Ecological and Geochemical Conditions for the Accumulation of Antioxidants in the Leaves of Lathyrus maritimus (L.) Bigel
Abstract
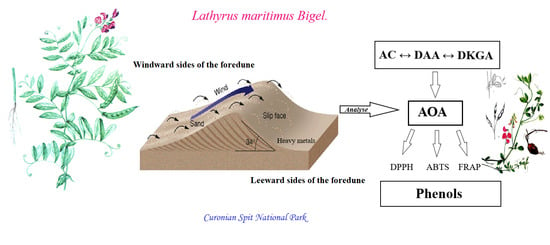
:1. Introduction
2. Results
2.1. A Geochemical Assessment of the Growth Conditions of L. maritimus
2.2. Accumulation of Ascorbic Acid and Its Derivatives in Leaves of L. maritimus
3. Discussion
4. Materials and Methods
4.1. Area and Species Characterization
4.2. Material Collection and Sample Preparation
4.3. Ascorbic, Dehydroascorbic and Diketogulonic Acid Content
4.4. Total Water-Soluble Antioxidants Content (TWAC)
4.5. Total Phenolic Content (TPC)
4.6. Total Flavonoids Content (TFC)
4.7. Total Antioxidant Capacity (TAC)
4.8. Ascorbate Peroxidase Activity (APA)
4.9. Catalase Activity
4.10. Hydrogen Peroxide Content
4.11. Heavy Metals Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N.; Feduraev, P.V.; Melnik, A.S. The Contribution of polyphenols to plant resistance to Pb soil pollution. Int. J. Environ. Stud. 2018, 75, 719–731. [Google Scholar] [CrossRef]
- Feduraev, P.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants 2019, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Tada, Y. Regulation of water, salinity and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Chupakhina, G.N.; Shansky, M.; Parol, A.; Chupakhina, N.Y.; Feduraev, P.V.; Skrypnik, L.N.; Maslennikov, P.V. Comparative characteristics of antioxidant capacity of some forage plants of the Baltic Sea Region (a case study of the Kaliningrad Region and Estonia). Agron. Res. 2018, 16, 1976–1985. [Google Scholar]
- Radyukina, N.L.; Mikheeva, L.E.; Karbysheva, E.A. Low molecular weight antioxidants in cyanobacteria and plant cells. Biol. Bull. Rev. 2019, 9, 520–531. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, S.S.; Mehta, I.K. Free radical scavenging potential of L-proline: Evidence from in vitro assay. Amino Acids 2008, 34, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Dickmanb, M.B.; Beckera, D. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N.; Feduraev, P.V.; Melnik, A.S. Estimation of the antioxidant potential of plants in urban ecosystems under the antropogenic pollution of the soil. Russ. J. Ecol. 2018, 49, 384–394. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Mazid, M.; Mohammad, F. Role of ascorbic acid against pathogenesis in plants. J. Stress Physiol. Biochem. 2011, 7, 222–234. [Google Scholar]
- Foyer, C.H.; Noctor, G. Defining robust redox signaling within the context of the plant cell. Plant Cell Environ. 2015, 38, 239. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Maslennikov, P.V. Ecological and geochemical evaluation of ecosystems of the Curonian Spit (Russia). Int. J. Environ. Stud. 2020, 77, 447–463. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Yu, J.; Gomez, F.; Fernandes, L.; Meintosh, L.; Foyer, C.H. Interrelationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef]
- Makavitskaya, M.; Svistunenko, D.; Navaselsky, I.; Hryvusevich, P.; Mackievic, V.; Rabadanova, C.; Tyutereva, E.; Samokhina, V.; Straltsova, D.; Sokolik, A.; et al. Novel roles of ascorbate in plants: Induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 2018, 69, 3477–3489. [Google Scholar] [CrossRef]
- Barth, C.; De Tullio, M.; Conklin, P.L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006, 57, 1657–1665. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, J.; Park, S. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.L.; Wei, Y.X.; Peng, C.L. Effects of endogenous ascorbic acid on resistance to high-temperature stress in excised rice leaves. Photosynthetica 2018, 56, 1453–1458. [Google Scholar] [CrossRef]
- Radyuk, M.S.; Domanskaya, I.N.; Shcherbakov, R.A.; Shalygo, N.V. Effect of low above-zero temperature on the content of low-molecular antioxidants and activities of antioxidant enzymes in green barley leaves. Russ. J. Plant Physiol. 2009, 56, 175–180. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, M.-T.; Li, X.; Zhou, Z.-Q.; Wang, X.-J.; Bai, J.-G. Exogenous sucrose increases chilling tolerance in cucumber seedlings by modulating antioxidant enzyme activity and regulating proline and soluble sugar contents. Sci. Hortic. 2014, 179, 67–77. [Google Scholar] [CrossRef]
- Yu, J.; Cang, J.; Li, Y.; Huang, R.; Lu, Q.; Wang, X.; Liu, L.; Xu, Q.; Zhang, K. Salicylic acid-induced antioxidant protection against low temperature in cold hardly winter wheat. Acta Physiol. Plant. 2016, 38, 261. [Google Scholar] [CrossRef]
- Borovik, O.A.; Grabelnych, O.I.; Koroleva, N.A.; Pobezhimova, T.P.; Voinikov, V.K. The Relationships Among an Activity of the Alternative Pathway Respiratory Flux, a Content of Carbohydrates and a Frost-Resistance of Winter Wheat. J. Stress Physiol. Biochem. 2013, 9, 241–250. [Google Scholar]
- Astakhova, N.V.; Popov, V.N.; Selivanov, A.A.; Burakhanova, E.A.; Alieva, G.P.; Moshkov, I.E. Reorganization of chloroplast ultrastructure associated with low-temperature hardening of arabidopsis plants. Russ. J. Plant Physiol. 2014, 61, 744–750. [Google Scholar] [CrossRef]
- Grabelnych, O.I.; Borovik, O.A.; Tauson, E.L.; Pobezhimova, T.P.; Katyshev, A.I.; Pavlovskaya, N.S.; Koroleva, N.A.; Lyubushkina, I.V.; Borovskii, G.B.; Voinikov, V.K.; et al. Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings. Biochemistry 2014, 79, 506–519. [Google Scholar] [CrossRef]
- Yamasaki, T.; Yamakawa, T.; Yamane, Y.; Koike, H.; Satoh, K.; Katoh, S. Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol. 2002, 128, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Tsonev, T.; Velikova, V.; Georgieva, K.; Hydde, P.F.; Jones, H.G. Low temperature enhances photosynthetic down-regulation in french bean (Phaseolus vulgaris L.). Plants Ann. Bot. 2003, 91, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göbel, L.; Coners, H.; Hertel, D.; Willinghöfer, S.; Leuschner, C. The role of low soil temperature for photosynthesis and stomatal conductance of three graminoids from different elevations. Front. Plant Sci. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isbilir, S.S.; Sagiroglu, A. Total phenolic content, antiradical and antioxidant activities of wild and cultivated Rumex acetosella L. extracts. Biol. Agric. Hortic. 2013, 29, 219–226. [Google Scholar] [CrossRef]
- Shah, A.; Singh, T.; Vijayvergia, R. In vitro antioxidant properties and total phenolic and flavonoid contents of Rumex vesicaius L. Int. J. Pharm. Pharm. Sci. 2015, 7, 81–84. [Google Scholar]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Aghdam, M.S. Salicylic acid treatment of peach trees maintains nutritional quality of fruits during cold storage. Adv. Hortic. Sci. 2018, 32, 33–40. [Google Scholar] [CrossRef]
- Chupakhina, G.N.; Maslennikov, P.V.; Skrypnik, L.N.; Chupakhina, N.Y.; Poltavskaya, R.L.; Feduraev, P.V. The influence of the Baltic regional conditions on the accumulation of water-soluble antioxidants in plants. Russ. Chem. Bull. 2014, 63, 1946–1953. [Google Scholar] [CrossRef]
- Shahidi, F.; Chavan, U.D.; Naczk, M.; Amarowicz, R. Nutrient distribution and phenolic antioxidants in air-classified fractions of beach pea (Lathyrus maritimus L.). J. Agric. Food Chem. 2001, 49, 926–933. [Google Scholar] [CrossRef]
- Chavan, U.D.; Amarowicz, R. Effect of various solvent systems on extraction of phenolics, tannins and sugars from beach pea (Lathyrus maritimus L.). Int. Food Res. J. 2013, 20, 1139–1144. [Google Scholar]
- Miteva, L.P.-E.; Ivanov, S.V.; Alexieva, V.S. Alterations in glutathione pool and some related enzymes in leaves and roots of pea plants treated with the herbicide glyphosate. Russ. J. Plant Physiol. 2010, 57, 131–136. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Pradedova, E.V.; Nimaeva, O.D.; Salyaev, R.K. Redox processes in biological systems. Russ. J. Plant Physiol. 2017, 64, 822–832. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, methods, and future considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Yashin, Y.I.; Yashin, A.Y.; Nemzer, B.V. Determination of antioxidant activity in tea extracts and their total antioxidant content. Am. J. Biomed. Sci. 2011, 3, 322–335. [Google Scholar] [CrossRef]
- Czyżowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Skrypnik, L.; Chupakhina, G.; Feduraev, P.; Chupakhina, N.; Maslennikov, P. Evaluation of the rose hips of Rosa canina L. and Rosa rugosa Thunb. as a valuable source of biological active compounds and antioxidants on the Baltic Sea coast. Pol. J. Nat. Sci. 2019, 34, 395–413. [Google Scholar]
- Kalinauskaitė, N.; Laaka-Lindberg, S. Grey dune ground layer vegetation in the curonian spit nature reserve, Lithuania. Arctoa 2013, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- HELCOM. Comprehensive Environmental Study of Marine and Coastal Areas of the Curonian Spit National Park for Granting These Areas the Legal Status of a Marine Protected Zone; BASE Project 2012–2014; HELCOM: Helsinki, Finland, 2014. [Google Scholar]
- Volkova, I.I.; Shaplygina, T.V.; Belov, N.S.; Danchenkov, A.R. Eolian coastal-marine natural systems in the Kaliningrad region. Terrestrial and inland water environment of the Kaliningrad region. Handb. Environ. Chem. 2018, 65, 147–178. [Google Scholar]
- Koroleva, Y.; Napreenko, M.; Baymuratov, R.; Schefer, R. Bryophytes as a bioindicator for atmospheric deposition in different coastal habitats (a case study in the Russian sector of the Curonian Spit, South-Eastern Baltic). Int. J. Environ. Stud. 2020, 77, 152–162. [Google Scholar] [CrossRef]
- Budantsev, A.L.; Yakovlev, G.P. Illyustrirovannyy Opredelitel’ Rasteniy Leningradskoy Oblasti; Tovarishchestvo Nauchnykh Izdaniy KMK Publ.: Moscow, Russia, 2006; pp. 795–796. [Google Scholar]
- Chupakhina, G.N.; Maslennikov, P.V.; Mosina, L.V.; Skrypnik, L.N.; Dedkov, V.P.; Chupakhina, N.Y.; Feduraev, P.V. Accumulation of biogenic metals in the plants of urbanised ecosystems in the city of Kaliningrad. Res. J. Chem. Environ. 2017, 21, 9–17. [Google Scholar]
- Golovina, E.Y. Sistema Askorbinovoy Kisloty Dominantov Flory Dyun Kurshskoy Kosy [The Ascorbic Acid System of the Flora Dominants of the Curonian Spit Dunes]. Sci. (Biol.) Dissertation, Kaliningrad State University, Kaliningrad, Russia, 2001. [Google Scholar]
- Padhi, E.M.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Sevket, A.L.P.; Ercisli, S.; Jurikova, T.; Cakir, O.; Gozlekci, S. Bioactive content of rose hips of different wildly grown Rosa dumalis genotypes. Not. Bot. Horti Agrobot. 2016, 44, 472–476. [Google Scholar]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Lukatkin, A.S.; Gar’kova, A.N.; Bochkarjova, A.S.; Nushtaeva, O.V.; Teixeira da Silva, J.A. Treatment with the herbicide TOPIK induces oxidative stress in cereal leaves. Pestic. Biochem. Physiol. 2013, 105, 44–49. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipperro, N.; Salvi, G.; Cervcone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef] [Green Version]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikov, P.; Golovina, E.; Artemenko, A. Ecological and Geochemical Conditions for the Accumulation of Antioxidants in the Leaves of Lathyrus maritimus (L.) Bigel. Plants 2020, 9, 746. https://doi.org/10.3390/plants9060746
Maslennikov P, Golovina E, Artemenko A. Ecological and Geochemical Conditions for the Accumulation of Antioxidants in the Leaves of Lathyrus maritimus (L.) Bigel. Plants. 2020; 9(6):746. https://doi.org/10.3390/plants9060746
Chicago/Turabian StyleMaslennikov, Pavel, Elena Golovina, and Anastasia Artemenko. 2020. "Ecological and Geochemical Conditions for the Accumulation of Antioxidants in the Leaves of Lathyrus maritimus (L.) Bigel" Plants 9, no. 6: 746. https://doi.org/10.3390/plants9060746
APA StyleMaslennikov, P., Golovina, E., & Artemenko, A. (2020). Ecological and Geochemical Conditions for the Accumulation of Antioxidants in the Leaves of Lathyrus maritimus (L.) Bigel. Plants, 9(6), 746. https://doi.org/10.3390/plants9060746