Cycas micronesica Stem Carbohydrates Decline Following Leaf and Male Cone Growth Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Methods
2.3. Tissue and Data Analysis
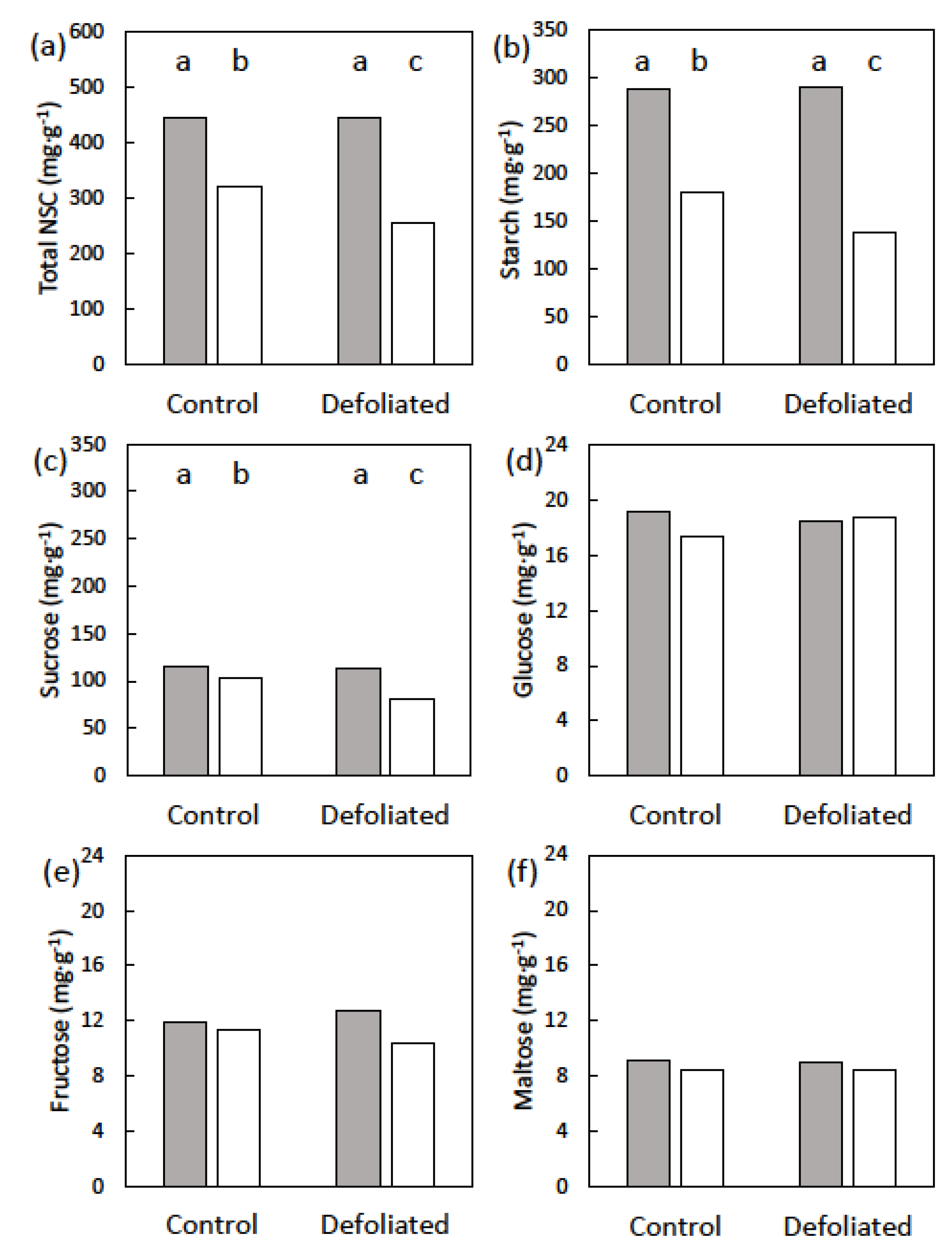
3. Results
3.1. Male Cone Trees
3.2. Leaf Flush Trees
4. Discussion
4.1. Stem NSC Behavior
4.2. Traditional Knowledge
4.3. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997. [Google Scholar]
- Niklas, K.J.; Cobb, E.D.; Marler, T. A comparison between the record height-to-stem diameter allometries of pachycaulis and leptocaulis species. Ann. Bot. 2006, 97, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrazas, T. Origin and activity of successive cambia in Cycas (Cycadales). Amer. J. Bot. 1991, 78, 1335–1344. [Google Scholar] [CrossRef]
- Fisher, J.B.; Lindström, A.; Marler, T. Tissue responses and solution movement after stem wounding in six Cycas species. HortScience 2009, 2009 44, 848–851. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.; Fisher, J.B. Stem tissue dimensions correlate with ease of horticultural management for six Cycas species. HortScience 2010, 2010 45, 1293–1296. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E. Axial and radial spatial patterns of non-structural carbohydrates in Cycas micronesica stems. Plants 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marler, T.E. Stem carbohydrates and adventitious root formation of Cycas micronesica following Aulacaspis yasumatsui infestation. HortScience 2018, 53, 1125–1128. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Cascasan, A.N.J. Carbohydrate depletion during lethal infestation of Aulacaspis yasumatsui on Cycas revoluta. Int. J. Plant Sci. 2018, 179, 497–504. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Free sugar profile in cycads. Front. Plant Sci. 2014, 5, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marler, T.E.; Dongol, N. Models to describe Cycas micronesica leaf and strobili development. HortScience 2011, 46, 1333–1337. [Google Scholar] [CrossRef]
- Dongol, N.; Marler, T.E. Season and frequency of Cycas micronesica leaf and reproductive events. Mem. N. Y. Bot. Gard. 2018, 117, 497–503. [Google Scholar]
- Hill, K.D. The Cycas rumpnii complex (Cycadaceae) in New Guinea and the Western Pacific. Aust. Syst. Bot. 1994, 7, 543–567. [Google Scholar] [CrossRef]
- Roemer, R.; Terry, I.; Walter, G.; Marler, T.E. Comparison of experimental measurements and predictions from biophysical models of thermogenic events in cones of Macrozamia lucida, M. macleayi and Cycas micronesica. Mem. N. Y. Bot. Gard. 2012, 106, 302–317. [Google Scholar]
- Roemer, R.B.; Terry, L.I.; Marler, T.E. Cone thermogenesis and its limits in the tropical Cycas micronesica (Cycadaceae): Association with cone growth, dehiscence, and post-dehiscence phases. Amer. J. Bot. 2013, 100, 1981–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marler, T.E.; Lee, V.; Shaw, C.A. Habitat heterogeneity of Cycas micronesica seed chemistry in Guam’s forest. Micronesica 2007, 39, 297–314. [Google Scholar]
- Marler, T.E.; Cruz, G.N. Source and sink relations mediate depletion of intrinsic cycad seed carbohydrates by Aulacaspis yasumatsui infestation. HortScience 2019, 54, 1712–1717. [Google Scholar] [CrossRef] [Green Version]
- Young, F.J. Soil survey of Territory of Guam; U.S. Dept. Agr. Soil Conservation Service: Washington, DC, USA, 1988. [Google Scholar]
- Prado, A.; Rubio-Mendez, G.; Yañez-Espinosa, L.; Bede, J.C. Ontogenetic changes in azoxyglycoside levels in the leaves of Dioon edule Lindl. J. Chem. Ecol. 2016, 42, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Krieg, C.; Watkins, J.E.; Chambers, S.; Husby, C.E. Sex-specific differences in functional traits and resource acquisition in five cycad species. AoB Plants 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Shaw, C.A. Distribution of free and glycosylated sterols within Cycas micronesica plants. Sci. Hortic. 2010, 123, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Holzman, G. Foam filler for cycad trunk wounds. Cycad Newsl. 2005, 28, 16. [Google Scholar]
- Schloter, M.; Winkler, J.B.; Aneja, M.; Koch, N.; Fleischmann, F.; Pritsch, K.; Heller, W.; Stich, S.; Grams, T.E.; Göttlein, A.; et al. Short term effects of ozone on the plant-rhizosphere-bulk soil system of young beech trees. Plant Biol. 2005, 7, 728–736. [Google Scholar] [CrossRef] [Green Version]
- American Association of Cereal Chemists (AACC). Method 76-11. In Approved Methods of the American Association of Cereal Chemists, 8th ed.; AACC: St. Paul, MN, USA, 1985. [Google Scholar]
- Carlquist, S. Living cells in wood 3. Overview; functional anatomy of the parenchyma network. Bot. Rev. 2018, 84, 242–294. [Google Scholar] [CrossRef]
- Marler, T.E. Cycad mutualist offers more than pollen transport. Amer. J. Bot. 2010, 97, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Niklas, N. Reproductive effort and success of Cycas micronesica K.D. Hill are affected by habitat. Int. J. Plant Sci. 2011, 172, 700–706. [Google Scholar] [CrossRef]
- Marler, T.E. Time-size trade-offs in responses of cycads to male cone herbivory. Communic. Integr. Biol. 2010, 3, 602–603. [Google Scholar] [CrossRef]
- Thieret, J.W. Economic botany of the cycads. Econ. Bot. 1958, 12, 3–41. [Google Scholar] [CrossRef]
- Whiting, M.G. Toxicity of cycads, a literature review. Econ. Bot. 1963, 17, 270–302. [Google Scholar] [CrossRef]
- Yumnam, J.Y.; Tripathi, O.P. Traditional knowledge of eating raw plants by the Meitei of Manipur as medicine/nutrient supplement in their diet. Indian J. Tradit. Knowl. 2012, 11, 45–50. [Google Scholar]
- Bonta, M.; Pulido-Silva, M.T.; Diego-Vargas, T.; Vite-Reyes, A.; Vovides, A.P.; Cibrián-Jaramillo, A. Ethnobotany of Mexican and northern Central American cycads (Zamiaceae). J. Ethnobiol. Ethnomed. 2019, 15, 4. [Google Scholar] [CrossRef] [Green Version]




| Variable | Cone Flush Defoliated | Cone Flush Control | Leaf Flush Defoliated | Leaf Flush Control |
|---|---|---|---|---|
| Stem height (m) | 2.29 ± 0.03 | 2.33 ± 0.04 | 2.38 ± 0.04 | 2.42 ± 0.02 |
| Base diameter (cm) | 25.8 ± 1.6 | 25.1 ± 1.5 | 25.9 ± 1.6 | 26.2 ± 1.3 |
| Pith diameter (cm) | 5.0 ± 0.5 | 5.2 ± 0.4 | 5.3 ± 0.3 | 5.2 ± 0.4 |
| Vascular diameter (cm) | 2.0 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.2 |
| Cortex diameter (cm) | 3.1 ± 0.3 | 3.2 ± 0.2 | 3.2 ± 0.2 | 3.1 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E.; Cruz, G.N. Cycas micronesica Stem Carbohydrates Decline Following Leaf and Male Cone Growth Events. Plants 2020, 9, 517. https://doi.org/10.3390/plants9040517
Marler TE, Cruz GN. Cycas micronesica Stem Carbohydrates Decline Following Leaf and Male Cone Growth Events. Plants. 2020; 9(4):517. https://doi.org/10.3390/plants9040517
Chicago/Turabian StyleMarler, Thomas E., and Gil N. Cruz. 2020. "Cycas micronesica Stem Carbohydrates Decline Following Leaf and Male Cone Growth Events" Plants 9, no. 4: 517. https://doi.org/10.3390/plants9040517






