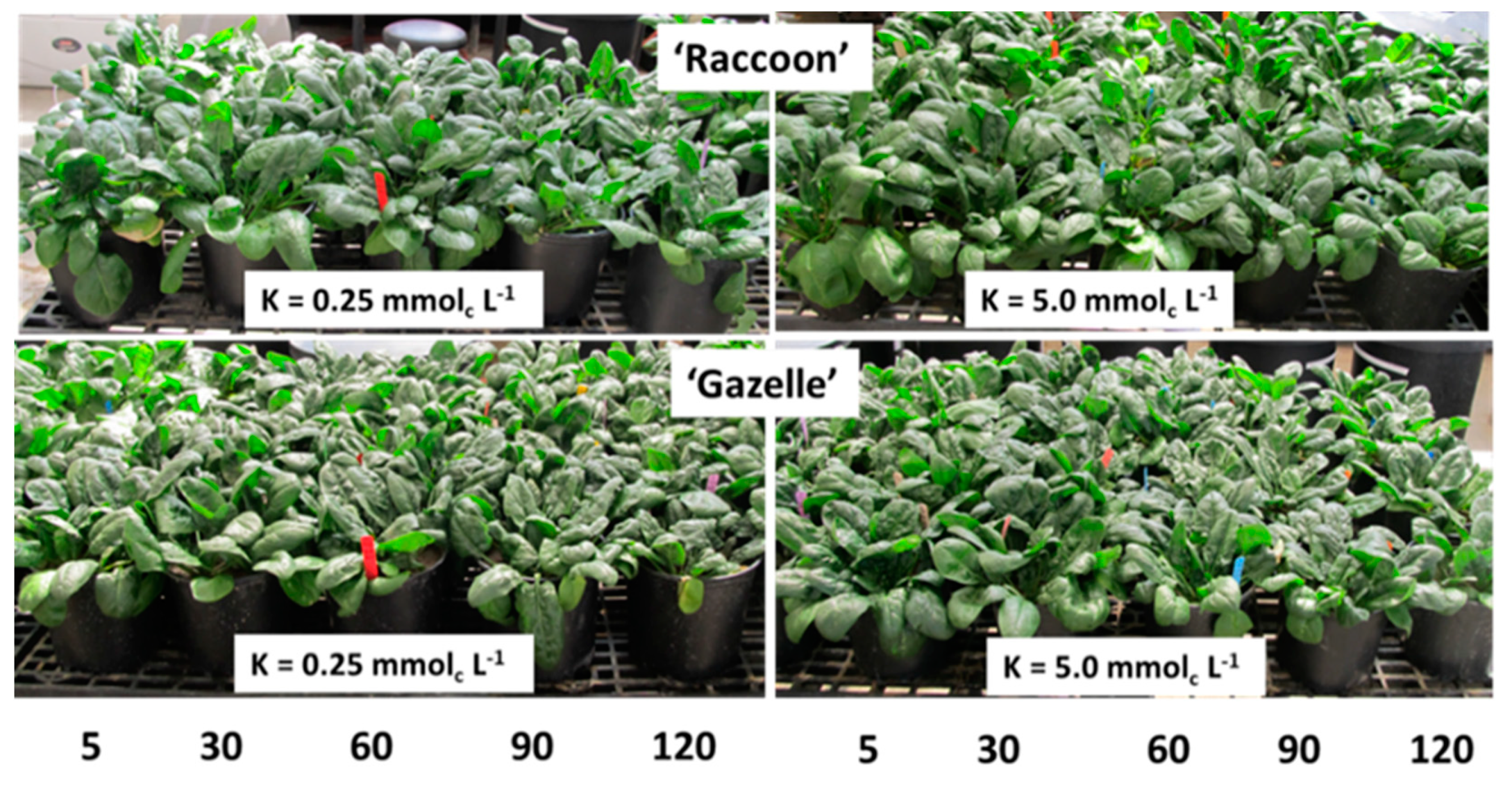
3.5. Effect of NaCl and K Doses on Spinach Biomass
4. Discussion
4.1. Salinity Effects on Soil Na, Cl, K, and NO3−
4.2. Shoot Na and Cl Concentrations and Their Relation to N and K
4.3. Mineral Shoot Composition in Response to NaCl and K Doses
4.4. Interaction among Potassium, Salinity and Macronutrients
4.5. Interaction among Potassium, Salinity, and Micronutrients
4.6. Effect of NaCl and K Doses on Spinach Biomass
4.7. Considerations on Salinity Tolerance Mechanisms of Spinach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Func. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hort. 1999, 78, 127–157. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Williams, M.C. Effect of sodium and potassium salts on growth and oxalate content of halogeton. Plant Physiol. 1960, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Mata-González, R.; Abdallah, M.A.B.; Trejo-Calzada, R.; Wan, C. Growth and leaf chemistry of Atriplex species from Northern Mexico as affected by salt stress. Arid Land Res. Manag. 2016, 1–14. [Google Scholar] [CrossRef]
- Marschner, P.; Broadley, M.; Buerkert, A.; Cakmak, I.; Others, S. Marschner's Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ Nutrition and Na+ Toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S.-R. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Faust, F.; Schubert, S. Protein synthesis is the most sensitive process when potassium is substituted by sodium in the nutrition of sugar beet (Beta vulgaris). Plant Physiol. Biochem. 2016, 107, 237–247. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wheeler, R.M.; Stutte, G.W.; Levine, L.H. How far can sodium substitute for potassium in red beet? J. Plant Nutr. 1999, 22, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. In ASCE Manual and Reports on Engineering Practice, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers: Riston, VA, USA, 2012; pp. 405–459. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance- current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Ors, S.; Suarez, D.L. Salt tolerance of spinach as related to seasonal climate. Hort. Sci. (Prague) 2016, 43, 33–41. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hort. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Speer, M.; Kaiser, W.M. Ion relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L. under salinity. Plant Physiol. 1991, 97, 990–997. [Google Scholar] [CrossRef]
- Lehr, J.J. Exploratory pot experiments on sensitiveness of different crops to sodium: A. Spinach. Plant Soil 1949, 2, 37–48. [Google Scholar] [CrossRef]
- Tomemori, H.; Hamamura, K.; Tanabe, K. Interactive effects of sodium and potassium on the growth and photosynthesis of spinach and komatsuna. Plant Prod. Sci. 2002, 5, 281–285. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional Value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–Status and perspectives. J. Plant. Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Ródenas, R.; Lara, A.; Martínez, V.; Rubio, F. The combination of K+ deficiency with other environmental stresses: What is the outcome? Physiol. Plant. 2019, 165, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wu, W.; Wu, W.-H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Simunek, J. UNSATCHEM: Unsaturated water and solute transport model with equilibrium and kinetic chemistry. Soil Sci. Soc. Am. J. 1997, 61, 1633–1646. [Google Scholar] [CrossRef]
- Ferreira, J.; Sandhu, D.; Liu, X.; Halvorson, J.J. Spinach (Spinacea oleracea L.) response to salinity: Nutritional value, physiological parameters, antioxidant capacity, and gene expression. Agriculture 2018, 8, 163. [Google Scholar] [CrossRef]
- Lacerda, C.F.; Ferreira, J.F.; Suarez, D.L.; Freitas, E.D.; Liu, X.; Ribeiro, A.A. Evidence of nitrogen and potassium losses in soil columns cultivated with maize under salt stress. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 553–557. [Google Scholar] [CrossRef]
- Wakeel, A.; Abd-El-Motagally, F.; Steffens, D.; Schubert, S. Sodium-induced calcium deficiency in sugar beet during substitution of potassium by sodium. J. Plant Nutr. Soil Sci. 2009, 172, 254–260. [Google Scholar] [CrossRef]
- White, P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- Öztekin, G.; Uludağ, T.; Tüzel, Y. Growing spinach (Spinacia oleracea L.) in a floating system with different concentrations of nutrient solution. Appl. Ecol. Env. Res. 2018, 16, 3333–3350. [Google Scholar] [CrossRef]
- Sheikhi, J.; Ronaghi, A. Growth and macro and micronutrients concentration in spinach (Spinacia oleracea L.) as influenced by salinity and nitrogen rates. Int. Res. J. Appl. Basic Sci. 2012, 3, 770–777. [Google Scholar]
- Díaz, F.J.; Grattan, S.R.; Reyes, J.A.; de la Roza-Delgado, B.; Benes, S.E.; Jiménez, C.; Dorta, M.; Tejedor, M. Using saline soil and marginal quality water to produce alfalfa in arid climates. Agric. Water Manag. 2018, 199, 11–21. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Cornacchione, M.V.; Liu, X.; Suarez, D.L. Nutrient composition, forage parameters, and antioxidant capacity of alfalfa (Medicago sativa, L.) in response to saline irrigation water. Agriculture 2015, 5, 577–597. [Google Scholar] [CrossRef]
- University of Florida. Nutrient Management of Vegetable and Row Crops handbook (SP500); University of Florida: Gainesville, FL, USA, 2015. [Google Scholar]
- Nemadodzi, L.E.; Araya, H.; Nkomo, M.; Ngezimana, W.; Mudau, N.F. Nitrogen, phosphorus, and potassium effects on the physiology and biomass yield of baby spinach (Spinacia oleracea L.). J. Plant Nutr. 2017, 40, 2033–2044. [Google Scholar] [CrossRef]
- Cachorro, P.; Ortiz, A.; Cerdá, A. Growth, water relations and solute composition of Phaseolus vulgaris L. under saline conditions. Plant Sci. 1993, 95, 23–29. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Fang, H.H. Inward-rectifying K+ channels in guard cells provide a mechanism for low-affinity K+ uptake. Proc. Natl. Acad. Sci. USA 1991, 88, 11583–11587. [Google Scholar] [CrossRef] [PubMed]
- Bar-Tal, A.; Feigenbaum, S.; Sparks, D.L. Potassium-salinity interactions in irrigated corn. Irrig. Sci. 1991, 12, 27–35. [Google Scholar] [CrossRef]
- Ashley, M.K.; Grant, M.; Grabov, A. Plant responses to potassium deficiencies: A role for potassium transport proteins. J. Exp. Bot. 2006, 57, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Jakobsen, S.T. Interaction between plant nutrients: III. Antagonism between potassium, magnesium and calcium. Acta Agric. Scand. Sect. B—Soil Plant Sci. 1993, 43, 1–5. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Past. Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotox. Environ. Saf. 2019, 184, 109671. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, V.; Rahemi, M.; Maftoun, M.; Panahi, B.; Karimi, S.; Ramezanian, A.; Vaezpour, M. Zinc influence and salt stress on photosynthesis, water relations, and carbonic anhydrase activity in pistachio. Sci. Hort. 2009, 123, 272–279. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Plett, D.C.; Møller, I.S. Na+ transport in glycophytic plants: What we know and would like to know. Plant Cell Environ. 2010, 33, 612–626. [Google Scholar] [CrossRef]
- Gattward, J.N.; Almeida, A.-A.F.; Souza, J.O.; Gomes, F.P.; Kronzucker, H.J. Sodium–potassium synergism in Theobroma cacao: Stimulation of photosynthesis, water-use efficiency and mineral nutrition. Physiol. Plant. 2012, 146, 350–362. [Google Scholar] [CrossRef]
- Almeida, J.C.R.; Laclau, J.-P.; Gonçalves, J.L.M.; Ranger, J.; Saint-André, L. A positive growth response to NaCl applications in Eucalyptus plantations established on K-deficient soils. For. Ecol. Manag. 2010, 259, 1786–1795. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride: From nutrient to toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 2009, 166, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kuroda, C.; Fujiyama, H. Growth promotion by sodium in amaranthaceous plants. J. Plant Nutr. 2016, 39, 1186–1193. [Google Scholar] [CrossRef]
- Yamada, M.; Kuroda, C.; Fujiyama, H. Function of sodium and potassium in growth of sodium-loving Amaranthaceae species. Soil Sci. Plant Nutr. 2016, 62, 20–26. [Google Scholar] [CrossRef]



| Treatment | * K+ | * Na+ | * Cl− | PO43− | * Ca2+ | * Mg2+ | * SO42− | * NO3− | ECiw | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| mmolc L−1 | dS m−1 | |||||||||
| T1_0 | 0.25 | 6.5 | 1.1 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 1.3 | 5.5 |
| T1_1 | 0.25 | 30.5 | 25.1 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 4.2 | 5.4 |
| T1_2 | 0.25 | 60.5 | 55.1 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 7.2 | 5.3 |
| T1_3 | 0.25 | 90.5 | 85.1 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 10.4 | 5.3 |
| T1_4 | 0.25 | 120.5 | 115.1 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 13.1 | 5.3 |
| T2_0 | 5.00 | 3.0 | 2.4 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 1.6 | 5.4 |
| T2_1 | 5.00 | 30.5 | 29.9 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 4.5 | 5.4 |
| T2_2 | 5.00 | 60.5 | 59.9 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 7.6 | 5.4 |
| T2_3 | 5.00 | 90.5 | 89.9 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 10.7 | 5.3 |
| T2_4 | 5.00 | 120.5 | 119.9 | 1.5 | 6.0 | 2.5 | 4.5 | 10.0 | 13.2 | 5.3 |
| ‘Raccoon’ | ‘Gazelle’ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K Doses | NaCl Doses (mmolc L−1) Used to Irrigate Pots | |||||||||
| Used | 5 | 30 | 60 | 90 | 120 | 5 | 30 | 60 | 90 | 120 |
| (mmolc L−1) | NO3−, mmolc L−1 | |||||||||
| 0.25 | 1.78aA | 1.32aA | 1.28aA | 2.96aA | 5.31aA | 1.51aBC | 0.98aC | 1.90aBC | 2.30aB | 5.83aA |
| 5 | 1.43aA | 1.24aA | 1.29aA | 1.64aA | 1.83aA | 2.28aAB | 1.77aB | 2.78aAB | 3.34aA | 3.34bA |
| PO4−, mmolc L−1 | ||||||||||
| 0.25 | 0.52aB | 0.50aB | 0.57aB | 0.69aA | 0.03bC | 0.46aA | 0.41aA | 0.59aA | 0.48aA | 0.00bA |
| 5 | 0.32bB | 0.53aA | 0.60aA | 0.52bA | 0.53aA | 0.33aA | 0.44aA | 0.50aA | 0.62aA | 0.65aA |
| K+, mmolc L−1 | ||||||||||
| 0.25 | 0.13bA | 0.25bA | 0.29bA | 0.33bA | 0.38bA | 0.13bA | 0.20bA | 0.22bA | 0.29bA | 0.33bA |
| 5 | 1.17aD | 1.56aC | 2.08aB | 2.36aAB | 2.41aA | 1.36aBC | 1.19aC | 1.64aB | 2.29aA | 2.46aA |
| Ca2+, mmolc L−1 | ||||||||||
| 0.25 | 3.44bAB | 3.57aAB | 3.19aB | 3.86aAB | 4.16aA | 4.11aAB | 5.40aA | 3.63aB | 4.82aAB | 3.96aB |
| 5 | 5.45aA | 3.60aB | 3.59aB | 4.07aB | 4.43aB | 5.31aA | 4.39aA | 4.74aA | 4.32aA | 4.41aA |
| Mg+, mmolc L−1 | ||||||||||
| 0.25 | 1.36bA | 1.25aA | 1.10aA | 1.28aA | 1.39aA | 1.66aAB | 1.90aA | 1.25aB | 1.53aAB | 1.36aB |
| 5 | 1.89aA | 1.13aB | 1.12aB | 1.22aB | 1.29aB | 2.02aA | 1.43bB | 1.46aB | 1.29aB | 1.30aB |
| SO4−, mmolc L−1 | ||||||||||
| 0.25 | 2.24aB | 2.13aB | 2.64aA | 2.72aA | 1.91aB | 2.82aA | 2.77aA | 2.76aA | 3.11aA | 1.90aB |
| 5 | 2.57aA | 1.86aB | 1.86bB | 1.93bB | 1.87aB | 2.45aA | 2.17bAB | 2.07bABC | 1.77bBC | 1.61aC |
| Na+,mmolc L−1 | ||||||||||
| 0.25 | 4.15aE | 26.97aD | 48.81aC | 73.51aB | 95.50aA | 5.03aE | 30.70aD | 49.33aC | 79.69aB | 94.69aA |
| 5 | 2.30aE | 24.68aD | 50.78aC | 76.42aB | 101.27aA | 2.01aE | 29.39aD | 54.31aC | 76.02aB | 94.35aA |
| Cl−, mmolc L−1 | ||||||||||
| 0.25 | 2.67aE | 24.24aD | 44.73aC | 71.54aB | 97.07bA | 2.55aE | 30.68aD | 47.84aC | 78.11aB | 94.09aA |
| 5 | 4.18aE | 24.46aD | 52.07aC | 81.56aB | 107.67aA | 1.62aE | 26.84aD | 54.38aC | 77.74aB | 97.34aA |
| pH | ||||||||||
| 0.25 | 8.06aA | 8.11aA | 8.12aA | 8.10aA | 8.09aA | 8.12aAB | 8.06aB | 8.09aAB | 8.22aAB | 8.30aA |
| 5 | 8.07aA | 8.08aA | 8.09aA | 8.05aA | 7.97bA | 8.24aA | 8.26aA | 8.23aA | 8.04aA | 8.12aA |
| EC, dS m−1 | ||||||||||
| 0.25 | 0.92aE | 3.46aD | 5.61aC | 8.04aB | 10.37aA | 1.06aE | 4.14aD | 5.86aC | 8.91aB | 10.24aA |
| 5 | 1.07aE | 3.46aD | 6.07aC | 8.85aB | 11.17aA | 1.08aE | 3.91aD | 6.53aC | 8.61aB | 10.41aA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.F.S.; da Silva Filho, J.B.; Liu, X.; Sandhu, D. Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil. Plants 2020, 9, 507. https://doi.org/10.3390/plants9040507
Ferreira JFS, da Silva Filho JB, Liu X, Sandhu D. Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil. Plants. 2020; 9(4):507. https://doi.org/10.3390/plants9040507
Chicago/Turabian StyleFerreira, Jorge F. S., Jaime Barros da Silva Filho, Xuan Liu, and Devinder Sandhu. 2020. "Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil" Plants 9, no. 4: 507. https://doi.org/10.3390/plants9040507
APA StyleFerreira, J. F. S., da Silva Filho, J. B., Liu, X., & Sandhu, D. (2020). Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil. Plants, 9(4), 507. https://doi.org/10.3390/plants9040507








