Application of Trichoderma harzianum, 6-Pentyl-?-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes
Abstract
:1. Introduction
2. Results
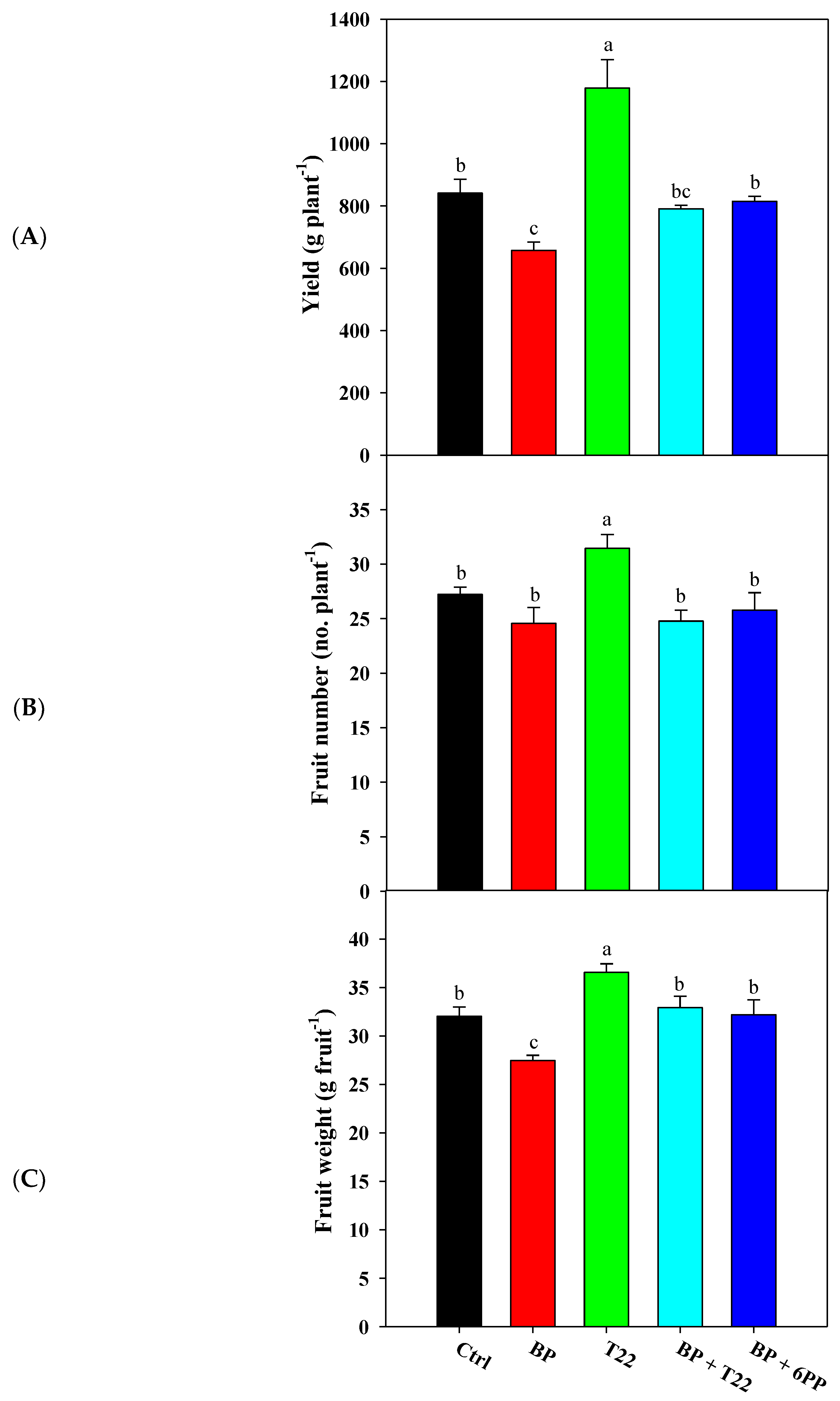
2.1. Yield, Yield Components, Shape Index and Juice Quality
2.2. Carbohydrates and Bioactive Metabolites Content
2.3. Soluble Proteins and Free Amino Acids Contents
2.4. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Biostimulants, Plant Material and Greenhouse Experimental Design
4.2. Yield Components, Sampling and Fruit Quality Assessments
4.3. Starch and Soluble Carbohydrate Analysis
4.4. Polyphenol and Lycopene Analysis
4.5. Soluble Proteins, Free and Total Amino Acid Analysis
4.6. Statistics, Principal Component Analysis and Percentage Increase
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shennan, C.; Krupnik, T.; Baird, G.; Cohen, H.; Forbush, K.; Lovell, R.; Olimpi, E. Organic and Conventional Agriculture: A Useful Framing? Annu. Rev. Environ. Resour. 2017, 42. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- FAO. Transforming the World through Food and Agriculture—FAO and the 2030 Agenda for Sustainable Development; FAO: Rome, Italy, 2019; p. 36. Available online: http://www.fao.org/documents/card/en/c/CA5299EN/ (accessed on 4 May 2020).
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and microorganisms boost the nutritional composition of buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Tranque, E.; Bettiol, W.; Monte, E.; Hermosa, R. Differential response of tomato plants to the application of three Trichoderma species when evaluating the control of Pseudomonas syringae populations. Plants 2020, 9, 626. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Hill, R. Chapter 31—Applications of Trichoderma in Plant Growth Promotion. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of combined applications of Trichoderma virens and a biopolymer-based biostimulant on lettuce agronomical, physiological, and qualitative properties under variable N regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Dell’Aversana, E.; Ruggiero, A.; Cirillo, V.; Gibon, Y.; Woodrow, P.; Maggio, A.; Carillo, P. Omeprazole treatment enhances nitrogen use efficiency through increased nitrogen uptake and assimilation in corn. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molla, A.H.; Manjurul Haque, M.; Amdadul Haque, M.; Ilias, G.N.M. Trichoderma-Enriched Biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum Mill.) and minimizes NPK fertilizer use. Agric. Res. 2012, 1, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Macías-Rodríguez, L.; Guzmán-Gómez, A.; García-Juárez, P.; Contreras-Cornejo, H.A. Trichoderma atroviride promotes tomato development and alters the root exudation of carbohydrates, which stimulates fungal growth and the biocontrol of the phytopathogen Phytophthora cinnamomi in a tripartite interaction system. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [Green Version]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [Green Version]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Bottiglieri, A.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; et al. Bivalent metal-chelating properties of harzianic acid produced by Trichoderma pleuroticola associated to the gastropod melarhaphe neritoides. Molecules 2020, 25, 2147. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Salwan, R.; Kumari, N.; Sharma, V. Bioactive Volatile Metabolites of Trichoderma: An Overview; Springer: Singapore, 2019; pp. 87–111. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and metabolome reprogramming in tomato plants by Trichoderma harzianum strain T22 primes and enhances defense responses against aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Raimondi, G.; Lucini, L.; Carillo, P.; Kyriacou, M.C.; Colla, G.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; De Pascale, S. Physiological and metabolic responses triggered by omeprazole improve tomato plant tolerance to NaCl Stress. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carillo, P.; Woodrow, P.; Raimondi, G.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.; Colla, G.; Mori, M.; Giordano, M.; De Pascale, S.; et al. Omeprazole promotes chloride exclusion and induces salt tolerance in greenhouse basil. Agronomy 2019, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.P.; Halim, A.F. Characterization of the major aroma constituent of the fungus Trichoderma viride. J. Agric. Food Chem. 1972, 20, 437–438. [Google Scholar] [CrossRef]
- Scarselletti, R.; Faull, J.L. In vitro activity of 6-pentyl-α-pyrone, a metabolite of Trichoderma harzianum, in the inhibition of Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici. Mycol. Res. 1994, 98, 1207–1209. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Woo, S.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.; Lorito, M. A novel role for the Trichoderma–plant interaction Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A Vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Le Mire, G.; Nguyen, M.L.; Fassotte, B.; du Jardin, P.; Verheggen, F.; Delaplace, P.; Jijakli, M. Review: Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. Biotechnol. Agron. Soc. Environ. 2016, 20, 299–313. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The Influence of Different Electrical Conductivity Values in a Simplified Recirculating Soilless System on Inner and Outer Fruit Quality Characteristics of Tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S. Trichoderma: A Multi-Purpose Tool for Integrated Pest Management. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–353. [Google Scholar] [CrossRef]
- Li, R.-X.; Cai, F.; Pang, G.; Shen, Q.-R.; Li, R.; Chen, W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-pependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Chacón, M.; Rodríguez-Galán, O.; Benítez, T.; Sousa, S.; Rey, M.; Llobell, A.; Delgado-Jarana, J. Microscopic and transcriptome analyses of early colonization of tomato roots by Trichoderma harzianum. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2007, 10, 19–27. [Google Scholar] [CrossRef]
- Davidson, D.; Verma, M.; Gu, F. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 318. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Chen, X.-F.; Deng, C.-L.; Yang, L.-T.; Lai, N.-W.; Guo, J.-X.; Chen, L.-S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Lubitz, W.; Chrysina, M.; Cox, N. Water oxidation in photosystem II. Photosynth. Res. 2019, 142, 105–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma asperellum T42 reprograms tobacco for enhanced nitrogen utilization efficiency and plant growth when fed with N nutrients. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, S.; Rubio, M.B.; Cardoza, R.E.; Gutiérrez, S.; Nicolás, C.; Bettiol, W.; Hermosa, R.; Monte, E. Nitrogen metabolism and growth enhancement in tomato plants challenged with Trichoderma harzianum expressing the Aspergillus nidulans Acetamidase amd S gene. Front. Microbiol. 2016, 7, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodrow, P.; Ciarmiello, L.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Poirot, E.; Flores-Mosquera, M. GABA, a non-protein amino acid ubiquitous in food matrices. Cogent Food Agric. 2018, 4, 1534323. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [Green Version]
- Tiansawang, K.; Luangpituksa, P.; Varanyanond, W.; Hansawasdi, C. GABA (γ-aminobutyric acid) production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci. Technol. (Camp.) 2016, 36. [Google Scholar] [CrossRef] [Green Version]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. yield and quality as influenced by cropping season, protein hydrolysates, and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H. Anticancer effect of lycopene in gastric carcinogenesis. J. Cancer Prev. 2015, 20, 92–96. [Google Scholar] [CrossRef]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2016, 76, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhash, K.; Bose, C.; Agrawal, B.K. Effect of short term supplementation of tomatoes on antioxidant enzymes and lipid peroxidation in type-II diabetes. Indian J. Clin. Biochem. 2007, 22, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Sipos, L.; Orbán, C.; Bálint, I.; Csambalik, L.; Divéky-Ertsey, A.; Gere, A. Colour parameters as indicators of lycopene and antioxidant activity traits of cherry tomatoes (Solanum lycopersicum L.). Eur. Food Res. Technol. 2017, 243, 1533–1543. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Sadler, G.; Davis, J.; Dezman, D. Rapid Extraction of lycopene and β-Carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Ciarmiello, L.; Piccirillo, P.; Carillo, P.; Luca, A.; Woodrow, P. Determination of the genetic relatedness of fig (Ficus carica L.) accessions using RAPD fingerprint and their agro-morphological characterization. S. Afr. J. Bot. 2015. [Google Scholar] [CrossRef]

| Treatment | Shape Index | Dry Matter Content | TSS | pH | EC |
|---|---|---|---|---|---|
| (%) | (°Brix) | (mS cm−1) | |||
| Ctrl | 0.81 a | 8.03 | 6.73 | 4.40 | 3.83 ab |
| BP | 0.81 a | 8.38 | 7.03 | 4.42 | 4.37 a |
| T22 | 0.79 ab | 8.00 | 6.63 | 4.39 | 3.87 ab |
| BP + T22 | 0.78 ab | 7.67 | 6.47 | 4.33 | 4.48 a |
| BP + 6PP | 0.77 b | 7.79 | 6.80 | 4.40 | 3.48 b |
| Significance | * | NS | NS | NS | * |
| Treatment | Starch | Glucose | Fructose | Sucrose | Polyphenols | Lycopene |
|---|---|---|---|---|---|---|
| (µmol g−1 dw) | (µmol g−1 dw) | (µmol g−1 dw) | (µmol g−1 dw) | (mg GAE g−1 dw) | (mg g−1 dw) | |
| Ctrl | 12.57 | 166.2 | 42.40 | 13.96 | 5.10 | 1.64 b |
| BP | 8.70 | 175.2 | 31.21 | 12.04 | 4.64 | 1.86 b |
| T22 | 13.26 | 170.2 | 36.23 | 13.58 | 5.39 | 2.45 a |
| BP + T22 | 9.19 | 171.9 | 32.42 | 11.90 | 5.25 | 2.29 a |
| BP + 6PP | 8.96 | 167.3 | 31.27 | 11.69 | 4.67 | 2.50 a |
| Significance | NS | NS | NS | NS | NS | *** |
| Chemical Compound | Treatment | Significance | ||||
|---|---|---|---|---|---|---|
| Ctrl | BP | T22 | BP + T22 | BP + 6PP | ||
| Soluble proteins (mg g−1 dw) | 20.66 c | 24.47 ab | 21.47 bc | 24.75 a | 23.46 abc | * |
| Alanine (µmol g−1 dw) | 6.23 | 6.49 | 6.64 | 5.57 | 7.44 | NS |
| Arginine (µmol g−1 dw) | 10.34 | 8.52 | 12.75 | 10.77 | 14.09 | NS |
| Asparagine (µmol g−1 dw) | 11.66 b | 13.58 b | 16.02 a | 12.01 b | 13.05 b | * |
| Aspartate (µmol g−1 dw) | 25.15 | 28.12 | 26.46 | 21.71 | 28.42 | NS |
| GABA (µmol g−1 dw) | 9.07 c | 18.62 a | 17.00 ab | 11.39 bc | 12.53 bc | * |
| Glutamine (µmol g−1 dw) | 28.49 | 29.82 | 37.35 | 27.12 | 28.27 | NS |
| Glutamate (µmol g−1 dw) | 179.9 ab | 231.3 a | 186.6 ab | 147.9 b | 189.1 ab | * |
| Glycine (µmol g−1 dw) | 3.48 a | 0.18 b | 0.15 b | 0.15 b | 0.27 b | *** |
| Histidine (µmol g−1 dw) | 4.72 | 4.90 | 5.01 | 4.48 | 5.03 | NS |
| Isoleucine (µmol g−1 dw) | 1.58 | 1.93 | 1.90 | 1.55 | 1.90 | NS |
| Leucine (µmol g−1 dw) | 2.29 | 2.84 | 2.71 | 2.17 | 2.75 | NS |
| Lysine (µmol g−1 dw) | 1.62 | 1.59 | 1.69 | 1.33 | 1.62 | ns |
| MEA (µmol g−1 dw) | 0.46 b | 0.86 a | 0.94 a | 0.65 ab | 0.81 a | * |
| Methionine (µmol g−1 dw) | 0.40 | 0.48 | 0.45 | 0.37 | 0.42 | NS |
| Ornithine (µmol g−1 dw) | 1.18 | 1.23 | 1.19 | 1.07 | 1.52 | NS |
| Phenylalanine (µmol g−1 dw) | 5.61 | 6.92 | 6.46 | 5.21 | 7.18 | NS |
| Proline (µmol g−1 dw) | 4.57 | 4.91 | 5.77 | 4.99 | 5.03 | NS |
| Serine (µmol g−1 dw) | 2.90 | 2.32 | 2.67 | 1.86 | 2.18 | NS |
| Threonine (µmol g−1 dw) | 1.86 | 1.54 | 2.04 | 1.23 | 1.80 | NS |
| Tryptophan (µmol g−1 dw) | 0.73 | 1.11 | 0.75 | 0.75 | 0.92 | NS |
| Tyrosine (µmol g−1 dw) | 1.82 | 1.99 | 2.30 | 1.69 | 2.01 | NS |
| Valine (µmol g−1 dw) | 0.88 | 1.13 | 1.11 | 0.83 | 0.99 | NS |
| Total amino acids (µmol g−1 dw) | 304.9 | 370.4 | 337.9 | 264.8 | 327.3 | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-?-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. https://doi.org/10.3390/plants9060771
Carillo P, Woo SL, Comite E, El-Nakhel C, Rouphael Y, Fusco GM, Borzacchiello A, Lanzuise S, Vinale F. Application of Trichoderma harzianum, 6-Pentyl-?-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants. 2020; 9(6):771. https://doi.org/10.3390/plants9060771
Chicago/Turabian StyleCarillo, Petronia, Sheridan L. Woo, Ernesto Comite, Christophe El-Nakhel, Youssef Rouphael, Giovanna Marta Fusco, Assunta Borzacchiello, Stefania Lanzuise, and Francesco Vinale. 2020. "Application of Trichoderma harzianum, 6-Pentyl-?-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes" Plants 9, no. 6: 771. https://doi.org/10.3390/plants9060771
APA StyleCarillo, P., Woo, S. L., Comite, E., El-Nakhel, C., Rouphael, Y., Fusco, G. M., Borzacchiello, A., Lanzuise, S., & Vinale, F. (2020). Application of Trichoderma harzianum, 6-Pentyl-?-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants, 9(6), 771. https://doi.org/10.3390/plants9060771











