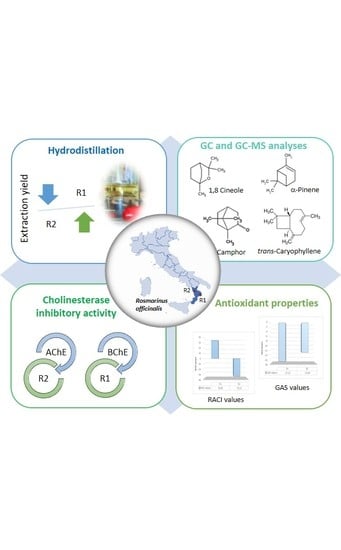
The Essential Oil of Salvia rosmarinus Spenn. from Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Profile
2.2. Cholinesterase Activity
2.3. Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Extraction Procedure
3.4. Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
3.5. In Vitro Anti-Cholinesterase Activity
3.6. In Vitro Antioxidant Activity
3.6.1. DPPH and ABTS Tests
3.6.2. FRAP Assay
3.6.3. β-Carotene Bleaching Test
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malvezzi de Macedo, L.; Mendes dos Santos, É.; Militão, L.; Lacalendola Tundisi, L.; Artem Ataide, J.; Barbosa Souto, E.; Gava Mazzola, P. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and its topical applications: A review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Drew, B.T.; Gonzàlez-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walker, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Morales, R. Rosmarinus L. In Flora Iberica; Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; REAL JARDÍN BOTÁNICO: Madrid, Spain, 2010; Volume 12, pp. 327–331. [Google Scholar]
- Rosúa, J.L. Contribución al estudio del género Rosmarinus L. en el Mediterráneo occidental. Lagascalia 1986, 14, 179–187. [Google Scholar]
- Greuter, W.; Burdet, H.M.; Long, G. Med-Checklist. 3. Dicotyledones (Convolvulaceae -Labiatae); C.B. de Geneve: Geneve, Switzerland; Berlin, Germany, 1986. [Google Scholar]
- Rosselló, J.A.; Cosín, R.; Boscaiu, M.; Vicente, O.; Martínez, I.; Soriano, P. Intragenomic diversity and phylogenetic systematics of wild rosemaries (Rosmarinus officinalis L. s.l., Lamiaceae) assessed by nuclear ribosomal DNA sequences (ITS). Plant Syst. Evol. 2006, 262, 1–12. [Google Scholar] [CrossRef]
- Mateu-Andrés, I.; Aguilella, A.; Boisset, F.; Currás, R.; Guara, M.; Laguna, E.; Marzo, A.; Puche, M.F.; Pedrola, J. Geographical patterns of genetic variation in rosemary (Rosmarinus officinalis) in the Mediterranean basin. Bot. J. Linn. Soc. 2013, 171, 700–712. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Antiproliferative activities on renal, prostate and melanoma cancer cell lines of Sarcopoterium spinosum aerial parts and its major constituent tormentic acid. Anti-Cancer Agents Med. Chem. 2013, 13, 768–776. [Google Scholar] [CrossRef]
- Kompelly, A.; Kompelly, S.; Vasudha, B.; Boggula, N. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. J. Drug Deliv. Ther. 2019, 9, 323–330. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Gardeli, C.; Mallouchos, A.; Papaioannou, M.; Komaitis, M. Variation of the chemical profile and antioxidant behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller grown in Greece. J. Agric. Food Chem. 2008, 56, 7254–7264. [Google Scholar] [CrossRef]
- Baydar, H.; Ozkan, G.; Erbas¸, S.; Altindal, D. IELD, Chemical composition and antioxidant properties of extracts and essential oils of sage and rosemary depending on seasonal variations. Acta Hort. 2009, 826, 383–390. [Google Scholar] [CrossRef]
- Zaouali, Y.; Boussaid, M. Isozyme markers and volatiles in Tunisian Rosmarinus officinalis L. (Lamiaceae): A comparative analysis of population structure. Biochem. Syst. Ecol. 2008, 36, 11–21. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, Y.; Messaoud, C.; Salah, A.; Boussaid, M. Oil composition variability among populations in relationship with their ecological areas in Tunisian Rosmarinus officinalis L. Flav. Fragr. J. 2015, 20, 512–520. [Google Scholar] [CrossRef]
- Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Jordàn, J.M.; Martìnez Conesa, C.; Monino, I.M.; Lax, V.; Quìlez, M.; Sotomayor, A.J. Effect of altitude on Rosmarinus officinalis L. essential oil in Murcia (Spain). Acta Hort. 2009, 826, 309–316. [Google Scholar]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coisson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: Effect of soil nutrients. Chem. Ecol. 2008, 34, 1219–1229. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Kántor, A.; Tokár, M.; Puchalski, C.; Ivanišová, E. Antimicrobial effect of sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.) essential oils on microbiota of chicken breast. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2017, 71, 461–467. [Google Scholar] [CrossRef][Green Version]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jager, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, 1465–1858. [Google Scholar]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Okonogi, S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine 2012, 19, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Magali Dumont, M.; Flint, B. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Bio. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.; Araújo, M.E. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168–184. [Google Scholar] [CrossRef]
- Ben Jemia, M.; Tundis, R.; Pugliese, A.; Menichini, F.; Senatore, F.; Bruno, M.; Kchouka, M.E.; Loizzo, M.R. Effect of bioclimatic area on the composition and bioactivity of Tunisian Rosmarinus officinalis essential oils. Nat. Prod. Res. 2015, 29, 213–222. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.; Conforti, F.; Passalacqua, N.G.; Statti, G.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzyme Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Bonesi, M.; Tenuta, M.C.; Loizzo, M.R.; Sicari, V.; Tundis, R. Potential application of Prunus armeniaca L. and P. domestica L. leaf essential oils as antioxidant and of cholinesterases inhibitors. Antioxidants 2019, 8, 2. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flav. Fragr. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Syst. Ecol. 2010, 38, 659–670. [Google Scholar] [CrossRef]
- Hcini, K.; Sotomayor, J.A.; Jordan, M.J.; Bouzid, S. Chemical composition of essential oil of rosemary (Rosmarinus officinalis L.) of Tunisian origin. Asian J. Chem. 2013, 25, 2601–2603. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi, M.N. Phytochemical characteristics of Citrus peels and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Cutillas, A.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosyst. 2018, 152, 1282–1292. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Farhat, A.; Benmoussa, H.; Bachoual, R.; Nasfi, Z.; Elfalleh, H.; Romdhane, M.; Bouajila, J. Efficiency of the optimized microwave assisted extractions on the yield, chemical composition and biological activities of Tunisian Rosmarinus officinalis L. essential oil. Food Bioprod. Process. 2017, 105, 224–233. [Google Scholar] [CrossRef]
- Orhan, I.; Aslan, S.; Kartal, M.; Şener, B.; Başer, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In Vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Nat. C J. Biosci. 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yamafuji, C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Calva, J.; Bec, N.; Gilardoni, G.; Larroque, C.; Cartuche, L.; Bicchi, C.; Montesinos, J.V. Acorenone B: AChE and BChE inhibitor as a major compound of the essential oil distilled from the Ecuadorian species Niphogeton dissecta (Benth.). Pharmaceuticals 2017, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Chraibi, M.; Farah, A.; Elamin, O.; Iraqui, H.M.; Fikri-Benbrahim, K. Characterization, antioxidant, antimycobacterial, antimicrobial effects of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020, 11, 25–29. [Google Scholar]
- El Kamli, T.; El Hamdani, M.; Errachidi, F.; Chabir, R.; Bour, A. Chemical composition, antioxidant, and antimicrobial activities of Rosmarinus officinalis essential oil from Moroccan middle atlas. Phytothérapie. in press. [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Beretta, G.; Artali, R.; Facino, R.M.; Gelmini, F. An analytical and theoretical approach for the profiling of the antioxidant activity of essential oils: The case of Rosmarinus officinalis L. J. Pharm. Biomed. Anal. 2011, 55, 1255–1264. [Google Scholar] [CrossRef]
- Hendel, N.; Napoli, E.; Sarri, M.; Saija, A.; Cristani, M.; Nostro, A.; Ginestra, G.; Ruberto, G. Essential oil from aerial parts of wild Algerian rosemary: Screening of chemical composition, antimicrobial and antioxidant activities. J. Essent. Oil-Bear. Plants 2019, 22, 1–17. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013, 5, 729–739. [Google Scholar]
- Kadri, A.; Zarai, Z.; Ben Chobba, I.; Békir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical constituents and antioxidant properties of Rosmarinus officinalis L. essential oil cultivated from the South-Western of Tunisia. J. Med. Plant Res. 2011, 5, 6502–6508. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.Y.; Li, R.; Jiang, Z.T.; Wang, Y.; Tan, J.; Tang, S.H.; Zhang, Y. Antioxidant activity screening and chemical constituents of the essential oil from rosemary by ultra-fast GC electronic nose coupled with chemical methodology. J. Sci. Food Agric. 2020, 100, 3481–3487. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; GómezSerranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Clevenger, J.F. Apparatus for the determination of volatile oils. J. Am. Pharm. Assoc. 1928, 17, 341–346. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Abouali, M.; Salehi, P.; Sonboli, A.; Kanani, M.; Menichini, F.; Tundis, R. In Vitro antioxidant and antiproliferative activities of nine Salvia species. Nat. Prod. Res. 2014, 28, 2278–2285. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Tundis, R.; Leporini, M.; Falco, T.; Calabrò, V. The Influence of ultrafiltration of Citrus limon L. burm. cv femminello comune juice on its chemical composition and antioxidant and hypoglycemic properties. Antioxidants 2019, 8, 23. [Google Scholar] [CrossRef]

| Nr. | Compound | Class | RI a | R1 | R2 | I.M b | Sign |
|---|---|---|---|---|---|---|---|
| 1 | Thujene | MH | 926 | 2.34 ± 0.03 a | 0.88 ± 0.05 b | 1,2 | ** |
| 2 | α-Pinene | MH | 938 | 10.37 ± 0.01 a | 10.96 ± 1.33 b | 1,2,3 | ** |
| 3 | Camphene | MH | 953 | 6.30 ± 0.24 a | 6.87 ± 0.21 b | 1,2,3 | ** |
| 6 | Sabinene | MH | 973 | 2.82 ± 0.11 a | 1.01 ± 0.06 b | 1,2,3 | ** |
| 4 | β-Pinene | MH | 980 | 7.89 ± 1.03 b | 8.23 ± 0.62 a | 1,2,3 | ** |
| 5 | Myrcene | MH | 993 | 1.32 ± 0.06 b | 2.73 ± 0.12 a | 1,2,3 | ** |
| 7 | α-Phellandrene | MH | 1005 | 0.70 ± 0.02 a | 0.39 ± 0.04 b | 1,2 | ** |
| 8 | δ-3-Carene | MH | 1009 | nd | 0.34 ± 0.02 a | 1,2 | ** |
| 9 | α-Terpinene | MH | 1012 | 1.19 ± 0.08 b | 1.35 ± 0.03 a | 1,2,3 | ** |
| 10 | o-Cymene | MH | 1020 | 0.25 ± 0.2 a | tr | 1,2 | ** |
| 11 | p-Cymene | MH | 1025 | 1.80 ± 0.18 a | 0.67 ± 0.07 b | 1,2 | ** |
| 12 | Limonene | MH | 1030 | 1.78 ± 0.06 b | 2.30 ± 0.09 a | 1,2,3 | ** |
| 13 | 1,8-Cineole | OM | 1034 | 16.98 ± 2.11 b | 21.89 ± 2.32 a | 1,2,3 | ** |
| 14 | γ-Terpinene | MH | 1057 | 2.76 ± 0.25 a | 2.42 ± 0.06 b | 1,2,3 | ** |
| 15 | Terpinolene | MH | 1086 | 1.18 ± 0.05 a | 1.17 ± 0.01 a | 1,2,3 | ns |
| 16 | Linalool | OM | 1098 | 0.35 ± 0.06 a | nd | 1,2,3 | ** |
| 17 | α-Thujone | OM | 1106 | 0.10 ± 0.01 a | nd | 1,2 | ** |
| 18 | Camphor | OM | 1145 | 7.27 ± 0.23 b | 11.08 ± 0.76 a | 1,2 | ** |
| 19 | Borneol | OM | 1167 | 5.30 ± 0.26 a | 3.31 ± 0.08 b | 1,2 | ** |
| 20 | Terpinen-4-ol | OM | 1176 | 2.03 ± 0.09 a | nd | 1,2 | ** |
| 21 | α-Terpineol | OM | 1189 | 4.05 ± 0.26 a | 3.19 ± 0.10 b | 1,2,3 | ** |
| 22 | (-)-Bornyl acetate | SH | 1286 | 2.40 ± 0.11 b | 4.26 ± 0.12 a | 1,2 | ** |
| 23 | α-Cubebene | SH | 1352 | nd | 0.43 ± 0.03 a | 1,2 | ** |
| 24 | α-Copaene | SH | 1377 | 0.24 ± 0.03 a | nd | 1,2 | ** |
| 25 | trans-Caryophyllene | SH | 1415 | 10.58 ± 1.98 a | 8.62 ± 0.17 b | 1,2,3 | ** |
| 26 | Aromadendrene | SH | 1437 | 0.30 ± 0.03 a | 0.25 ± 0.04 a | 1,2 | ns |
| 27 | α-Humulene | SH | 1455 | 1.95 ± 0.05 a | 1.49 ± 0.10 b | 1,2 | ** |
| 28 | γ-Muurolene | SH | 1478 | 0.32 ± 0.05 a | 0.32 ± 0.01 a | 1,2 | ns |
| 29 | α-Amorphene | SH | 1487 | 0.25 ± 0.02 a | tr | 1,2 | ** |
| 30 | δ-Selinene | SH | 1493 | tr | 0.58 ± 0.02 a | 1,2 | ** |
| 31 | β-Bisabolene | SH | 1508 | tr | 0.41 ± 0.01 a | 1,2 | ** |
| 32 | γ-Cadinene | SH | 1515 | tr | 0.44 ± 0.05 a | 1,2 | ** |
| 33 | δ-Cadinene | SH | 1526 | 0.46 ± 0.03 b | 1.41 ± 0.01 a | 1,2 | ** |
| 34 | Caryophyllene oxide | OS | 1580 | 0.65 ± 0.04 a | 0.54 ± 0.02 b | 1,2 | ** |
| 35 | Viridiflorol | OS | 1591 | 1.65 ± 0.07 a | tr | 1,2 | ** |
| 36 | Manool | OS | 2055 | 1.90 ± 0.08 a | 1.24 ± 0.06 b | 1,2 | ** |
| MH | 40.70 | 39.32 | |||||
| OM | 36.08 | 39.47 | |||||
| SH | 16.50 | 18.21 | |||||
| OS | 4.20 | 1.78 | |||||
| Total identified | 97.48 | 98.78 |
| Sample | AChE IC50 (µg/mL) | BChE IC50 (µg/mL) | SI (BChE/AChE) |
|---|---|---|---|
| R1 | 85.96 ± 3.12 **** | 46.71 ± 1.85 **** | 0.54 |
| R2 | 41.86 ± 1.63 **** | 48.29 ± 1.90 **** | 1.15 |
| Positive control | |||
| Physostigmine | 0.12 ± 0.01 | 0.21 ± 0.03 | 2.0 |
| Sample | DPPH Test a IC50 (µg/mL) | ABTS Test IC50 (µg/mL) | β-carotene Bleaching Test IC50 (µg/mL) | FRAP Test μM Fe (II)/g | |
|---|---|---|---|---|---|
| R1 | 33.21% | 35.43 ± 2.83 **** | 45.21 ± 2.76 **** | 49.48 ± 3.08 **** | 2.95 ± 1.46 **** |
| R2 | 29.84% | 22.61 ± 1.91 **** | 33.18 ± 2.11 **** | 44.81 ± 2.75 **** | 5.59 ± 1.95 **** |
| Positive control | |||||
| Ascorbic acid | 5.02 ± 0.80 | 1.70 ± 0.06 | |||
| Propyl gallate | 0.09 ± 0.004 | 0.09 ± 0.004 | |||
| BHT | 63.22 ± 4.3 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leporini, M.; Bonesi, M.; Loizzo, M.R.; Passalacqua, N.G.; Tundis, R. The Essential Oil of Salvia rosmarinus Spenn. from Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity. Plants 2020, 9, 798. https://doi.org/10.3390/plants9060798
Leporini M, Bonesi M, Loizzo MR, Passalacqua NG, Tundis R. The Essential Oil of Salvia rosmarinus Spenn. from Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity. Plants. 2020; 9(6):798. https://doi.org/10.3390/plants9060798
Chicago/Turabian StyleLeporini, Mariarosaria, Marco Bonesi, Monica Rosa Loizzo, Nicodemo Giuseppe Passalacqua, and Rosa Tundis. 2020. "The Essential Oil of Salvia rosmarinus Spenn. from Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity" Plants 9, no. 6: 798. https://doi.org/10.3390/plants9060798
APA StyleLeporini, M., Bonesi, M., Loizzo, M. R., Passalacqua, N. G., & Tundis, R. (2020). The Essential Oil of Salvia rosmarinus Spenn. from Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity. Plants, 9(6), 798. https://doi.org/10.3390/plants9060798