Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Light Treatments, and Culture Conditions
2.2. Measurements of the Growth Parameters
2.3. Scanning Electron Microscopy (SEM) of Stomata
2.4. Stomatal Conductance and Quantum Yield (Fv/Fm)
2.5. Soluble Sugars, Starch, and Soluble Proteins
2.6. Measurements of Chlorophyll A and B Contents
2.7. Data Collection
3. Results
3.1. Stomatal Morphology and Characteristics
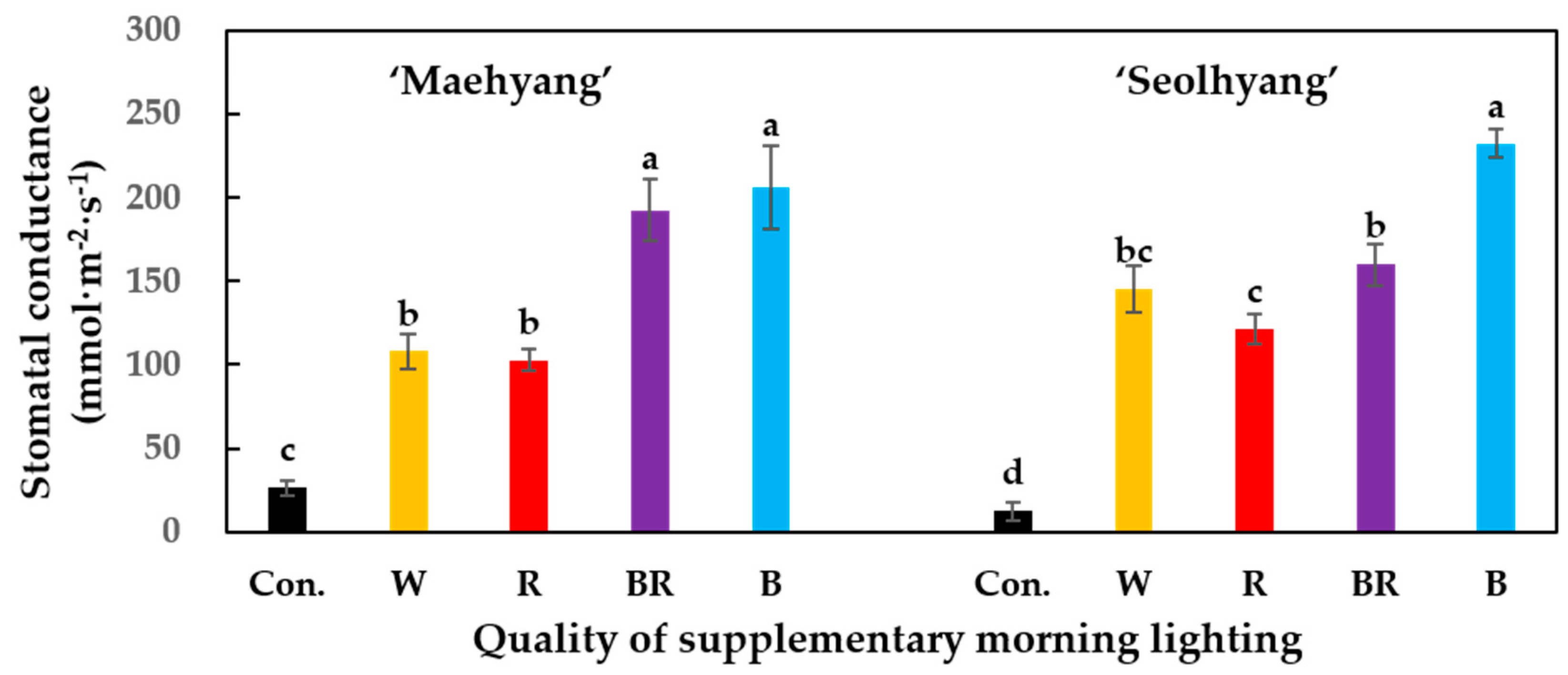
3.2. Stomatal Conductance and Quantum Yield (Fv/Fm)
3.3. Growth and Development Parameters
3.4. Chlorophyll Contents
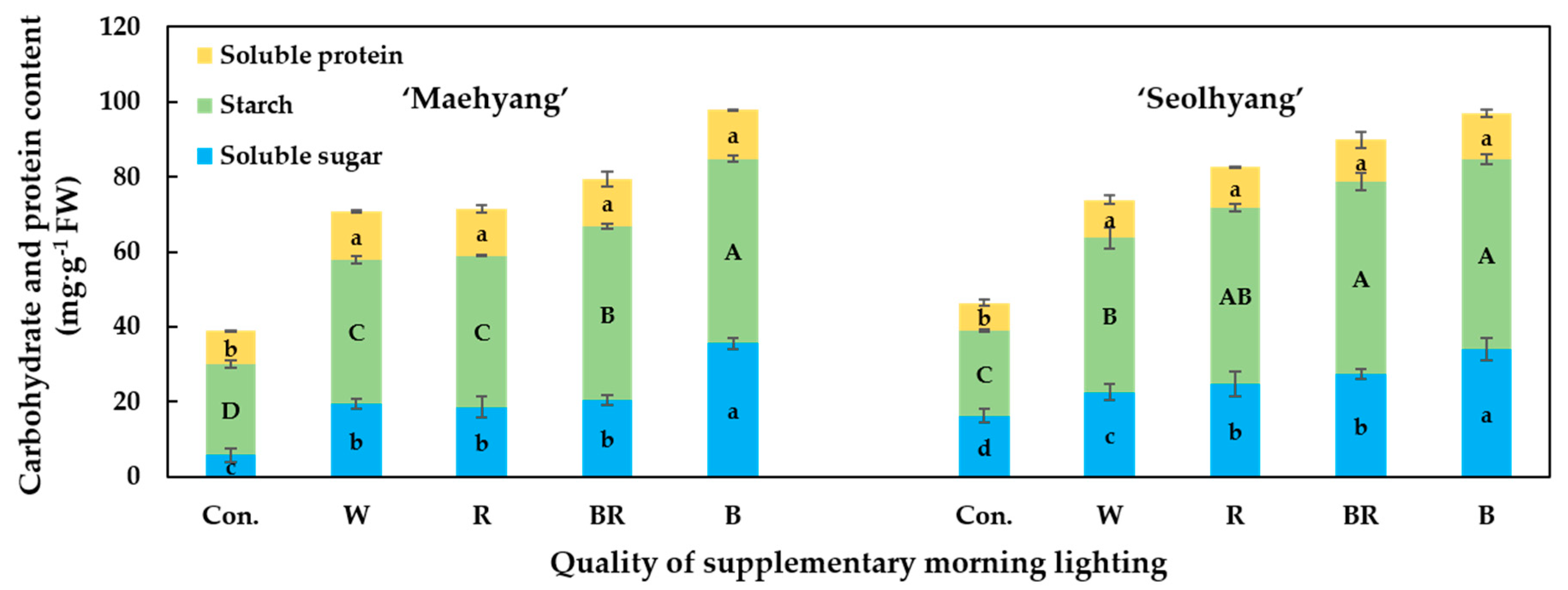
3.5. Contents of the Soluble Sugars, Starch, and Soluble Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korea Rural Revitalization Hall. 2016. Available online: http://www.rda.go.kr/ (accessed on 27 April 2020).
- Korean Statistical Information Service. 2017. Available online: http://kosis.kr (accessed on 27 April 2020).
- Wojciechowska, R.; Kurpaska, S.; Malinowski, M.; Sikora, J.; Krakowiak-Bal, A.; Długosz-Grochowska, O. Effect of supplemental led lighting on growth and quality of Valerianella locusta L. and economic aspects of cultivation in autumn cycle. Acta Sci. Pol-Hortoru 2016, 15, 233–244. [Google Scholar]
- Wei, H.; Hu, J.; Liu, C.; Wang, M.; Zhao, J.; Kang, D.; Jeong, B.R. Effect of supplementary light source on quality of grafted tomato seedlings and expression of two photosynthetic genes. Agronomy 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Zhao, J.; Hu, J.; Jeong, B.R. Effect of supplementary light intensity on quality of grafted tomato seedlings and expression of two photosynthetic genes and proteins. Agronomy 2019, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, M.; Jeong, B.R. Effect of Supplementary Lighting Duration on Growth and Activity of Antioxidant Enzymes in Grafted Watermelon Seedlings. Agronomy 2020, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, R.E.; Kronenberg, G.H. Photomorphogenesis in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 17–24. [Google Scholar]
- Tichà, I. Photosynthetic characteristics during ontogenesis of leaves. 7. Stomatal density and sizes. Photosynthetica 1982, 16, 375–471. [Google Scholar]
- Mishra, G.; Zhang, W.; Deng, F.; Zhao, J.; Wang, X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 2006, 312, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.O.; Coombs, J. Techniques in Bioproductivity and Photosynthesis; Pergamon press: Oxford, UK, 1982; pp. 85–88. [Google Scholar]
- Hanyu, H.; Shoji, K. Acceleration of growth in spinach by short-term exposure to red and blue light at the beginning and at the end of the daily dark period. Acta Hortic. 2000, 580, 145–150. [Google Scholar] [CrossRef]
- Xue, J.; Wang, S.; Zhang, P.; Zhu, F.; Ren, X.; Liu, C.; Zhang, X. On the role of physiological substances, abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii ‘Feng Dan’). Sci. Hortic. 2015, 182, 92–101. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. Forest. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Cordeiro, K.V.; Costa, N.A.; Andrade, H.A.F.D.; Oliveira-Neto, E.D.D.; Rocha, B.R.D.S.; Farias Machado, N.A.; Albano, F.G.; Furtado, M.B.; Silva-Matos, R.R.S. Inclusion of babassu decomposed stem substrates on the pattern of the vegetative growth of passion fruit seedlings. Commun. Soil Sci. Plant Anal. 2019, 50, 2777–2786. [Google Scholar] [CrossRef]
- Erhioui, B.M.; Gosselin, A.; Hao, X.; Papadopoulos, A.P.; Dorais, M. Greenhouse covering materials and supplemental lighting affect growth, yield, photosynthesis, and leaf carbohydrate synthesis of tomato plants. J. Am. Soc. Hortic. Sci. 2002, 127, 819–824. [Google Scholar] [CrossRef]
- Hidaka, K.; Okamoto, A.; Araki, T.; Miyoshi, Y.; Dan, K.; Imamura, H.; Kitano, M.; Sameshima, K.; Okimura, M. Effect of photoperiod of supplemental lighting with light-emitting diodes on growth and yield of strawberry. Environ. Control Biol. 2014, 52, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Smith, H. Phytochromes and light signal perception by plants-an emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Assmann, S.M. Signal transduction in guard cells. Annu. Rev. Cell Biol. 1993, 9, 345–375. [Google Scholar] [CrossRef]
- Blatt, M.R. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 221–241. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.A. Stomatal opening: Role of potassium uptake by guard cells. Science 1968, 160, 784–785. [Google Scholar] [CrossRef]
- Fujino, M. Role of ATP and ATPase in stomatal movements. Sci. Bull. Fac. Educ. Nagasaki Univ. 1967, 18, 1–47. [Google Scholar]
- Talbott, L.D.; Zeiger, E. The role of sucrose in guard cell osmoregulation. J. Exp. Bot. 1998, 49, 329–337. [Google Scholar] [CrossRef]
- Costa, J.M.; Monnet, F.; Jannaud, D.; Leonhardt, N.; Ksas, B.; Reiter, I.M.; Pantin, F.; Genty, B. Open all night long: The dark side of stomatal control. Plant Physiol. 2015, 167, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.; Cheung, M.K.; Clarke, A.; Golding, S.; Sagi, M.; Fluhr, R.; Rock, C.; Hancock, J.; Neill, S. Hydrogen peroxide is a common signal for darkness-and ABA-induced stomatal closure in Pisum sativum. Funct. Plant Biol. 2004, 31, 913–920. [Google Scholar] [CrossRef]
- Wilkinson, S.; Clephan, A.L.; Davies, W.J. Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol. 2001, 126, 1566–1578. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, E.; Hepler, P.K. Light and stomatal function: Blue light stimulates swelling of guard cell protoplasts. Science 1977, 196, 887–889. [Google Scholar] [CrossRef]
- Takemiya, A.; Kinoshita, T.; Asanuma, M.; Shimazaki, K.I. Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba. Proc. Natl. Acad. Sci. USA 2006, 103, 13549–13554. [Google Scholar] [CrossRef] [Green Version]
- Huala, E.; Oeller, P.W.; Liscum, E.; Han, I.S.; Larsen, E.; Briggs, W.R. Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef]
- Briggs, W.R.; Beck, C.F.; Cashmore, A.R.; Christie, J.M.; Hughes, J.; Jarillo, J.A.; Kagawa, T.; Kanegae, H.; Liscum, E.; Nagatani, A.; et al. The phototropin family of photoreceptors. Plant Cell 2001, 13, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Shimazaki, K.I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, M.U.F.; Pearcy, R.W. Gas exchange analysis of the relative importance of stomatal and biochemical factors in photosynthetic induction in Alocasia macrorrhiza. Plant Physiol. 1988, 86, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.T.; MacKintosh, C.; Kaiser, W.M. Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol. Biol. 2002, 50, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Zhu, J.X.; Han, S.W.; Zeiger, E. Phytochrome and blue light-mediated stomatal opening in the orchid, Paphiopedilum. Plant Cell Physiol. 2002, 43, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Messinger, S.M.; Buckley, T.N.; Mott, K.A. Evidence for involvement of photosynthetic processes in the stomatal responses to CO2. Plant Physiol. 2006, 140, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Ishikawa, H.; Shimada, K.; Shibata, K. Synergistic action of red and blue light and action spectra for malate formation in guard cells of Vicia faba L. Planta 1978, 142, 61–65. [Google Scholar] [CrossRef]
- Karlsson, P.E. Blue light regulation of stomata in wheat seedlings. I. Influence of red background illumination and initial conductance level. Physiol. Plant. 1986, 66, 202–206. [Google Scholar] [CrossRef]
- Outlaw, W.H.J.; Manchester, J.; DiCamelli, C.A.; Randall, D.D.; Rapp, B.; Veith, G.M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc. Natl. Acad. Sci. USA 1979, 76, 6371–6375. [Google Scholar] [CrossRef] [Green Version]
- Iino, M.; Ogawa, T.; Zeiger, E. Kinetic properties of the blue-light response of stomata. Proc. Natl. Acad. Sci. USA 1985, 82, 8019–8023. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, E. Blue light and stomatal function. In Blue Light Effects in Biological Systems; Senger, H., Ed.; Springer: New York, NY, USA; Tokyo, Japan, 1984; pp. 484–494. [Google Scholar]
- Assmann, S.M. Enhancement of the stomatal response to blue light by red light, reduced intercellular concentrations of CO2, and low vapor pressure differences. Plant Physiol. 1988, 87, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.X. Effect of different light quality on chloroplast structure and photosynthetic characteristics of panax. Acta Bot. Sin. 1993, 25, 588–592. (In Chinese) [Google Scholar]
- Voskresenskaya, N.P.; Nechaeva, E.P.; Vlasova, M.P.; Nichiporovich, A.A. The significance of blue light and kinetin for restoration of the photosynthetic apparatus in aging nearly leaves. Fizid Rast 1968, 15, 890. [Google Scholar]
- Albertsson, P.A. A quantitative model of the domain structure of photosynthetic membrane. Trends Plant Sci. 2001, 6, 349–354. [Google Scholar] [CrossRef]
- Anderson, J.M. Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: A personal perspective. Aust. J. Plant Physiol. 1999, 26, 625–639. [Google Scholar] [CrossRef]
- Danielsson, R.; Albertsson, P.Å.; Mamedov, F.; Styring, S. Quantification of photosystem I and II in different parts of the thylakoid membrane from spinach. Biochim. Biophys. Acta 2004, 1608, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J. Rapid suppression of growth by blue light. Plant Physiol. 1981, 67, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, L.M.; StrOmme, E. Effects of light quality on some greenhouse crops. Scientia Hortic. 1987, 33, 27–36. [Google Scholar] [CrossRef]
- Sung, I.K.; Takano, T. Effects of supplemental blue-and red-lights in the morning twilight on the growth and physiological responses of cucumber seedlings. Environ. Control Biol. 1997, 35, 261–265. [Google Scholar] [CrossRef]
- Sung, I.K.; Kiyota, M.; Hirano, K. Intensity dependence of the growth promotion of cucumber seedlings by supplemental blue-lighting at morning twilight. J. SHITA 1997, 9, 271–277. [Google Scholar]
- Sung, I.K.; Kiyota, M.; Tani, A.; Hirano, K.; Murakami, K.; Taira, T. Time dependence of the growth promotion of cucumber seedlings by blue-lighting during morning twilight. Environ. Control Biol. 1998, 36, 85–90. [Google Scholar] [CrossRef]
- Lopez-Juez, E.; Hughes, M.J.G. Effect of blue light and red light on the control of chloroplast acclimation of light-grown pea leaves to increased fluence rate. Photochem. Photobiol. 1995, 61, 106–111. [Google Scholar] [CrossRef]
- Ishii, Y.; Yamazaki, K. Diurnal and annual variations in the spectral photon flux of daylight at Gifu city. Environ. Control Biol. 2002, 40, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Sawbridge, T.I.; Lopez-Juez, E.; Knight, M.R.; Jenkins, G.I. A blue-light photoreceptor mediates the fluence-rate-dependent expression of genes encoding the small subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase in light-grown Phaseolus vulgaris primary leaves. Planta 1994, 192, 1–8. [Google Scholar] [CrossRef]





| Cultivar | Light Quality | Stomatal Density (mm−2) | Guard Cell Size (μm) | Stomatal Pore Size (μm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | ||||||||
| ‘Maehyang’ | Con. | 194.3 | b | 12.3 | b | 2.4 | a | 0.0 | e |
| W | 186.2 | b | 13.7 | a | 2.6 | a | 0.2 | d | |
| R | 160.6 | c | 14.2 | a | 2.4 | a | 0.8 | c | |
| BR | 184.0 | b | 14.3 | a | 2.9 | a | 2.0 | b | |
| B | 226.5 | a | 13.7 | a | 3.0 | a | 3.3 | a | |
| ‘Seolhyang’ | Con. | 190.5 | b | 13.0 | b | 3.1 | a | 0.0 | d |
| W | 180.0 | b | 13.6 | b | 2.9 | a | 0.6 | c | |
| R | 210.0 | ab | 14.6 | a | 2.9 | a | 1.2 | b | |
| BR | 236.7 | a | 12.5 | c | 2.6 | a | 3.1 | a | |
| B | 200.2 | ab | 15.4 | a | 3.2 | a | 3.4 | a | |
| Cultivar | Light Quality | Shoot Length (cm) | Shoot Fresh Weight (g) | Shoot Dry Weigh (g) | Crown Diameter (mm) | Leaf No. | Root Length (cm) | Root Fresh Weight (g) | Root Dry Weight (g) | Runner No. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Maehyang’ | Con. | 32.3 | a | 15.20 | d | 1.08 | c | 2.56 | d | 8 | b | 20.6 | c | 24.60 | c | 1.68 | c | 2 | b |
| W | 28.3 | b | 18.52 | bc | 1.22 | b | 2.86 | c | 10 | a | 22.6 | b | 32.31 | b | 1.75 | b | 2 | b | |
| R | 26.9 | b | 16.52 | c | 1.16 | b | 2.94 | b | 9 | a | 19.9 | c | 30.60 | b | 1.66 | c | 3 | a | |
| BR | 26.4 | bc | 19.36 | b | 1.45 | a | 3.15 | ab | 10 | a | 23.5 | ab | 36.62 | a | 1.95 | a | 3 | a | |
| B | 24.6 | c | 20.46 | a | 1.48 | a | 3.26 | a | 10 | a | 24.8 | a | 35.80 | a | 1.84 | ab | 3 | a | |
| ‘Seolhyang’ | Con. | 30.6 | a | 14.41 | d | 0.91 | d | 2.61 | b | 9 | a | 18.5 | c | 26.94 | d | 1.62 | c | 2 | c |
| W | 27.5 | b | 17.38 | c | 1.23 | c | 2.84 | b | 11 | a | 22.3 | b | 34.64 | b | 1.69 | c | 3 | b | |
| R | 27.3 | b | 16.55 | c | 1.18 | c | 2.78 | b | 10 | a | 23.6 | ab | 32.85 | c | 1.52 | c | 3 | b | |
| BR | 25.9 | b | 20.25 | b | 1.52 | b | 3.29 | a | 12 | a | 24.5 | a | 35.90 | b | 2.04 | b | 4 | a | |
| B | 26.8 | b | 21.22 | a | 1.69 | a | 3.31 | a | 11 | a | 24.8 | a | 38.70 | a | 2.21 | a | 4 | a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Liu, C.; Hu, J.; Jeong, B.R. Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants. Plants 2020, 9, 638. https://doi.org/10.3390/plants9050638
Wei H, Liu C, Hu J, Jeong BR. Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants. Plants. 2020; 9(5):638. https://doi.org/10.3390/plants9050638
Chicago/Turabian StyleWei, Hao, Chen Liu, Jiangtao Hu, and Byoung Ryong Jeong. 2020. "Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants" Plants 9, no. 5: 638. https://doi.org/10.3390/plants9050638
APA StyleWei, H., Liu, C., Hu, J., & Jeong, B. R. (2020). Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants. Plants, 9(5), 638. https://doi.org/10.3390/plants9050638







