Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress
Abstract
:1. Introduction
2. Results
2.1. Growth and Yield Responses
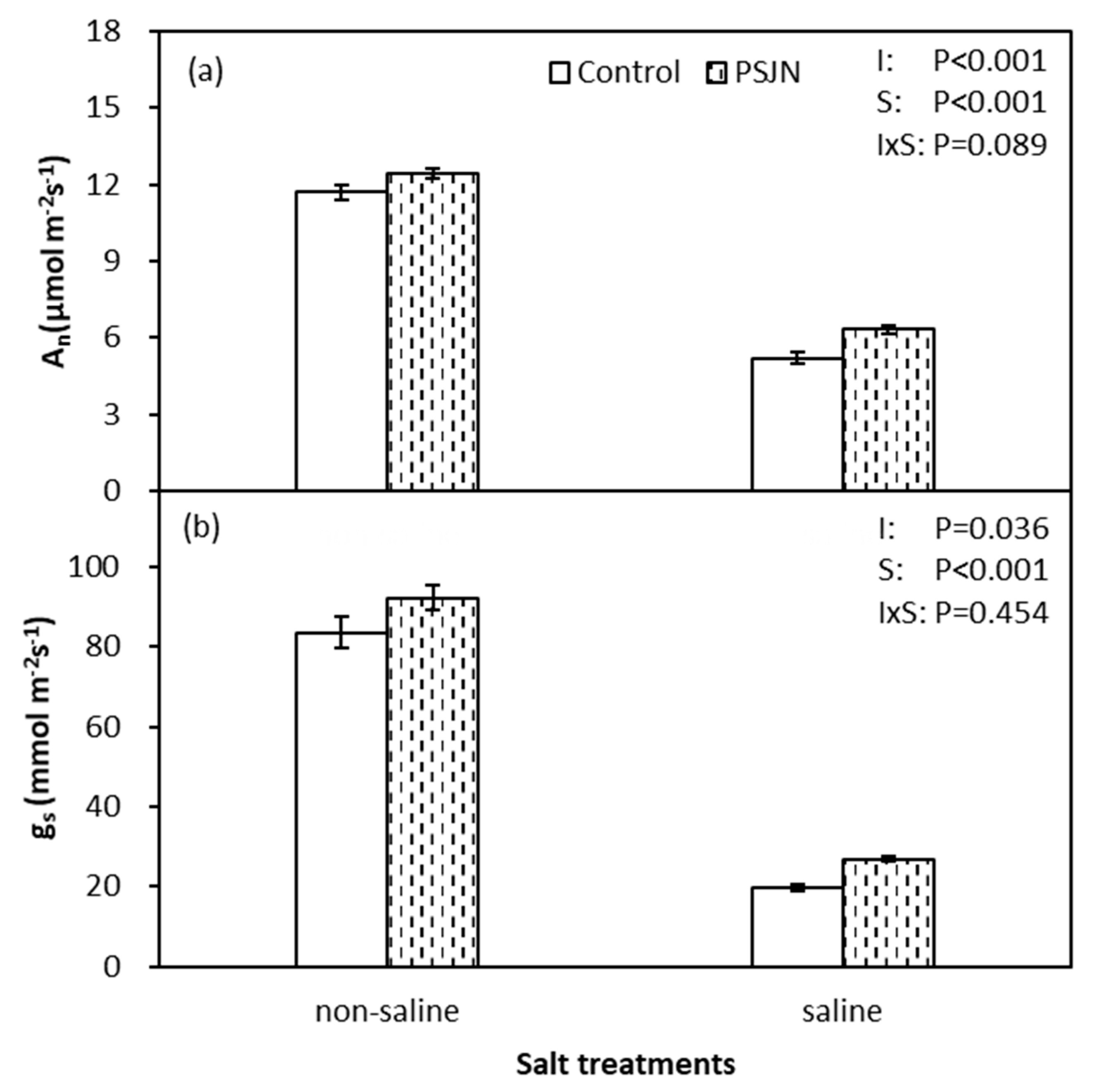
2.2. Leaf Stomatal Conductance (gs) and Photosynthetic Rate (An)
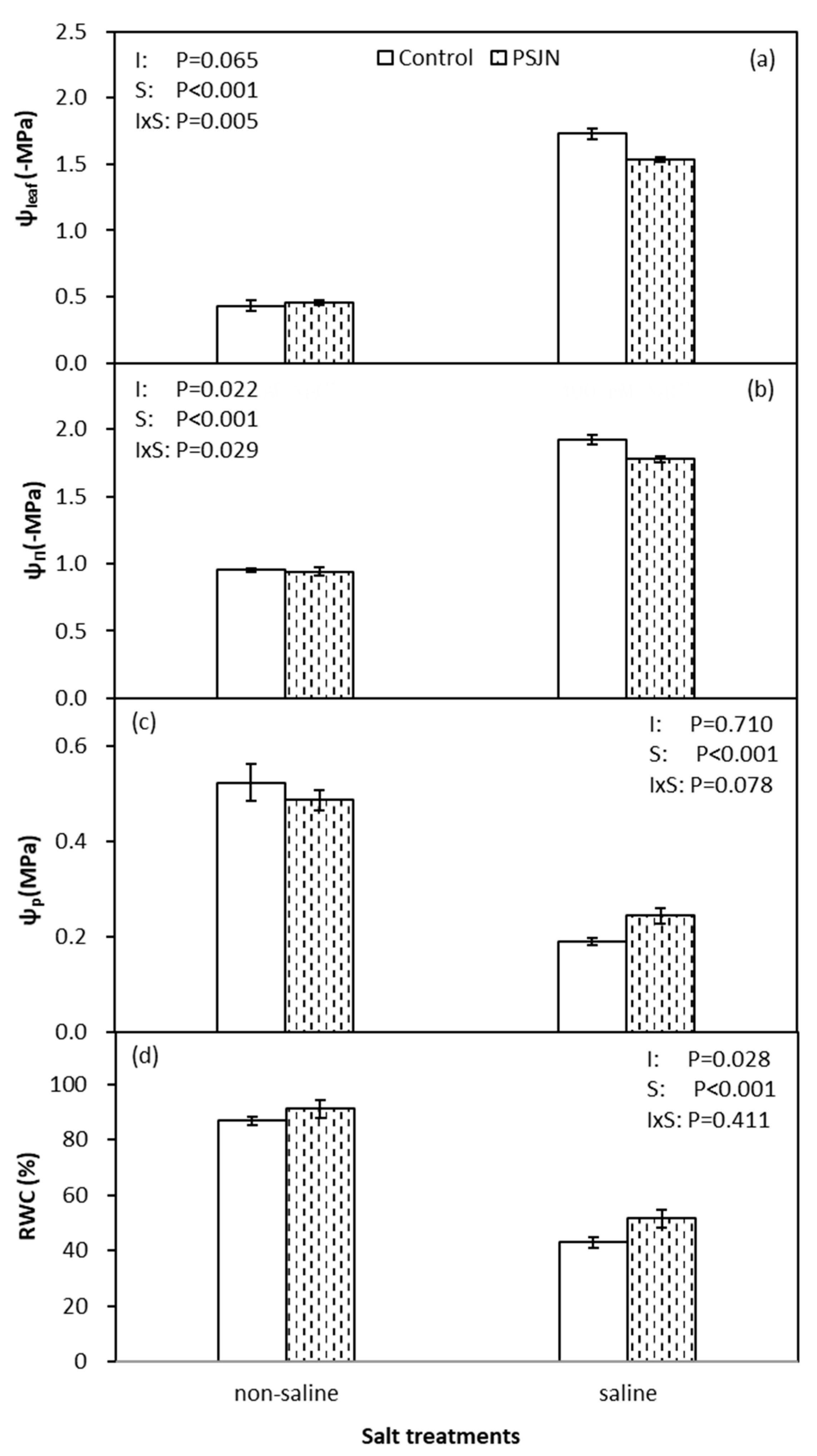
2.3. Plant–Water Relations
2.4. Leaf Na+ and K+
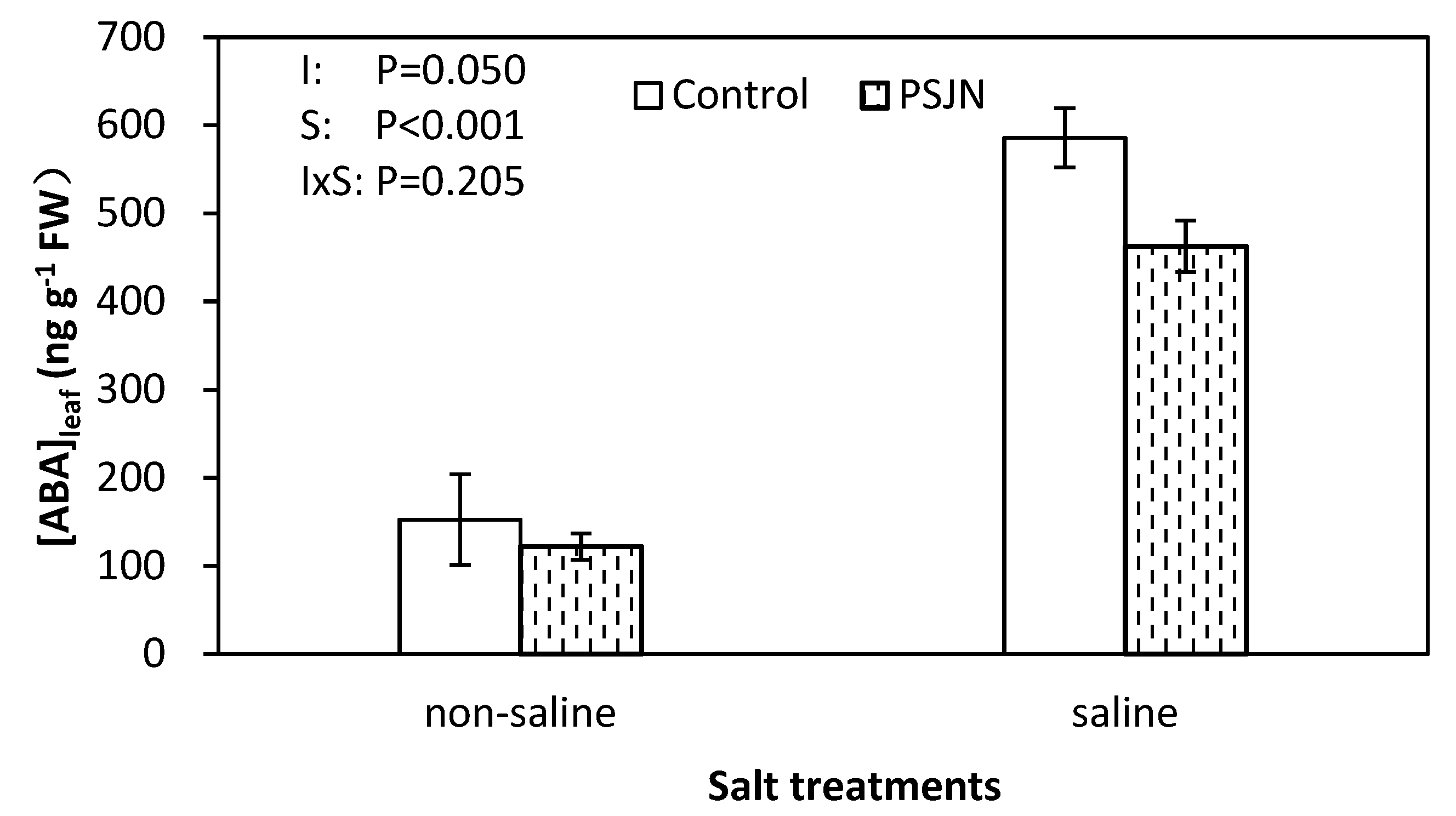
2.5. Leaf Abscisic Acid (ABA) Concentration
2.6. Key Reactive Oxygen Species (ROS) Scavenging Enzyme Response
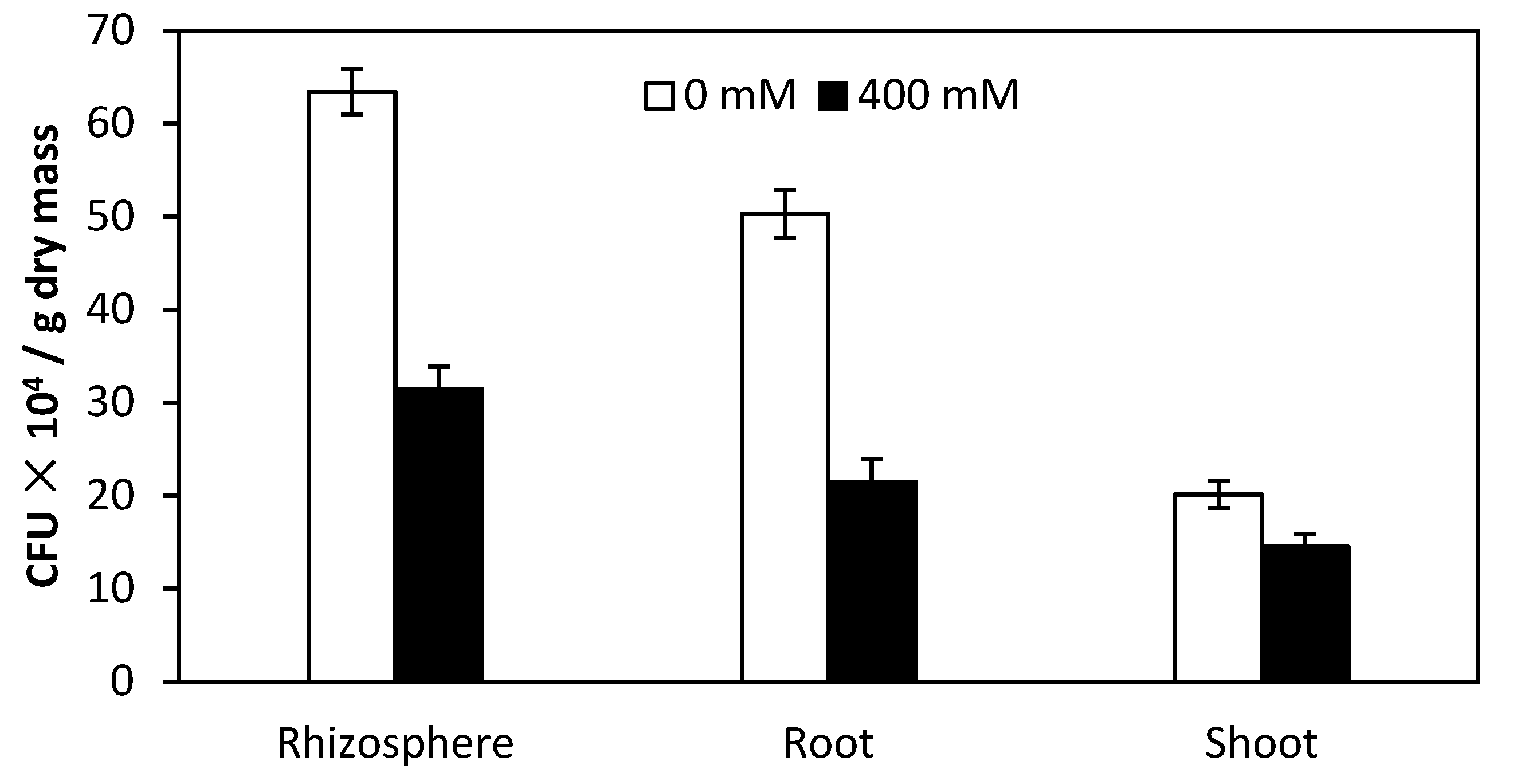
2.7. Colony-Forming Units (CFU) of Burkholderia Phytofirmans PsJN in Rhizosphere and Root Interior
2.8. Salt Tolerance of PsJN
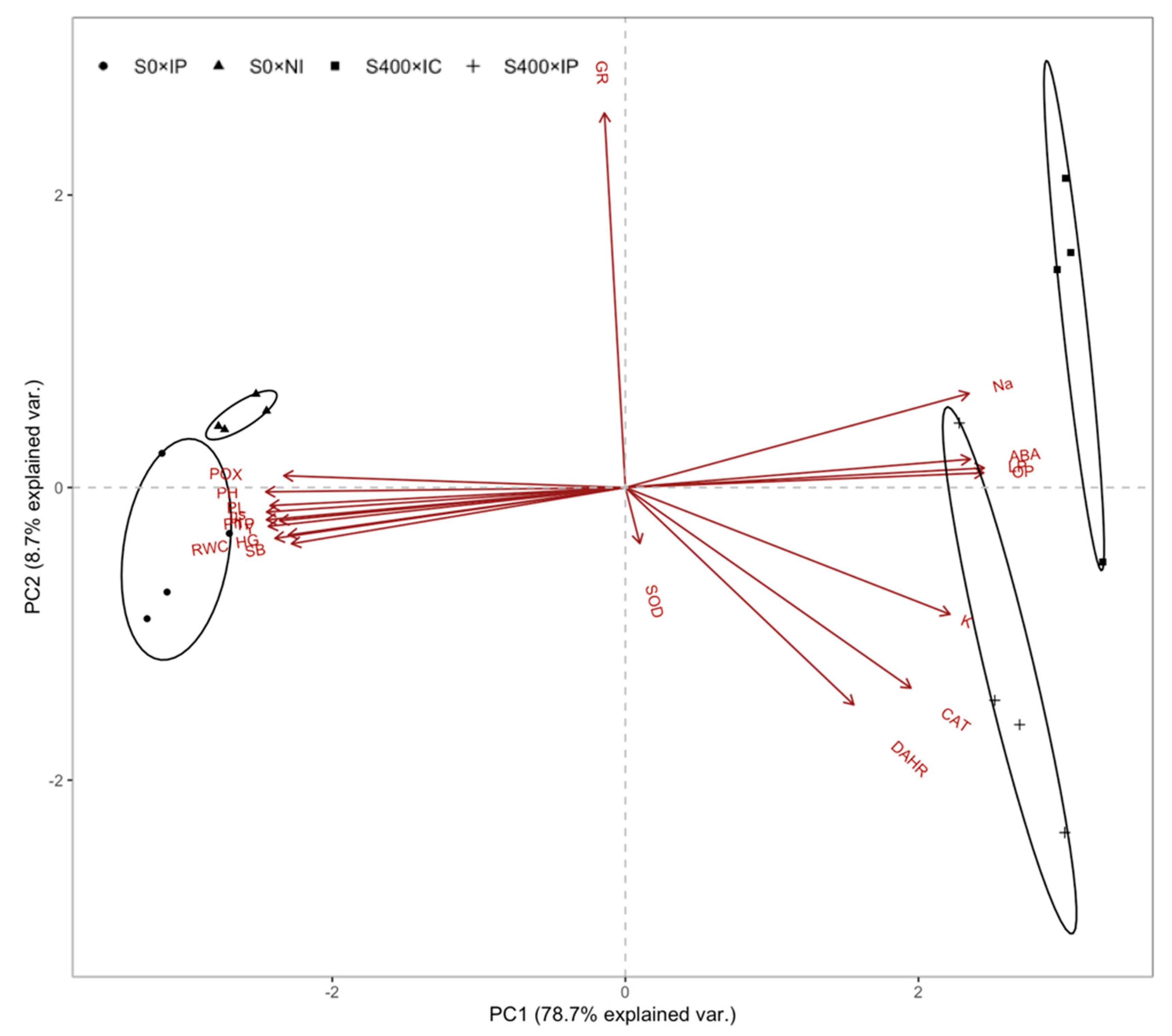
2.9. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Bacterial Strains
4.2. Inoculum Preparation and Seed Bacterization
4.3. Pot Study
4.4. Saline Irrigation Treatments
4.5. Growth and Yield Measurements
4.6. Physiological Measurements
4.7. Measurements of Water Relations
4.8. Leaf ABA Determination
4.9. Enzyme Activity Signature of Antioxidant Metabolism
4.10. Leaf Na+ and K+ Contents
4.11. Colonization of PsJN in Rhizosphere and Root Interior
4.12. Osmoadaptation Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Panta, S.; Flowers, T.J.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.-E. Growth and Physiological Responses of Quinoa to Drought and Temperature Stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Bruning, B.; Van Logtestijn, R.; Broekman, R.; De Vos, A.; González, A.P.; Rozema, J. Growth and nitrogen fixation of legumes at increased salinity under field conditions: Implications for the use of green manures in saline environments. AoB PLANTS 2015, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Panuccio, M.R.S.; Jacobsen, S.-E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS 2014, 6, plu047. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Brown, J.J.; Glenn, E.P.; Smith, S.E. Feasibility of Halophyte Domestication for High-Salinity Agriculture. In Sabkha Ecosystems; Springer: Dordrecht, The Netherlands, 2014; Volume 47, pp. 73–80. [Google Scholar]
- Jacobsen, S.-E.; Monteros, C.; Christiansen, J.; A Bravo, L.; Corcuera, L.; Mujica, A. Plant responses of quinoa (Chenopodium quinoa Willd.) to frost at various phenological stages. Eur. J. Agron. 2005, 22, 131–139. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Razzaghi, F.; Ahmadi, S.H.; Adolf, V.I.; Jensen, C.R.; Jacobsen, S.-E.; Andersen, M.N. Water Relations and Transpiration of Quinoa (Chenopodium quinoa Willd.) Under Salinity and Soil Drying. J. Agron. Crop Sci. 2011, 197, 348–360. [Google Scholar] [CrossRef]
- Razzaghi, F.; Ahmadi, S.H.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effects of Salinity and Soil-Drying on Radiation Use Efficiency, Water Productivity and Yield of Quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2011, 198, 173–184. [Google Scholar] [CrossRef]
- Razzaghi, F.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought—Mechanisms of tolerance. Funct. Plant Biol. 2015, 42, 136–148. [Google Scholar] [CrossRef]
- Iqbal, S.; Basra, S.M.A.; Afzal, I.; Wahid, A.; Saddiq, M.S.; Hafeez, M.B.; Jacobsen, S.-E. Yield potential and salt tolerance of quinoa on salt-degraded soils of Pakistan. J. Agron. Crop Sci. 2018, 205, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Shabala, L.; Mackay, A.; Tian, Y.; Jacobsen, S.-E.; Zhou, D.; Shabala, S. Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa Willd). Physiol. Plant. 2012, 146, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Pulvento, C.; Lavini, A.; D’Andria, R.; Jacobsen, S.-E. Growth and Ionic Content of Quinoa Under Saline Irrigation. J. Agron. Crop Sci. 2014, 200, 246–260. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Science 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.; Gomes, N.C.M.; Almeida, A.; Silva, A.M.S.; Silva, H.; Cunha, A. Microbe-Assisted Phytoremediation of Hydrocarbons in Estuarine Environments. Microb. Ecol. 2014, 69, 1–12. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.-E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2014, 22, 4907–4921. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Subramanian, S.; Smith, D. Bacteriocins from the rhizosphere microbiome—From an agriculture perspective. Front. Plant Sci. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liao, D.; Hu, A.; Wang, H.; Chen, J.; Khan, S.; Su, J.; Li, H. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Can. J. Microbiol. 2015, 61, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, A.P.; Chaudhary, D.; Jha, B.; Jha, A.B. Biomass production, nutrient cycling, and carbon fixation bySalicornia brachiataRoxb.: A promising halophyte for coastal saline soil rehabilitation. Int. J. Phytoremediation 2016, 18, 801–811. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.; Zhang, G.; Wu, F. Difference in Yield and Physiological Features in Response to Drought and Salinity Combined Stress during Anthesis in Tibetan Wild and Cultivated Barleys. PLoS ONE 2013, 8, e77869. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Flowers, T.J.; Wang, S.-M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2009, 326, 45–60. [Google Scholar] [CrossRef]
- Volkov, V. Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant Sci. 2015, 6, 5. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Mackay, A. Ion Transport in Halophytes. In Plant Responses to Drought and Salinity Stress: Developments in a Post-Genomic Era; Turkan, I., Ed.; Elsevier Ltd.: Kidlington, UK, 2011; Volume 57, pp. 151–199. [Google Scholar]
- Sneha, S.; Rishi, A.; Dadhich, A.; Chandra, S. Effect of salinity on seed germination, accumulation of proline and free amino acid in Pennisetum glaucum (L.) R. Br. Pak. J. Biol. Sci. 2013, 16, 877–881. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Llanes, A.; Masciarelli, O.; Ordoñez, R.; Isla, M.I.; Luna, M.V. Differential growth responses to sodium salts involve different abscisic acid metabolism and transport in Prosopis strombulifera. Biol. Plant. 2013, 58, 80–88. [Google Scholar] [CrossRef]
- Hetherington, A.M. Guard Cell Signaling. Cell 2001, 107, 711–714. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.; Srivastava, S.; Lokhande, V.H.; D’Souza, S.F.; Suprasanna, P. Salt stress reveals differential antioxidant and energetics responses in glycophyte (Brassica juncea L.) and halophyte (Sesuvium portulacastrum L.). Front. Environ. Sci. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fimognari, L.; Dölker, R.; Kaselyte, G.; Jensen, C.N.G.; Akhtar, S.S.; Großkinsky, D.K.; Roitsch, T. Simple semi-high throughput determination of activity signatures of key antioxidant enzymes for physiological phenotyping. Plant Methods 2020, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Azhar, S.Q.-T.-A.; Kamran, M.; Zahir, Z.A.; Núñez-Delgado, A. Burkholderia phytofirmans PsJN and tree twigs derived biochar together retrieved Pb-induced growth, physiological and biochemical disturbances by minimizing its uptake and translocation in mung bean (Vigna radiata L.). J. Environ. Manag. 2020, 257, 109974. [Google Scholar] [CrossRef]
- Miotto-Vilanova, L.; Courteaux, B.; Padilla, R.; Rabenoelina, F.; Jacquard, C.; Clément, C.; Comte, G.; Lavire, C.; Barka, E.A.; Kerzaon, I.; et al. Impact of Paraburkholderia phytofirmans PsJN on Grapevine Phenolic Metabolism. Int. J. Mol. Sci. 2019, 20, 5775. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Svensgaard, J.; Christensen, S.; Roitsch, T. Plant phenomics and the need for physiological phenotyping across scales to narrow the genotype-to-phenotype knowledge gap. J. Exp. Bot. 2015, 66, 5429–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Großkinsky, D.K.; Syaifullah, S.J.; Roitsch, T.; Lim, P. Integration of multi-omics techniques and physiological phenotyping within a holistic phenomics approach to study senescence in model and crop plants. J. Exp. Bot. 2017, 69, 825–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2013, 73, 121–131. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770. [Google Scholar] [CrossRef]
- Philosoph-Hadas, S.; Hadas, E.; Aharoni, N. Characterization and use in ELISA of a new monoclonal antibody for quantitation of abscisic acid in senescing rice leaves. Plant Growth Regul. 1993, 12, 71–78. [Google Scholar] [CrossRef]
- Jammer, A.; Gasperl, A.; Luschin-Ebengreuth, N.; Heyneke, E.; Chu, H.; Cantero-Navarro, E.; Großkinsky, D.; Albacete, A.; Stabentheiner, E.; Franzaring, J.; et al. Simple and robust determination of the activity signature of key carbohydrate metabolism enzymes for physiological phenotyping in model and crop plants. J. Exp. Bot. 2015, 66, 5531–5542. [Google Scholar] [CrossRef] [Green Version]
- Gorham, J.; McDonnell, E.; Jones, R.G.W. Salt Tolerance in the Triticeae:Leymus sabulosus. J. Exp. Bot. 1984, 35, 1200–1209. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]


| Attributes | 0 mM NaCl | 400 mM NaCl | P Values | ||||
|---|---|---|---|---|---|---|---|
| Control | PsJN | Control | PsJN | I | S | I × S | |
| Plant height (cm) | 140.63 ± 2.07 a | 140.13 ± 1.43 a | 65.75 ± 1.01 b | 73.38 ± 1.91 c | 0.052 | <0.001 | 0.031 |
| Shoot biomass (g) | 28.70 ± 1.14 a | 33.73 ± 1.36 b | 19.83 ± 0.59 c | 22.19 ± 0.69 d | 0.003 | <0.001 | 0.207 |
| Panicle length (cm) | 25.75 ± 1.11 a | 25.5 ± 1.94 a | 8.20 ± 0.21 b | 11.13 ± 0.35 c | 0.073 | <0.001 | 0.038 |
| 100-grain weight (g) | 0.32 ± 0.02 a | 0.34 ± 0.02 a | 0.16 ± 0.01 b | 0.19 ± 0.01 c | 0.144 | <0.001 | 0.515 |
| Grain yield /plant (g) | 13.33 ± 0.26 a | 14.43 ± 0.69 b | 4.28 ± 0.04 c | 6.05 ± 0.10 d | 0.002 | <0.001 | 0.386 |
| 0 mM | 400 mM | |||
|---|---|---|---|---|
| Control | PsJN | Control | PsJN | |
| DHAR | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 |
| CAT | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| GR | 9.27 ± 0.47 | 7.99 ± 0.47 | 11.39 ± 1.83 | 7.17 ± 1.90 |
| POX | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.1 ± 0.00 | 0.02 ± 0.00 |
| SOD | 10.53 ± 0.91 | 10.26 ± 2.36 | 9.86 ± 2.18 | 10.04 ± 2.28 |
 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.; Akhtar, S.S.; Fu, Q.; Naveed, M.; Iqbal, S.; Roitsch, T.; Jacobsen, S.-E. Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress. Plants 2020, 9, 672. https://doi.org/10.3390/plants9060672
Yang A, Akhtar SS, Fu Q, Naveed M, Iqbal S, Roitsch T, Jacobsen S-E. Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress. Plants. 2020; 9(6):672. https://doi.org/10.3390/plants9060672
Chicago/Turabian StyleYang, Aizheng, Saqib Saleem Akhtar, Qiang Fu, Muhammad Naveed, Shahid Iqbal, Thomas Roitsch, and Sven-Erik Jacobsen. 2020. "Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress" Plants 9, no. 6: 672. https://doi.org/10.3390/plants9060672
APA StyleYang, A., Akhtar, S. S., Fu, Q., Naveed, M., Iqbal, S., Roitsch, T., & Jacobsen, S.-E. (2020). Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress. Plants, 9(6), 672. https://doi.org/10.3390/plants9060672







