Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent
Abstract
:1. Introduction
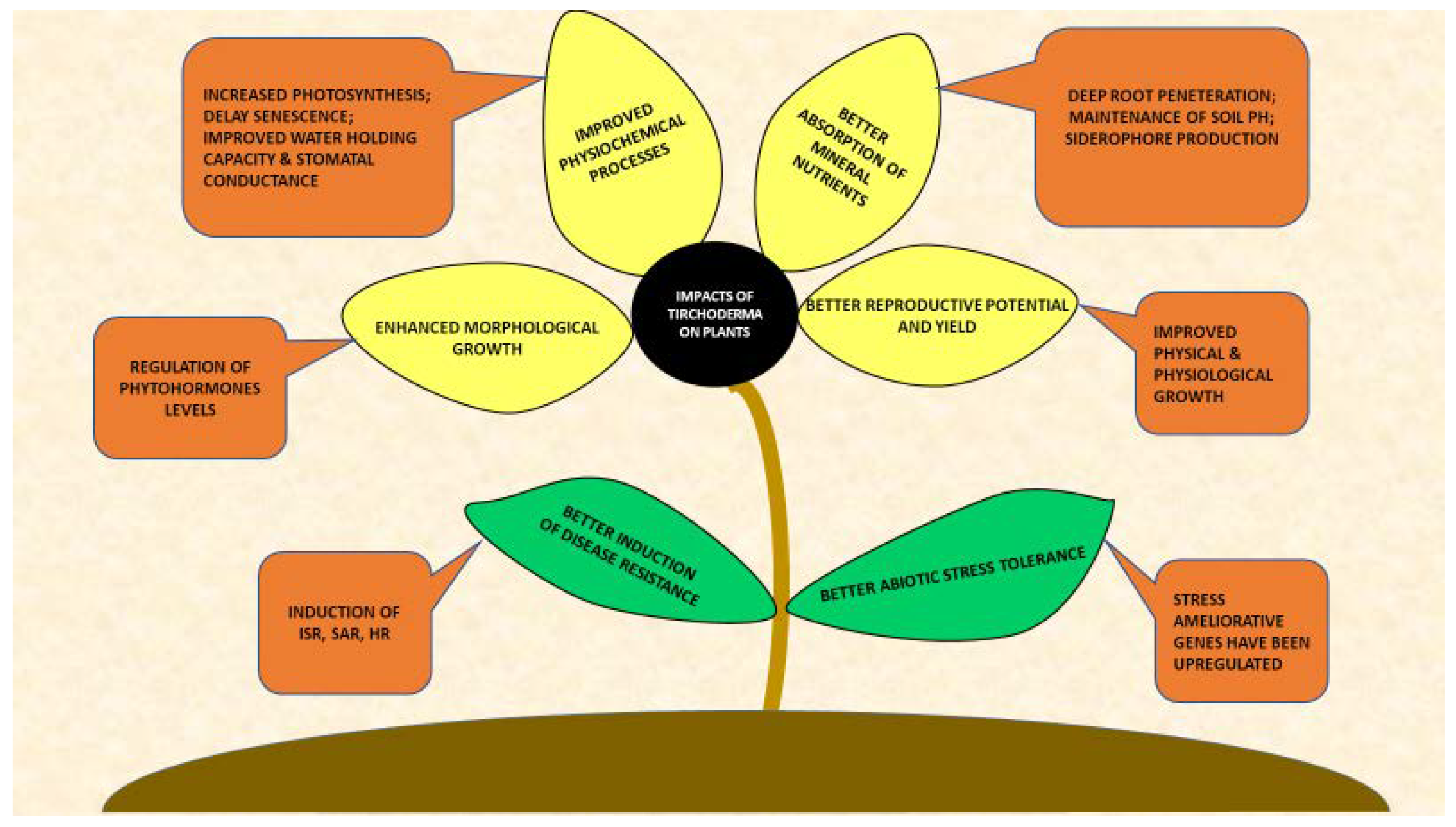
2. Trichoderma-Plants Interactions
2.1. Impacts on Plant Morphology
2.2. Impacts on Plant Physiology
2.3. Impacts on Nutrient Solubilization and Absorption
2.4. Yield Improvement
2.5. Impacts on Abiotic Stress Tolerance
2.6. Induction of Disease Resistance
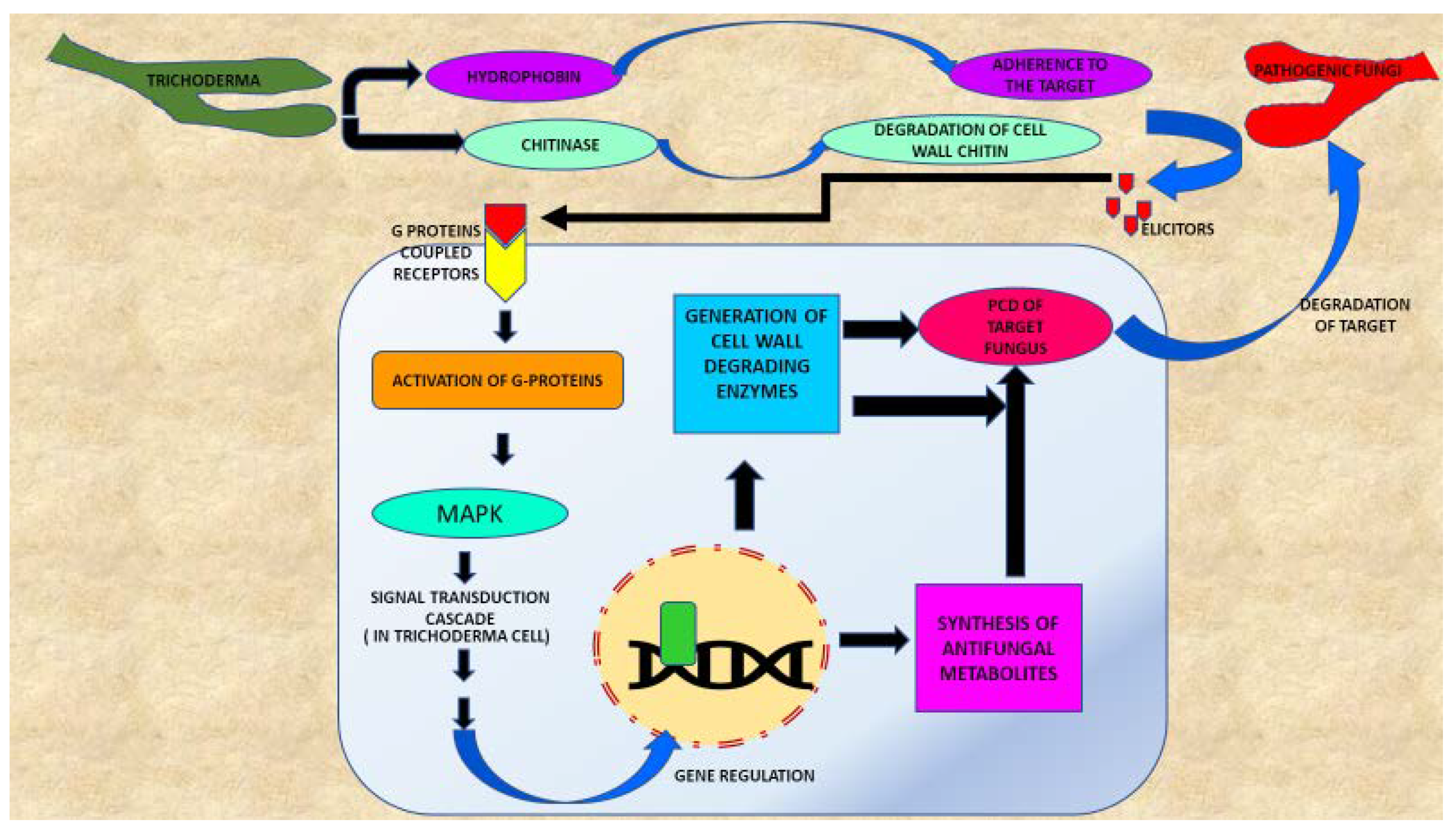
3. Trichoderma-Pathogen Interactions
3.1. Mycoparasitism
3.2. Competition
3.3. Antibiosis
4. Effect of Trichoderma Inoculation
4.1. Destruction of Pathogenic Organism
4.2. Plant Growth Promotion
| Sr. No. | Category | Sub-Category | Function Performed | Trichoderma Species | References |
|---|---|---|---|---|---|
| 1. | Phytohormones | ||||
| IAA | Growth and development of plants and their root system. | T. virens | [35] | ||
| GA3 | Growth promotion by degradation of growth repressing DELLA proteins and reduction in ethylene level. | Trichoderma spp. | [13,137] | ||
| ABA | Alteration in transpiration and regulation of stomatal aperture via induction of an ABA receptor. | T. virens and T. atroviride | [33] | ||
| Ethylene | Improved tolerance to biotic as well as abiotic stresses by regulation of levels of SA and JA as well as their signaling pathways. | T. atroviride | [138,139,140] | ||
| JA | JA and/or ET are the signaling molecule for Tichoderma-induced ISR. | T. asperellum | [141] | ||
| SA | Enhances disease resistance in plants through induction of SAR. | T. atroviride | [26,142,143] | ||
| 2. | Enzymes | ||||
| Hydrolytic | |||||
| Cellulolytic enzymes | Cleavage of β-1,4-D-glycosidic bonds in cellulose molecule. | [120] | |||
| Exo-β-1,4-glucanases | Breakdown of cellulose by forming a cellobiose molecule either from the reducing or nonreducing terminals. | T. viride, T. harzianum, T. reesei, T. koningii | [144] | ||
| Endo-β-1,4-glucanases | At the time of enzymatic lysis of cellulose, break the β-1,4- glycosidic bonds in a random way probably in the amorphous areas of cellulose and thereby cause formation of cellulodextrines with variable chain lengths. | T. viride, T. longibrachiatum, T. pseudokoningii and T. reesei | [145,146,147] | ||
| β-Glucosidases | Promote lysis of short length oligosaccharides and cellobiose into glucose. | T. viride, T. harzianum, T. reesei and T. longibrachiatum. | [148,149] | ||
| Xylanase | Catalyze breakdown of xylans to form xylo-oligomers, xylobiose and xylose. | T. harzianum, T. koningii, T. lignorum, T. longibrachiatum, T. pseudokoningii, T. reesei, T. viride Trichoderma harzianum, T. virens, T. asperellum, T. atroviride | [150] | ||
| Chitinase | Catalyze degradation of chitin to chitooligomers of low molecular weight. | [83,151,152,153,154] | |||
| Endochitinases | Randomly hydrolyses chitin at internal sites and form dimer of diacetylchitobiose and low molecular weight multimers of GlcNAc like chitotriose and chitotetraose. | ||||
| Exochitinases | Divided into 2 subcategories: 1. Chitobiosidases, involved in catalyzing the sequential release of diacetylchitobiose starting from the non-reducing end of the chitin microfibril 2. 1-4-β-glucosaminidases, splitting the oligomeric products of endochitinases and chitobiosidases, thereby producing GlcNAc monomers. | ||||
| Proteases | |||||
| Exopeptidases | Cause the cleaving of peptide bond either at the amino or carboxy terminal. | T. viride, T. harzianum, T. aureoviride, T. atroviride | [155,156] | ||
| Endopeptidases | Split the peptide bonds away from the ends. | ||||
| Lipase | Lipase hydrolyses ester bonds of triacylglycerols, resulting in the formation of mono- and diacylglycerols, free fatty acids and, in some cases, glycerol also. | T. lanuginosus, Trichoderma reesei, Trichoderma koningii, T. harzianum, T. virens, m T. viride | [157] | ||
| Glucose oxidase | Cause generation of reactive oxygen species (ROS). | T. virens, T. asperelloides | [123,124,125] | ||
| Antioxidative enzymes (e.g., SOD, CAT, POD etc.) | Enhance antioxidative defense mechanism in plants. | Trichoderma spp. | [59,158] | ||
| Biosynthetic and signaling | |||||
| PAL & CHS | Production of phytoalexins. | Trichoderma spp. | [60] | ||
| Glucan and Chitin synthases | Produced by the Trichoderma to repair their self-cell wall damage by pathogen during Trichoderma–pathogen interaction. | Trichoderma spp. | [159] | ||
| MAPK | Convey information from receptor to generate cellular signaling and defense responses. | Trichoderma spp. | [126,131] | ||
| ETR1 and CTR1 | Involved in ethylene (ET) signaling. | Trichoderma spp. | [131] | ||
| LOX1 (Lipoxygenase 1) PAL1 (phenylalanine ammonia lyase), | Participate in jasmonic acid (JA) biosynthetic pathway. Involved in biosynthetic pathway for salicylic acid (SA) | Trichoderma spp. | [160] | ||
| ACC synthase ACC oxidase | Promote ethylene biosynthesis. | Trichoderma spp. | [134] | ||
| δ-cadinene synthase | Act as precursor for phytoalexin synthesis. | T. virens | [123,136] | ||
| 3. | Soil modifiers | ||||
| Gluconic, citric and fumaric acids | Reduce the pH of soil and facilitate the solubilization of phosphates and micronutrients. | Trichoderma spp. | [18,41] | ||
| Siderophore | Chelate with insoluble Fe (III) and convert them to soluble Fe (II). | Trichoderma spp. | [44,94,95] | ||
| 4. | Secondary metabolites | ||||
| Pyrones | Antimicrobial | Trichoderma spp. | [161] | ||
| Lactones | Participate in IAA and ethylene-mediated signaling and improve plant growth and root architecture. | T. harzianum, Trichoderma cremeum | [162] | ||
| Koninginins | Antimicrobial | T. koningii, T. harzianum, T. aureoviride | [163,164] | ||
| Trichodermamides | Antifungal and exhibit cytotoxicity to human colon carcinoma. | T. virens | [165,166] | ||
| Viridins | Antifungal | Trichoderma virens, T. koningii, T. viride | [99,167,168] | ||
| Nitrogen heterocyclic compounds (harzianopyridone, harzianic acid) | Antifungal | T. harzianum | [169,170,171] | ||
| Azaphilones | Antifungal | T. harzianum T22 | [171,172] | ||
| Butenolides and hydroxy-Lactones (cerinolactone, trichosordarin A, harzianol A and harzianone) | Antifungal | T. cerinum, Trichoderma cremeum, Trichoderma longibrachiatum A-WH-20-2 | [163,173,174] | ||
| Isocyano metabolites (dermadin and trichoviridin) | Antifungal | T. viride T. koningii and T. hamatum | [164,175,176] | ||
| Diketopiperazines (gliotoxin and gliovirin) | Antifungal | Trichoderma (Gliocladium) virens | [177] | ||
| Peptaibol (alamethicin, trichokonin VI) | Non-ribosomal short peptides, rich in 2-amino-isobutyric acid involved in plant defense and antimicrobial in nature. | T. virens, T. longibrachiatum | [178,179] | ||
| Polyketides | Participate in SA mediated signaling pathway and exhibit antimicrobial activities. | T. virens, Trichoderma sp. SCSIO41004 | [180,181] | ||
| Terpenes cyclonerane sesquiterpenoids, trichocitrin, trichosordarin A | Antimicrobial | T. virens, Trichoderma harzianum P1-4, Trichoderma citrinoviride cf-27, Trichoderma harzianum R5 | [182,183,184,185] | ||
| Volatile organic compounds (VOCs) (trichodiene) | Facilitate the plant-microbe interactions in rhizosphere | T. arundinaceum, T. atroviride | [186,187,188] | ||
| Hydrophobins | Plant growth promotion, signaling and defense | T. virens and T. atroviride, T. asperellum | [189,190] | ||
5. Other Applications of Trichoderma
5.1. Bioremediation
5.2. Animal Feed
5.3. Industrial Applications
5.4. Second Generation Biofuels
5.5. Wood Preservation
5.6. Agricultural and Horticultural Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Raney, T. The State of Food and Agriculture: Livestock in the Balance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009.
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, N.S.; Niranjana, S.R.; Shetty, H.S. Effect of Pseudomonas fluoriescens and Trichoderma harzianum on head moulds and seed qualitites of Sorghum. Crop Improv. (India) 2003, 30, 6–12. [Google Scholar]
- Atreya, K.; Sitaula, B.K.; Bajracharya, R.M. Pesticide use in agriculture: The philosophy, complexities and opportunities. Sci. Res. Essays 2012, 7, 2168–2173. [Google Scholar]
- Meszka, B.; Broniarek-Niemiec, A.; Bielenin, A. The status of dodine resistance of Venturia inaequalis populations in Poland. Phytopathol. Pol. 2008, 47, 57–61. [Google Scholar]
- Matson, M.E.H.; Small, I.M.; Fry, W.E.; Judelson, H.S. Metalaxyl resistance in Phytophthora infestans: Assessing role of RPA190 gene and diversity within clonal lineages. Phytopathology 2015, 105, 1594–1600. [Google Scholar] [CrossRef] [Green Version]
- Slabaugh, W.R.; Grove, M.D. Postharvest diseases of bananas and their control. Plant Dis. 1982, 66, 746–750. [Google Scholar] [CrossRef]
- Spalding, D.H. Resistance of mango pathogens to fungicides used to control postharvest diseases. Plant Dis. 1982, 66, 1185–1186. [Google Scholar] [CrossRef]
- Farungsang, U.; Farungsang, N. Benomyl resistance of Colletotrichum spp. Associated with rambutan and mango fruit rot in Thailand. Front. Trop. Fruit Res. 1991, 321, 891–897. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Hyder, S.; Inam-ul-Haq, M.; Bibi, S.; Humayun, A.; Ghuffar, S.; Iqbal, S. Novel potential of Trichoderma spp. as biocontrol agent. J. Entomol. Zool. Stud. 2017, 5, 214–222. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persoon, C.H. Disposita methodical fungorum. Romers. Neues. Mag. Bot. 1794, 1, 81–128. [Google Scholar]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol. Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Singh, H.B.; Singh, B.N.; Singh, S.P.; Singh, S.R.; Sarma, B.K. Biological control of plant diseases: Status and prospects. In Recent Advances in Biopesticides: Biotechnological Applications; New India Pub.: New Delhi, India, 2009; Volume 322. [Google Scholar]
- Van Wees, S.C.M.; der Ent, S.; Pieterse, C.M.J. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Wilson, P.S.; Ketola, E.O.; Ahvenniemi, P.M.; Lehtonen, M.J.; Valkonen, J.P.T. Dynamics of soilborne Rhizoctonia solani in the presence of Trichoderma harzianum: Effects on stem canker, black scurf, and progeny tubers of potato. Plant Pathol. 2008, 57, 152–161. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From’omics to the field. Ann. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, G.-Y.; Li, X.-Z.; Hu, M.; Wang, B.-Y.; Ruan, B.-H.; Zhou, H.; Zhao, L.-X.; Zhou, J.; Ding, Z.-T.; et al. Phytotoxic, antibacterial, and antioxidant activities of mycotoxins and other metabolites from Trichoderma sp. Nat. Prod. Res. 2017, 31, 2745–2752. [Google Scholar] [CrossRef]
- Kumar, S. Trichoderma: A biological weapon for managing plant diseases and promoting sustainability. Int. J. Agric. Sci. Med. Vet. 2013, 1, 106–121. [Google Scholar]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Viterbo, A.D.A.; Chet, I. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 2006, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol. Plant-Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masunaka, A.; Hyakumachi, M.; Takenaka, S. Plant growth-promoting fungus, Trichoderma koningi suppresses isoflavonoid phytoalexin vestitol production for colonization on/in the roots of Lotus japonicus. Microbes Environ. 2009, 1102230277. [Google Scholar] [CrossRef] [Green Version]
- Lace, B.; Genre, A.; Woo, S.; Faccio, A.; Lorito, M.; Bonfante, P. Gate crashing arbuscular mycorrhizas: In vivo imaging shows the extensive colonization of both symbionts by Trichoderma atroviride. Environ. Microbiol. Rep. 2015, 7, 64–77. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Bacterial and Fungal Endophytes: Tiny Giants with Immense Beneficial Potential for Plant Growth and Sustainable Agricultural Productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef] [Green Version]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of Two Trichoderma Strains on Plant Growth, Rhizosphere Soil Nutrients, and Fungal Community of Pinus sylvestris var. mongolica Annual Seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef] [Green Version]
- Sajeesh, P.K. Cu-Chi-Tri: A Triple Combination for the Management of Late Blight Disease of Potato (Solanum tuberosum L.). Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar, India, 2015. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Vergara, A.G.; López-Bucio, J. Trichoderma modulates stomatal aperture and leaf transpiration through an abscisic acid-dependent mechanism in Arabidopsis. J. Plant Growth Regul. 2015, 34, 425–432. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabeendran, N.; Moot, D.J.; Jones, E.E.; Stewart, A. Inconsistent growth promotion of cabbage and lettuce from Trichoderma isolates. New Zeal. Plant Prot. 2000, 53, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Ariffin, S.M.; Mohamad, W.N.W.; Yusoff, W.M.W. Formulation of Trichoderma sp. SL2 inoculants using different carriers for soil treatment in rice seedling growth. Springerplus 2014, 3, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Mishra, A.; Salokhe, V.M. Rice root growth and physiological responses to SRI water management and implications for crop productivity. Paddy Water Environ. 2011, 9, 41–52. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [Green Version]
- Li, R.-X.; Cai, F.; Pang, G.; Shen, Q.-R.; Li, R.; Chen, W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Haque, M.M.; Ilias, G.N.M.; Molla, A.H. Impact of Trichoderma-enriched biofertilizer on the growth and yield of mustard (Brassica rapa L.) and tomato (Solanum lycopersicon Mill.). Agriculturists 2012, 10, 109–119. [Google Scholar] [CrossRef] [Green Version]
- El-Katatny, M.H.; Idres, M.M. Effects of single and combined inoculations with Azospirillum brasilense and Trichoderma harzianum on seedling growth or yield parameters of wheat (Triticum vulgaris L., Giza 168) and corn (Zea mays L., hybrid 310). J. Plant Nutr. 2014, 37, 1913–1936. [Google Scholar] [CrossRef]
- Naznin, A.; Hossain, M.M.; Ara, K.A.; Hoque, A.; Islam, M. Influence of organic amendments and bio-control agent on yield and quality of tuberose. J. Hort. 2015, 2, 1–8. [Google Scholar]
- Srivastava, S.N.; Singh, V.; Awasthi, S.K. Trichoderma induced improvement in growth, yield and quality of sugarcane. Sugar Tech. 2006, 8, 166–169. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; de Masi, L.; de Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Idowu, O.O.; Olawole, O.I.; Idumu, O.O.; Salami, A.O. Bio-control effect of Trichoderma asperellum (Samuels) Lieckf. and Glomus intraradices Schenk on okra seedlings infected with Pythium aphanidermatum (Edson) Fitzp and Erwinia carotovora (Jones). J. Exp. Agric. Int. 2016, 1–12. [Google Scholar] [CrossRef]
- Mahmood, A.; Kataoka, R. Potential of biopriming in enhancing crop productivity and stress tolerance. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 127–145. [Google Scholar]
- Ousley, M.A.; Lynch, J.M.; Whipps, J.M. The effects of addition of Trichoderma inocula on flowering and shoot growth of bedding plants. Sci. Hortic. 1994, 59, 147–155. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Alam, M.J.; Islam, M.M. Effect of Trichoderma on seed germination and seedling parameters of chili. J. Sci. Found. 2010, 8, 141–150. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Seed biopriming with salinity tolerant isolates of Trichoderma harzianum alleviates salt stress in rice: Growth, physiological and biochemical characteristics. J. Plant Pathol. 2012, 94, 353–365. [Google Scholar]
- Qi, W.; Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 2013, 53, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, A.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Montero-Barrientos, M.; Hermosa, R.; Cardoza, R.E.; Gutierrez, S.; Nicolas, C.; Monte, E. Transgenic expression of the Trichoderma harzianum hsp70 gene increases Arabidopsis resistance to heat and other abiotic stresses. J. Plant Physiol. 2010, 167, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Stacey, G.; Keen, N.T. (Eds.) Plant-Microbe Interactions Vol 4; American Phytopathological Society Press: St. Paul Minnesota, MN, USA, 1999. [Google Scholar]
- McIntyre, M.; Nielsen, J.; Arnau, J.; van der Brink, H.; Hansen, K.; Madrid, S. Proceedings of the 7th European Conference on Fungal Genetics, Copenhagen, Denmark, 7–20 April 2004.
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-H.; Zheng, L.-J.; Liu, F.-F.; Dang, L.-Z.; Li, L.; Huang, R.; Zhang, K.-Q. New cyclopentenones from strain Trichoderma sp. YLF-3. Nat. Prod. Res. 2009, 23, 1431–1435. [Google Scholar] [CrossRef]
- Karuppiah, V.; Li, T.; Vallikkannu, M.; Chen, J. Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 causes differential gene expression and improvement in the wheat growth and biocontrol activity. Front. Microbiol. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R. Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathology 2006, 96, 178–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliatti, F.C.; Rezende, A.A.; Juliatti, B.C.M.; Morais, T.P. Trichoderma as a Biocontrol Agent against Sclerotinia Stem Rot or White Mold on Soybeans in Brazil: Usage and Technology. In Trichoderma-The Most Widely Used Fungicide; IntechOpen: London, UK, 2019. [Google Scholar]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2019, 1–9. [Google Scholar] [CrossRef]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [Green Version]
- de La Cruz, J.; Hidalgo-Gallego, A.; Lora, J.M.; Benitez, T.; Pintor-Toro, J.A.; Llobell, A. Isolation and characterization of three chitinases from Trichoderma harzianum. Eur. J. Biochem. 1992, 206, 859–867. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Henis, Y. Degradation of plant pathogenic fungi by Trichoderma harzianum. Can. J. Microbiol. 1982, 28, 719–725. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Degradation of fungal cell walls by lytic enzymes of Trichoderma harzianum. Microbiology 1989, 135, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Geremia, R.A.; Goldman, G.H.; Jacobs, D.; Ardrtes, W.; Vila, S.B.; van Montagu, M.; Herrera-Estrella, A. Molecular characterization of the proteinase-encoding gene, prb1, related to mycoparasitism by Trichoderma harzianum. Mol. Microbiol. 1993, 8, 603–613. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kobayashi, R.; Nagasaki, S. Purification and Properties of an Endo β-1, 6-Glucanase from Rhizopus chinensis R-69. Agric. Biol. Chem. 1974, 38, 1493–1500. [Google Scholar] [CrossRef] [Green Version]
- Chet, I.; Harman, G.E.; Baker, R. Trichoderma hamatum: Its hyphal interactions with Rhizoctonia solani and Pythium spp. Microb. Ecol. 1981, 7, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.M.; Phaff, H.J. Lysis of Yeast Cell Walls Lytic β-(1→ 6)-Glucanase from Bacillus circulans WL-12: Lytic β-(1→ 6)-Glucanase from Bacillus circulans WL-12. Eur. J. Biochem. 1976, 63, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Galhaup, C.; Payer, K.; Woo, S.L.; Mach, R.L.; Fekete, C.; Lorito, M.; Kubicek, C.P. Chitinase Gene Expression during Mycoparasitic Interaction of Trichoderma harzianum with Its Host. Fungal Genet. Biol. 1999, 26, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Peterbauer, C.K.; Mach, R.L.; Lorito, M.; Zeilinger, S.; Kubicek, C.P. The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr. Genet. 2003, 43, 289–295. [Google Scholar] [PubMed]
- Viterbo, A.; Montero, M.; Ramot, O.; Friesem, D.; Monte, E.; Llobell, A.; Chet, I. Expression regulation of the endochitinase chit36 from Trichoderma asperellum (T. harzianum T-203). Curr. Genet. 2002, 42, 114–122. [Google Scholar] [CrossRef]
- Inbar, J.; Menendez, A.N.A.; Chet, I. Hyphal interaction between Trichoderma harzianum and Sclerotinia sclerotiorum and its role in biological control. Soil Biol. Biochem. 1996, 28, 757–763. [Google Scholar] [CrossRef]
- Dotson, B.R.; Soltan, D.; Schmidt, J.; Areskoug, M.; Rabe, K.; Swart, C.; Widell, S.; Rasmusson, A.G. The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonized by cellulase-induced resistance to alamethicin. BMC Plant Biol. 2018, 18, 165. [Google Scholar] [CrossRef]
- Benitez, T.; Limon, C.; Delgado-Jarana, J.; Rey, M. Glucanolytic and other enzymes and their genes. Trichoderma Gliocladium 1998, 2, 101–127. [Google Scholar]
- Lorito, M. Chitinolytic enzymes and their genes. Trichoderma Gliocladium 1998, 2, 73–99. [Google Scholar]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Jarana, J.; Moreno-Mateos, M.A.; Benítez, T. Glucose uptake in Trichoderma harzianum: Role of gtt1. Eukaryot. Cell 2003, 2, 708–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef]
- Sarrocco, S.; Guidi, L.; Fambrini, S.; Degl’Innocenti, E.; Vannacci, G. Competition for cellulose exploitation between Rhizoctonia solani and two Trichoderma isolates in the decomposition of wheat straw. J. Plant Pathol. 2009, 91, 331–338. [Google Scholar]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef] [Green Version]
- Miethke, M. Molecular strategies of microbial iron assimilation: From high-affinity complexes to cofactor assembly systems. Metallomics 2013, 5, 15–28. [Google Scholar] [CrossRef]
- Srivastava, M.P.; Gupta, S.; Sharm, Y.K. Detection of siderophore production from different cultural variables by CAS-agar plate assay. Asian J. Pharm. Pharmacol. 2018, 4, 66–69. [Google Scholar] [CrossRef]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [Green Version]
- Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal metabolites antagonists towards plant pests and human pathogens: Structure-activity relationship studies. Molecules 2018, 23, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Hu, M.; Li, Q.-L.; Yang, Y.-B.; Liu, K.; Miao, C.-P.; Zhao, L.-X.; Ding, Z.-T. Koninginins RS from the endophytic fungus Trichoderma koningiopsis. Nat. Prod. Res. 2017, 31, 835–839. [Google Scholar] [CrossRef]
- Turaga, V.N.R. Peptaibols: Antimicrobial Peptides from Fungi. In Bioactive Natural Products in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 713–730. [Google Scholar]
- Howell, C.R.; Hanson, L.E.; Stipanovic, R.D.; Puckhaber, L.S. Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 2000, 90, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma–a genomic perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, R.W.; Simon, A.; Sivasithamparam, K.; Ghisalberti, E.L. An antibiotic from Trichoderma koningii active against soilborne plant pathogens. J. Nat. Prod. 1989, 52, 67–74. [Google Scholar] [CrossRef]
- Singh, S.; Dureja, P.; Tanwar, R.S.; Singh, A. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pestic. Res. J. 2005, 17, 26–29. [Google Scholar]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- Brito, J.P.C.; Ramada, M.H.S.; de Magalhães, M.T.Q.; Silva, L.P.; Ulhoa, C.J. Peptaibols from Trichoderma asperellum TR356 strain isolated from Brazilian soil. SpringerPlus 2014, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar]
- Zeilinger, S.; Reithner, B.; Scala, V.; Peissl, I.; Lorito, M.; Mach, R.L. Signal transduction by Tga3, a novel G protein α subunit of Trichoderma atroviride. Appl. Environ. Microbiol. 2005, 71, 1591–1597. [Google Scholar] [CrossRef] [Green Version]
- Omann, M.R.; Lehner, S.; Rodr\’\iguez, C.E.; Brunner, K.; Zeilinger, S. The seven-transmembrane receptor Gpr1 governs processes relevant for the antagonistic interaction of Trichoderma atroviride with its host. Microbiology 2012, 158, 107. [Google Scholar] [CrossRef] [PubMed]
- Reithner, B.; Schuhmacher, R.; Stoppacher, N.; Pucher, M.; Brunner, K.; Zeilinger, S. Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet. Biol. 2007, 44, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Scher, K.; Mukherjee, M.; Pardovitz-Kedmi, E.; Sible, G.V.; Singh, U.S.; Kale, S.P.; Mukherjee, P.K.; Horwitz, B.A. Overlapping and distinct functions of two Trichoderma virens MAP kinases in cell-wall integrity, antagonistic properties and repression of conidiation. Biochem. Biophys. Res. Commun. 2010, 398, 765–770. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, N.; Kabadwal, B.; Tewari, A.; Kumar, J. Review on plant-Trichoderma-pathogen interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Buchenauer, H. Trichoderma harzianum and its metabolite 6-pentyl-alpha-pyrone suppress fusaric acid produced by Fusarium moniliforme. J. Phytopathol. 2008, 156, 79–87. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wiest, A.; Ruiz, N.; Keightley, A.; Moran-Diez, M.E.; McCluskey, K.; Pouchus, Y.F.; Kenerley, C.M. Two classes of new peptaibols are synthesized by a single non-ribosomal peptide synthetase of Trichoderma virens. J. Biol. Chem. 2011, 286, 4544–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Chen, L.; Wang, X.-W.; Zhang, T.; Zhao, P.-B.; Song, X.-Y.; Sun, C.-Y.; Chen, X.-L.; Zhou, B.-C.; Zhang, Y.-Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Tijerino, A.; Cardoza, R.E.; Moraga, J.; Malmierca, M.G.; Vicente, F.; Aleu, J.; Collado, I.G.; Gutiérrez, S.; Monte, E.; Hermosa, R. Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet. Biol. 2011, 48, 285–296. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Strakowska, J.; Błaszczyk, L.; Chełkowski, J. The significance of cellulolytic enzymes produced by Trichoderma in opportunistic lifestyle of this fungus. J. Basic Microbiol. 2014, 54, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Kaur, T.; Singh, B.; Chauhan, V.S.; Kumar, A.; Rastegari, A.A.; Yadav, N.; Yadav, A.N.; Gupta, V.K. Extremophiles for Hydrolytic Enzymes Productions: Biodiversity and Potential Biotechnological Applications. Bioprocess. Biomol. Prod. 2019, 321–372. [Google Scholar] [CrossRef]
- Khatabi, B.; Molitor, A.; Lindermayr, C.; Pfiffi, S.; Durner, J.; von Wettstein, D.; Kogel, K.-H.; Schäfer, P. Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica. PLoS ONE 2012, 7, e35502. [Google Scholar] [CrossRef]
- Djonović, S.; Pozo, M.J.; Dangott, L.J.; Howell, C.R.; Kenerley, C.M. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant-Microbe Interact. 2006, 19, 838–853. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.J.; Mur, L.A.J.; Brotman, Y. Trichoderma asperelloides suppresses nitric oxide generation elicited by Fusarium oxysporum in Arabidopsis roots. Mol. Plant-Microbe Interact. 2014, 27, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J.S.; López-Bucio, J. Enhanced plant immunity using Trichoderma. In BioTechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 495–504. [Google Scholar]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Shoresh, M.; Gal-On, A.; Leibman, D.; Chet, I. Characterization of a mitogen-activated protein kinase gene from cucumber required for Trichoderma-conferred plant resistance. Plant Physiol. 2006, 142, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal-and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Uresti-Rivera, E.E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flores, S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Pascual, J.A.; Pérez-Alfocea, F.; Albacete, A.; Roldán, A. Trichoderma harzianum and Glomus intraradices modify the hormone disruption induced by Fusarium oxysporum infection in melon plants. Phytopathology 2010, 100, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piel, J.; Atzorn, R.; Gäbler, R.; Kühnemann, F.; Boland, W. Cellulysin from the plant parasitic fungus Trichoderma viride elicits volatile biosynthesis in higher plants via the octadecanoid signalling cascade. FEBS Lett. 1997, 416, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Sharon, A.; Fuchs, Y.; Anderson, J.D. The elicitation of ethylene biosynthesis by a Trichoderma xylanase is not related to the cell wall degradation activity of the enzyme. Plant Physiol. 1993, 102, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Polko, J.K. 1-aminocyclopropane 1-carboxylic acid and its emerging role as an ethylene-independent growth regulator. Front. Plant Sci. 2019, 10, 1602. [Google Scholar]
- Kurusu, T.; Hamada, J.; Nokajima, H.; Kitagawa, Y.; Kiyoduka, M.; Takahashi, A.; Hanamata, S.; Ohno, R.; Hayashi, T.; Okada, K.; et al. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010, 153, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Yoshikuni, Y.; Martin, V.J.J.; Ferrin, T.E.; Keasling, J.D. Engineering cotton (+)-δ-cadinene synthase to an altered function: Germacrene D-4-ol synthase. Chem. Biol. 2006, 13, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; der Does, D.; Zamioudis, C.; Leon-Reyes, A.; van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Wang, K.L.-C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, Y.; Ichikawa, H.; Naznin, H.A.; Kogure, A.; Hyakumachi, M. Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seedborne diseases of rice. Pest. Manag. Sci. 2012, 68, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple roles and effects of a novel Trichoderma hydrophobin. Mol. Plant-Microbe Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Garcidueñas, S.; Leal-Morales, C.A.; Herrera-Estrella, A. Analysis of the β-1, 3-glucanolytic system of the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1998, 64, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, H.; Tada, K.; Sekiya, T.; Yokoyama, K.; Takahashi, A.; Tohda, H.; Kumagai, H.; Morikawa, Y. Molecular characterization and heterologous expression of the gene encoding a low-molecular-mass endoglucanase from Trichoderma reesei QM9414. Appl. Environ. Microbiol. 1998, 64, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Sandgren, M.; Shaw, A.; Ropp, T.H.; Wu, S.; Bott, R.; Cameron, A.D.; Ståhlberg, J.; Mitchinson, C.; Jones, T.A. The X-ray crystal structure of the Trichoderma reesei family 12 endoglucanase 3, Cel12A, at 1.9 Å resolution. J. Mol. Biol. 2001, 308, 295–310. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Wang, M.; Zhou, F.; Malik, F.A.; Yang, H.; Bhaskar, R.; Hu, J.; Sun, C.; Miao, Y. Expression of Trichoderma viride endoglucanase III in the larvae of silkworm, Bombyx mori L. and characteristic analysis of the recombinant protein. Mol. Biol. Rep. 2011, 38, 3897–3902. [Google Scholar] [CrossRef]
- Chandra, M.; Kalra, A.; Sangwan, N.S.; Sangwan, R.S. Biochemical and proteomic characterization of a novel extracellular β-glucosidase from Trichoderma citrinoviride. Mol. Biotechnol. 2013, 53, 289–299. [Google Scholar] [CrossRef]
- Sternberg, D.; Vuayakumar, P.; Reese, E.T. β-Glucosidase: Microbial production and effect on enzymatic hydrolysis of cellulose. Can. J. Microbiol. 1977, 23, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.Y.; Saddler, J.N. Trichoderma xylanases, their properties and application. Crit. Rev. Biotechnol. 1992, 12, 413–435. [Google Scholar] [CrossRef]
- Lorito, M.; Harman, G.E.; Hayes, C.K.; Broadway, R.M.; Tronsmo, A.; Woo, S.L.; di Pietro, A. Chitinolytic enzymes produced by Trichoderma harzianum: Antifungal activity of purified endochitinase and chitobiosidase. Phytopathology 1993, 83, 302–307. [Google Scholar] [CrossRef]
- Peterbauer, C.K.; Lorito, M.; Hayes, C.K.; Harman, G.E.; Kubicek, C.P. Molecular cloning and expression of the nag1 gene (N-acetyl-β-D-glucosaminidase-encoding gene) from Trichoderma harzianum P1. Curr. Genet. 1996, 30, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-J.; Baek, J.-M.; Uribe, P.; Kenerley, C.M.; Cook, D.R. Cloning and characterization of multiple glycosyl hydrolase genes from Trichoderma virens. Curr. Genet. 2002, 40, 374–384. [Google Scholar] [CrossRef]
- Harman, G.E.; Hayes, C.K.; Lorito, M.; Broadway, R.M.; di Pietro, A.; Peterbauer, C.; Tronsmo, A. Chitinolytic enzymes of Trichoderma harzianum: Purification of chitobiosidase and endochitinase. Phytopathology 1993, 83, 313–318. [Google Scholar] [CrossRef]
- Flores, A.; Chet, I.; Herrera-Estrella, A. Improved biocontrol activity of Trichoderma harzianum by over-expression of the proteinase-encoding gene prb1. Curr. Genet. 1997, 31, 30–37. [Google Scholar] [CrossRef]
- Goldman, M.H.S.; Goldman, G.H. Trichoderma harzianum transformant has high extracellular alkaline proteinase expression during specific mycoparasitic interactions. Genet. Mol. Biol. 1998, 21. [Google Scholar] [CrossRef]
- Bhale, U.N.; Rajkonda, J.N. Enzymatic activity of Trichoderma species. Nov. Nat. Sci. Res. 2012, 1, 1–8. [Google Scholar]
- Mastouri, F.; Björkman, T.; Harman, G.E. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant-Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Suriani Ribeiro, M.; de Paula, R.; Raquel Voltan, A.; de Castro, R.G.; Carraro, C.B.; de Assis, L.; Stecca Steindorff, A.; Goldman, G.H.; Silva, R.N.; Ulhoa, C.J.; et al. Endo-β-1, 3-glucanase (GH16 Family) from Trichoderma harzianum Participates in Cell Wall Biogenesis but Is Not Essential for Antagonism Against Plant Pathogens. Biomolecules 2019, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Salwan, R.; Al-Ani, L.K.T. Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Cardoza, R.-E.; Hermosa, M.-R.; Vizcaíno, J.-A.; Sanz, L.; Monte, E.; Gutiérrez, S. Secondary metabolites produced by Trichoderma and their importance in the biocontrol process. Microorg. Ind. Enzym. Biocontrol 2005, 1–22. [Google Scholar]
- Vinale, F.; Girona, I.A.; Nigro, M.; Mazzei, P.; Piccolo, A.; Ruocco, M.; Woo, S.; Rosa, D.R.; Herrera, C.L.; Lorito, M. Cerinolactone, a hydroxy-lactone derivative from Trichoderma cerinum. J. Nat. Prod. 2012, 75, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Pyke, T.R.; Dietz, A. U-21,963, a New Antibiotic: I. Discovery and Biological Activity. Appl. Environ. Microbiol. 1966, 14, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Almassi, F.; Ghisalberti, E.L.; Narbey, M.J.; Sivasithamparam, K. New antibiotics from strains of Trichoderma harzianum. J. Nat. Prod. 1991, 54, 396–402. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Rowland, C.Y. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 1993, 56, 1799–1804. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef]
- Liu, R.; Gu, Q.-Q.; Zhu, W.-M.; Cui, C.-B.; Fan, G.-T. Trichodermamide A and aspergillazine A, two cytotoxic modified dipeptides from a marine-derived fungus Spicaria elegans. Arch. Pharm. Res. 2005, 28, 1042–1046. [Google Scholar] [CrossRef]
- Brian, P.W.; McGowan, J.G. Viridin: A highly fungistatic substance produced by Trichoderma viride. Nature 1945, 156, 144–145. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Ghisalberti, E.L. Secondary metabolism in Trichoderma. Trichoderma Gliocladium. Vol 1 Basic Biol. Taxon. Genet. 2002, 1, 139. [Google Scholar]
- Dickinson, J.M.; Hanson, J.R.; Hitchcock, P.B.; Claydon, N. Structure and biosynthesis of harzianopyridone, an antifungal metabolite of Trichoderma harzianum. J. Chem. Soc. Perkin Trans. 1989, 1, 1885–1887. [Google Scholar] [CrossRef]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a new antifungal, 10-member lactone from Trichoderma cremeum with plant growth promotion activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.-X.; Song, Y.-P.; Ji, N.-Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.E. U-21,963, a New Antibiotic: II. Isolation and Characterization. Appl. Environ. Microbiol. 1966, 14, 511–512. [Google Scholar] [CrossRef] [Green Version]
- Tamura, A.; Kotani, H.; Naruto, S. Trichoviridin and dermadin from Trichoderma sp. TK-1. J. Antibiot. 1975, 28, 161–162. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.R. Selective isolation from soil and separation in vitro of P and Q strains of Trichoderma virens with differential media. Mycologia 1999, 91, 930–934. [Google Scholar] [CrossRef]
- Rippa, S.; Eid, M.; Formaggio, F.; Toniolo, C.; Béven, L. Hypersensitive-Like Response to the Pore-Former Peptaibol Alamethicin in Arabidopsis Thaliana. ChemBioChem 2010, 11, 2042–2049. [Google Scholar] [CrossRef]
- Shi, W.-L.; Chen, X.-L.; Wang, L.-X.; Gong, Z.-T.; Li, S.; Li, C.-L.; Xie, B.-B.; Zhang, W.; Shi, M.; Li, C.; et al. Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp. J. Exp. Bot. 2016, 67, 2191–2205. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Buensanteai, N.; Moran-Diez, M.E.; Druzhinina, I.S.; Kenerley, C.M. Functional analysis of non-ribosomal peptide synthetases (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NRPS hybrid enzyme involved in the induced systemic resistance response in maize. Microbiology 2012, 158, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Lin, X.; Tian, Y.; Liang, R.; Wang, J.; Yang, B.; Zhou, X.; Kaliyaperumal, K.; Luo, X.; Tu, Z.; et al. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat. Prod. Res. 2018, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valdespino, C.A.; Porras-Troncoso, M.D.; Corrales-Escobosa, A.R.; Wrobel, K.; Mart\’\inez-Hernández, P.; Olmedo-Monfil, V. Functional characterization of TvCyt2, a member of the p450 monooxygenases from Trichoderma virens relevant during the association with plants and mycoparasitism. Mol. Plant-Microbe Interact. 2018, 31, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.-T.; Wang, Y.-J.; Ma, X.-Y.; Yin, X.-L.; Ji, N.-Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1-4. Nat. Prod. Res. 2019, 33, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-R.; Miao, F.-P.; Song, Y.-P.; Guo, Z.-Y.; Ji, N.-Y. Trichocitrin, a new fusicoccane diterpene from the marine brown alga-endophytic fungus Trichoderma citrinoviride cf-27. Nat. Prod. Res. 2016, 30, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-R.; Ma, X.-Y.; Ji, N.-Y. Trichosordarin A, a norditerpene glycoside from the marine-derived fungus Trichoderma harzianum R5. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Schenkel, D.; Lemfack, M.C.; Piechulla, B.; Splivallo, R. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 2015, 6, 707. [Google Scholar] [CrossRef]
- Malmierca, M.G.; McCormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Monte, E.; Gutiérrez, S. Production of trichodiene by Trichoderma harzianum alters the perception of this biocontrol strain by plants and antagonized fungi. Environ. Microbiol. 2015, 17, 2628–2646. [Google Scholar] [CrossRef]
- Cruz-Magalhães, V.; Nieto-Jacobo, M.F.; van Zijll de Jong, E.; Rostás, M.; Padilla-Arizmendi, F.; Kandula, D.; Kandula, J.; Hampton, J.; Herrera-Estrella, A.; Steyaert, J.M.; et al. The NADPH oxidases Nox1 and Nox2 differentially regulate volatile organic compounds, fungistatic activity, plant growth promotion and nutrient assimilation in Trichoderma atroviride. Front. Microbiol. 2019, 9, 3271. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mijiti, G.; Wang, Z.; Yu, W.; Fan, H.; Zhang, R.; Liu, Z. Functional analysis of the class II hydrophobin gene HFB2-6 from the biocontrol agent Trichoderma asperellum ACCC30536. Microbiol. Res. 2015, 171, 8–20. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, A.; Matsumura, F. Photochemically enhanced microbial degradation of environmental pollutants. Environ. Sci. Technol. 1991, 25, 1329–1333. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Kumar, R.; Bishnoi, K. Biosorption of Cr (VI) with Trichoderma viride immobilized fungal biomass and cell free Ca-alginate beads. Indian J. Exp. Biol. 2007, 45, 657–664. [Google Scholar] [PubMed]
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 2008, 187, 327–336. [Google Scholar] [CrossRef]
- Faedda, R.; Puglisi, I.; Sanzaro, V.; Petrone, G.; Cacciola, S.O. Expression of genes of Trichoderma harzianum in response to the presence of cadmium in the substrate. Nat. Prod. Res. 2012, 26, 2301–2308. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. BioTechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef]
- Shafique, S.; Bajwa, R.; Shafique, S. Molecular characterisation of UV and chemically induced mutants of Trichoderma reesei FCBP-364. Nat. Prod. Res. 2010, 24, 1438–1448. [Google Scholar] [CrossRef]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M. Optimization of conditions for the efficient production of mutants in streptococcal cultures and post-culture liquids. Acta Biol. Hung. 2005, 56, 137–150. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Singh, J.; Singh, S.; Nain, L. Prospecting the Potential of Agroresidues as Substrate for Microbial Flavor Production. Front. Sustain. Food Syst. 2020, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Marra, R.; Nicoletti, R.; Pagano, E.; DellaGreca, M.; Salvatore, M.M.; Borrelli, F.; Lombardi, N.; Vinale, F.; Woo, S.L.; Andolfi, A. Inhibitory effect of trichodermanone C, a sorbicillinoid produced by Trichoderma citrinoviride associated to the green alga Cladophora sp., on nitrite production in LPS-stimulated macrophages. Nat. Prod. Res. 2019, 33, 3389–3397. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Rangsan, J.; Siripong, P.; Tip-Pyang, S. 9-epi-Viridiol, a novel cytotoxic furanosteroid from soil fungus Trichoderma virens. Nat. Prod. Res. 2006, 20, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niaz, S.I.; Wang, Z.; Zhu, Y.; Lin, Y.; Li, J.; Liu, L. α-Glucosidase inhibitory and cytotoxic botryorhodines from mangrove endophytic fungus Trichoderma sp. 307. Nat. Prod. Res. 2018, 32, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Qin, D.; Ye, T.; Yan, X.; Wang, J.; Duan, X.; Dong, J. An endophytic fungus from Trichoderma harzianum SWUKD3. 1610 that produces nigranoic acid and its analogues. Nat. Prod. Res. 2019, 33, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Iqtedar, M.; Nadeem, M.; Naeem, H.; Abdullah, R.; Naz, S.; Syed, Q.U.A.; Kaleem, A. Bioconversion potential of Trichoderma viride HN1 cellulase for a lignocellulosic biomass Saccharum spontaneum. Nat. Prod. Res. 2015, 29, 1012–1019. [Google Scholar] [CrossRef]
- Arthe, R.; Rajesh, R.; Rajesh, E.M.; Rajendran, R.; Jeyachandran, S. Production of bio-ethanol from cellulosic cotton waste through microbial extracellular enzymatic hydrolysis and fermentation. Electron. J. Environ. Agric. Food chem. 2008, 7, 2948–2958. [Google Scholar]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.M. Chemical modification of wood: Advantages and disadvantages. In Proceedings of the American Wood-Preservers’ Association, San Francisco, CA, USA, 28–30 April 1975; Volume 71, pp. 41–51. [Google Scholar]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Lebow, S. Leaching of Wood Preservative Components and Their Mobility in the Environment: Summary of Pertinent Literature; (General Technical Report FPL; GTR-93): 36 p.; 28 cm; United States Department of Agriculture: Washington, DC, USA, 1996; Volume 93.
- Hingston, J.A.; Collins, C.D.; Murphy, R.J.; Lester, J.N. Leaching of chromated copper arsenate wood preservatives: A review. Environ. Pollut. 2001, 111, 53–66. [Google Scholar] [CrossRef]
- Schultz, T.P.; Militz, H.; Freeman, M.H.; Goodell, B.; Nicholas, D.D. Development of Commercial Wood Preservatives: Efficacy, Environmental, and Health Issues. ACS Sympos. Ser. 2008, 982, 655. [Google Scholar]
- Namyslo, J.C.; Kaufmann, D.E. Chemical improvement of surfaces. Part 1: Novel functional modification of wood with covalently bound organoboron compounds. Holzforschung 2009, 63, 627–632. [Google Scholar] [CrossRef]
- Verma, P.; Junga, U.; Militz, H.; Mai, C. Protection mechanisms of DMDHEU treated wood against white and brown rot fungi. Holzforschung 2009, 63, 371–378. [Google Scholar] [CrossRef]
- Lee, M.J.; Cooper, P. Copper monoethanolamine adsorption in wood and its relation with cation exchange capacity (CEC). Holzforschung 2010, 64, 653–658. [Google Scholar] [CrossRef]
- Pilgård, A.; Alfredsen, G.; Hietala, A. Quantification of fungal colonization in modified wood: Quantitative real-time PCR as a tool for studies on Trametes versicolor. Holzforschung 2010, 64, 645–651. [Google Scholar] [CrossRef]
- Robinson, S.C.; Laks, P.E. The effects of subthreshold loadings of tebuconazole, DDAC, and boric acid on wood decay by Postia placenta. Holzforschung 2010, 64, 537–543. [Google Scholar] [CrossRef]
- Chirkova, J.; Andersone, I.; Irbe, I.; Spince, B.; Andersons, B. Lignins as agents for bio-protection of wood. Holzforschung 2011, 65, 497–502. [Google Scholar] [CrossRef]
- Freitag, C.; Morrell, J.J.; Love, C.S. Long-term performance of fused borate rods for limiting internal decay in Douglas-fir utility poles. Holzforschung 2011, 65, 429–434. [Google Scholar] [CrossRef]
- Pankras, S.; Cooper, P.A. Effect of ammonia addition to alkaline copper quaternary wood preservative solution on the distribution of copper complexes and leaching. Holzforschung 2012, 66, 397–406. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Relative fungal efficacy results from the soil block test with a long incubation period of three commercial copper wood preservatives. Holzforschung 2012, 66, 245–250. [Google Scholar] [CrossRef]
- Ejechi, B.O. Biological control of wood decay in an open tropical environment with Penicillium sp. and Trichoderma viride. Int. Biodeterior. Biodegrad. 1997, 39, 295–299. [Google Scholar] [CrossRef]
- Tucker, E.J.B.; Bruce, A.; Staines, H.J. Application of modified international wood preservative chemical testing standards for assessment of biocontrol treatments. Int. Biodeterior. Biodegrad. 1997, 39, 189–197. [Google Scholar] [CrossRef]
- Mortuza, M.G.; Ilag, L.L. Potential for biocontrol of Lasiodiplodia theobromae (Pat.) Griff. & Maubl. in banana fruits by Trichoderma species. Biol. Control. 1999, 15, 235–240. [Google Scholar]
- Batta, Y.A. Effect of treatment with Trichoderma harzianum Rifai formulated in invert emulsion on postharvest decay of apple blue mold. Int. J. Food microbiol. 2004, 96, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Bankole, S.A.; Adebanjo, A. Biocontrol of brown blotch of cowpea caused by Colletotrichum truncatum with Trichoderma viride. Crop Prot. 1996, 15, 633–636. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. https://doi.org/10.3390/plants9060762
Sood M, Kapoor D, Kumar V, Sheteiwy MS, Ramakrishnan M, Landi M, Araniti F, Sharma A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants. 2020; 9(6):762. https://doi.org/10.3390/plants9060762
Chicago/Turabian StyleSood, Monika, Dhriti Kapoor, Vipul Kumar, Mohamed S. Sheteiwy, Muthusamy Ramakrishnan, Marco Landi, Fabrizio Araniti, and Anket Sharma. 2020. "Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent" Plants 9, no. 6: 762. https://doi.org/10.3390/plants9060762








