Quantitative Profiling of Arabidopsis Polar Glycerolipids under Two Types of Heat Stress
Abstract
:1. Introduction
2. Results
2.1. Contents and Alterations of Glycerolipids During the Heat Acclimation Process in Wild-Type Arabidopsis and Hot-1
2.2. Heat Acclimation Reduced the Degradation of Glycerolipids During Heat Shock in Wild-Type Arabidopsis and Hot-1
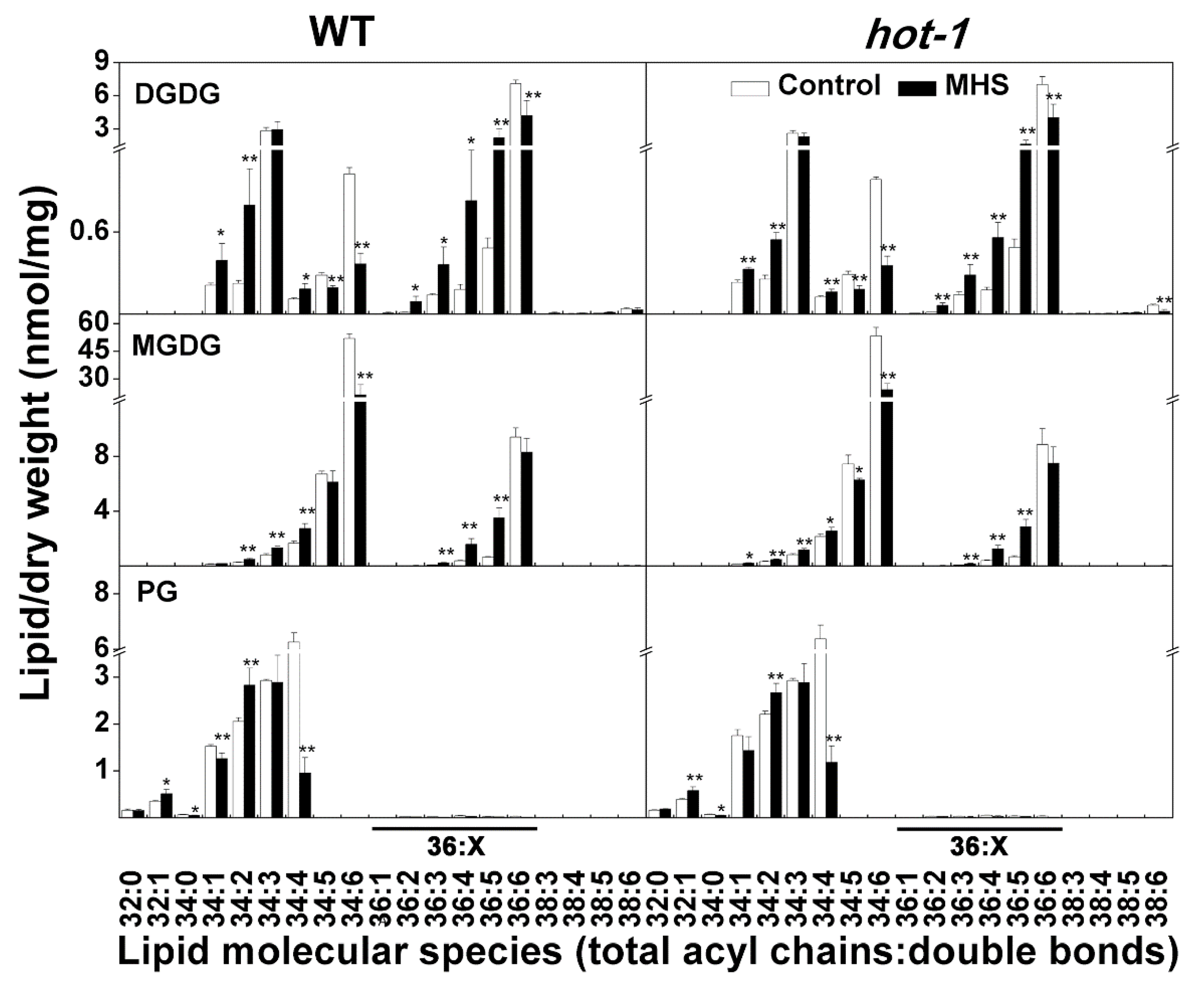
2.3. The Contents of Glycerolipids Changed Profoundly Under Moderate Heat Stress in Wild-Type Arabidopsis and Hot-1
2.4. The Alterations of Plastidic Lipids Molecular Species under Moderate Heat Stress in Wild-Type Arabidopsis and Hot-1
2.5. The Alterations of Extraplastidic Lipids Molecular Species Under Moderate Heat Stress in Wild-Type Arabidopsis and Hot-1
2.6. Changes in the Double Bond Index Under the Two Types of Heat Stress in Wild-Type Arabidopsis and Hot-1
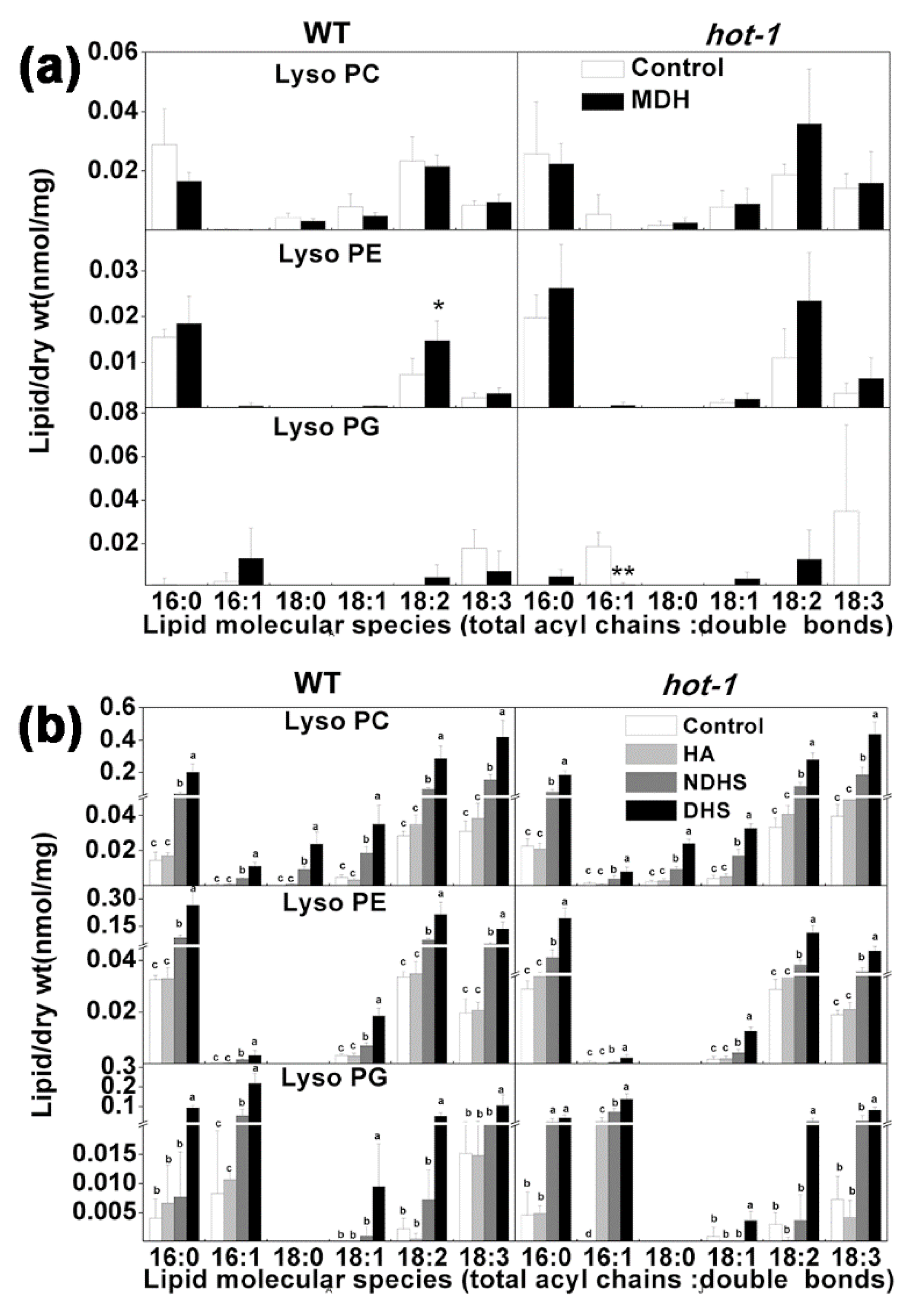
2.7. The Alterations of LysoPLs under Two Types of Heat Stress in Wild-Type Arabidopsis and Hot-1
3. Discussion
4. Materials and Methods
4.1. Plant Materials
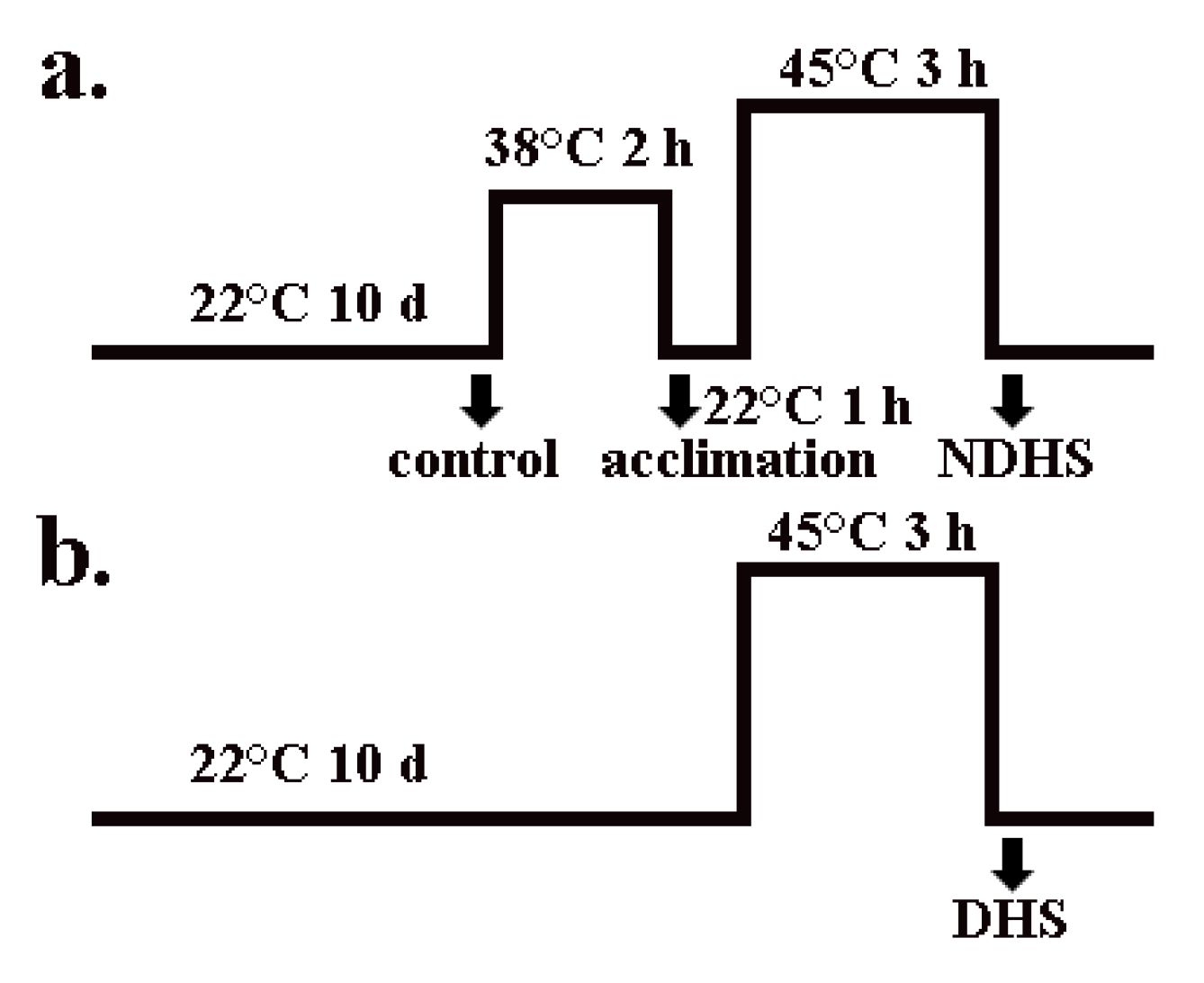
4.2. Growth Conditions and Treatments
4.3. Lipid Extraction and ESI-MS/MS Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DBI | double bond index |
| DGDG | digalactosyl diacylglycerol |
| DHS | direct heat shock |
| HA | heat acclimation |
| LysoPC | lysophosphatidylcholine |
| LysoPE | lysophosphatidylethanolamine |
| LysoPG | lysophosphatidylglycerol |
| MGDG | Monogalactosyl diacylglycerol |
| MHS | moderate heat stress |
| NDHS | non-direct heat shock |
| PA | phosphatidic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
References
- Christensen, J.H.; Christensen, O.B. A summary of the PRUDENCE model projections of changes in European climate by the end of this century. Clim. Chang. 2007, 81, 7–30. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, A.; Mittal, D.; Negi, M.; Lavania, D. Generating high temperature tolerant transgenic plants: Achievements and challenges. Plant Sci. 2013, 205, 38–47. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed. 2014, 133, 679–701. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Pro. Lipid Res. 2019, 75, 15. [Google Scholar] [CrossRef]
- Tang, T.; Liu, P.L.; Zheng, G.W.; Li, W.Q. Two phases of response to long-term moderate heat: Variation in thermotolerance between Arabidopsis thaliana and its relative Arabis paniculata. Phytochemistry 2016, 122, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.R.; Vincent, J.R.; Auffhammer, M.; Moya, P.F.; Dobermann, A.; Dawe, D. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 14562–14567. [Google Scholar] [CrossRef] [Green Version]
- You, L.Z.; Rosegrant, M.W.; Wood, S.; Sun, D.S. Impact of growing season temperature on wheat productivity in China. Agr. For. Meteorol. 2009, 149, 1009–1014. [Google Scholar] [CrossRef]
- Higashi, Y.; Okazaki, Y.; Takano, K.; Myouga, F.; Shinozaki, K.; Knoch, E.; Fukushima, A.; Saito, K. HEAT INDUCIBLE LIPASE1 remodels chloroplastic monogalactosyldiacylglycerol by liberating alpha-Linolenic acid in Arabidopsis leaves under heat stress. Plant Cell 2018, 30, 1887–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zheng, Q.; Shen, W.Y.; Cram, D.; Fowler, D.B.; Wei, Y.D.; Zou, J.T. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 2015, 27, 86–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.Z.; Li, W.Q. Comparative profiling of membrane lipids during water stress in Thellungiella salsuginea and its relative Arabidopsis thaliana. Phytochemistry 2014, 108, 77–86. [Google Scholar] [CrossRef]
- Chen, J.P.; Burke, J.J.; Xin, Z.G.; Xu, C.C.; Velten, J. Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ. 2006, 29, 1437–1448. [Google Scholar] [CrossRef] [Green Version]
- Welti, R.; Li, W.Q.; Li, M.Y.; Sang, Y.M.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X.M. Profiling membrane lipids in plant stress responses—Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [Green Version]
- Vigh, L.; Maresca, B.; Harwood, J.L. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 1998, 23, 369–374. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.; Li, M.; Li, L.; Wang, C.; Welti, R.; Wang, X. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J. Biol. Chem. 2008. [Google Scholar]
- Dormann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Thorlby, G.; Fourrier, N.; Warren, G. The sensitive to freezing2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a beta-Glucosidase. Plant Cell 2004, 16, 2192–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourrier, N.; Bedard, J.; Lopez-Juez, E.; Barbrook, A.; Bowyer, J.; Jarvis, P.; Warren, G.; Thorlby, G. A role for SENSITIVE TO FREEZING2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. Plant J. 2008, 55, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing Tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, D.; Ogas, J.; Somerville, C. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004, 4, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos, A.; Szymanski, J.; Seiwert, B.; Degenkolbe, T.; Hannah, M.A.; Giavalisco, P.; Willmitzer, L. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. Plant J. 2011, 66, 656–668. [Google Scholar] [CrossRef]
- Parsell, D.A.; Kowal, A.S.; Singer, M.A.; Lindquist, S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 1994, 372, 475–478. [Google Scholar] [CrossRef]
- Torok, Z.; Goloubinoff, P.; Horvath, I.; Tsvetkova, N.M.; Glatz, A.; Balogh, G.; Varvasovszki, V.; Los, D.A.; Vierling, E.; Crowe, J.H.; et al. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA 2001, 98, 3098–3103. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, M.; Katiyar-Agarwal, S.; Grover, A. Plant Hsp100 proteins: Structure, function and regulation. Plant Sci. 2002, 163, 397–405. [Google Scholar] [CrossRef]
- Sun, W.N.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta-Gene Struct. Expr. 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, H.; Li, B. Research development for plant heat-shock proteins. Biotechnol. Bull. 2003, 13, 6–9. [Google Scholar]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Vierling, E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Natl. Acad. Sci. USA 2000, 97, 4392–4397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queitsch, C.; Hong, S.W.; Vierling, E.; Lindquist, S. Heat shock protein 101 plays a crucial role in thermotolerance in arabidopsis. Plant Cell 2000, 12, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Q.; Gao, Y.; Pan, H.; Shi, S.; Wang, Y. Overexpression of heat shock protein gene PfHSP21.4 in Arabidopsis thaliana enhances heat tolerance. Acta Physiol. Plant. 2014, 36, 1555–1564. [Google Scholar] [CrossRef]
- Mueller, S.P.; Krause, D.M.; Mueller, M.J.; Fekete, A. Accumulation of extra-chloroplastic triacylglycerols in Arabidopsis seedlings during heat acclimation. J. Exp. Bot. 2015, 66, 4517–4526. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-X.; Wang, C.; Yang, C.-Y.; Wang, J.-Y.; Chen, L.; Bao, X.-M.; Zhao, Y.-X.; Zhang, H.; Liu, J. The role of arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010, 62, 539–548. [Google Scholar] [CrossRef]
- Pearcy, R.W. Effect of growth temperature on fatty acid composition of leaf lipids in a Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1978, 61, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Zaharieva, I.; Markova, T.; Velitchkova, M. Thylakoid Membrane Fluidity Changes the Response of Isolated Pea Chloroplasts to High Temperature; Springer: Dordrecht, The Netherland, 1998; pp. 1823–1826. [Google Scholar]
- Wang, X.M.; Li, W.Q.; Li, M.Y.; Welti, R. Profiling lipid changes in plant response to low temperatures. Physiol. Plant. 2006, 126, 90–96. [Google Scholar] [CrossRef]
- Munnik, T.; Irvine, R.F.; Musgrave, A. Phospholipid signalling in plants. Biochim. Biophys. Acta-Lipids Lipid Metab. 1998, 1389, 222–272. [Google Scholar] [CrossRef]
- Laxalt, A.M.; Munnik, T. Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 2002, 5, 332–338. [Google Scholar] [CrossRef]
- Wang, X.M. Lipid signaling. Curr. Opin. Plant Biol. 2004, 7, 329–336. [Google Scholar] [CrossRef]
- Ryu, S.B. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci. 2004, 9, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Smith, M.A.; Kunst, L. All fatty acids are not equal: Discrimination in plant membrane lipids. Trends Plant Sci. 2000, 5, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, G.W.; Jia, Y.X.; Yu, X.M.; Zhang, X.D.; Yu, B.Z.; Wang, D.D.; Zheng, Y.L.; Tian, X.J.; Li, W.Q. Acyl chain length of phosphatidylserine is correlated with plant lifespan. PLoS ONE 2014, 9, e103227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Gasulla, F.; vom Dorp, K.; Dombrink, I.; Zahringer, U.; Gisch, N.; Dormann, P.; Bartels, D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Deme, B.; Cataye, C.; Block, M.A.; Marechal, E.; Jouhet, J. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB J. 2014, 28, 3373–3383. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkova, N.M.; Horvath, I.; Torok, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef] [Green Version]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid-synthesis in the 16:3 plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Heemskerk, J.W.M.; Storz, T.; Schmidt, R.R.; Heinz, E. Biosynthesis of digalactosyldiacylglycerol in plastids from 16-3 and 18-3 Plants. Plant Physiol. 1990, 93, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Balfagon, D.; Sengupta, S.; Gomez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging layers in controlling temperature stress tolerance. Front. Plant Sci. 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Buseman, C.M.; Tamura, P.; Sparks, A.A.; Baughman, E.J.; Maatta, S.; Zhao, J.; Roth, M.R.; Esch, S.W.; Shah, J.; Williams, T.D.; et al. Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol. 2006, 142, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Lin, L.; Jia, Y.; Li, W.; Yu, B. Quantitative Profiling of Arabidopsis Polar Glycerolipids under Two Types of Heat Stress. Plants 2020, 9, 693. https://doi.org/10.3390/plants9060693
Qin F, Lin L, Jia Y, Li W, Yu B. Quantitative Profiling of Arabidopsis Polar Glycerolipids under Two Types of Heat Stress. Plants. 2020; 9(6):693. https://doi.org/10.3390/plants9060693
Chicago/Turabian StyleQin, Feng, Liang Lin, Yanxia Jia, Weiqi Li, and Buzhu Yu. 2020. "Quantitative Profiling of Arabidopsis Polar Glycerolipids under Two Types of Heat Stress" Plants 9, no. 6: 693. https://doi.org/10.3390/plants9060693
APA StyleQin, F., Lin, L., Jia, Y., Li, W., & Yu, B. (2020). Quantitative Profiling of Arabidopsis Polar Glycerolipids under Two Types of Heat Stress. Plants, 9(6), 693. https://doi.org/10.3390/plants9060693




