Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives
Abstract
1. Introduction
2. COVID-19 Is Now Officially a Pandemic
3. An Overview of COVID-19
4. Antiviral Activity of Herbal Medicines and Phytochemicals against Coronaviruses
5. Mode of Antiviral Action
6. Phytomedicine and Clinical Trials for Coronavirus Infections
- Radix astragali (dried root of Astragalus membranaceus (Fisch.) (Figure 18) Bunge and Astragalus mongholicus Bunge (Fabaceae)) is a popular traditional Chinese medicine, and its active compounds may help fortify the immune system and decrease inflammation. Astragalus is occasionally also administrated as an injection in hospitals [102].
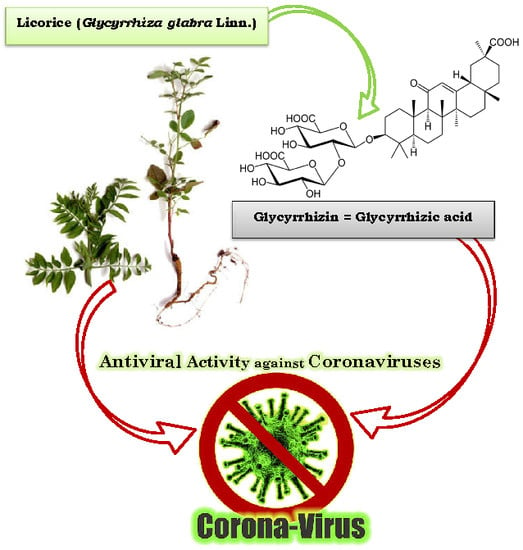
- Radix glycyrrhizae (dried roots and rhizomes of Glycyrrhiza glabra) or liquorice root is one of the 50 important plants used in phytomedicine [102].
- Radix saposhnikoviae, Saposhnikovia divaricate, recognized as fángfēng meaning “defend against the wind” in Chinese, is the single species in the genus Saposhnikovia [102].
- Atractylodis macrocephalae rhizome (Figure 19) is hailed as “the most essential Qi herb (vital energy in Chinese medicine) that tonifies and enhances the spleen”. It is the dried rhizome of Atractylodes lancea (Thunb.), Atractylodes chinensis Koidz, or any other nearby plant like Japonica atractylodes [32].
- Lonicera japonica Flos, member of the family Caprifoliaceae, is among the most widely used traditional medicines. It includes bioactive components such as caffeic acid derivatives, essential oils (EOs), flavonoids, iridoid glycosides, and terpenoids and it has anti-inflammatory, antimicrobial, anticancer, antioxidant, and immune-modulating properties [102].
- Golden Bell (Fructus forsythia) has long been recognized as a cure-all for patients who are especially vulnerable to skin infection. The plant has demonstrated broad-spectrum antibacterial activity and some suppression of influenza virus, leptospira, as well as other viruses. The plant also exhibits antipyretic and anti-inflammatory properties [32].
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Vehik, K.; Dabelea, D. The changing epidemiology of type 1 diabetes: Why is it going through the roof? Diab. Metabol. Res. Rev. 2011, 27, 3–13. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.D.; Guo, W.P.; Zhou, R.H.; Wang, M.R.; Wang, C.Q.; Holmes, E.C. Discovery, diversity and evolution of novel coronaviruses sampled from rodents in China. Virology 2015, 474, 19–27. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; Van Der Werf, S.; Brodt, H.R.; Becker, S.; Berger, A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Liang, G.; Chen, Q.; Xu, J.; Liu, Y.; Lim, W.; Peiris, J.S.M.; Di, B. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg. Infect. Dis. 2004, 10, 1774. [Google Scholar] [CrossRef]
- Hilgenfeld, R.; Peiris, M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013, 100, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Kostaki, E.G.; Magiorkinis, G.; Panayiotakopoulos, G.; Sourvinos, G.; Tsiodras, S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020, 79, 104212. [Google Scholar] [CrossRef] [PubMed]
- Roosa, K.; Lee, Y.; Luo, R.; Kirpich, A.; Rothenberg, R.; Hyman, J.M.; Chowell, G. Real-time forecasts of the COVID-19 epidemic in China from February 5 to 24 February 2020. Infect. Dis. Model. 2020, 5, 256–263. [Google Scholar] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Milit. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Hu, B.; Ge, X.; Wang, L.F.; Shi, Z. Bat origin of human coronaviruses. Virol. J. 2015, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Chang, C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell. Discov. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod Comm. 2012, 7, 1934578X1200701103. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.T.; Lu, H.T.; Fan, K.W.; Guan, Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nakamura, T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother. Res. 2004, 18, 592–594. [Google Scholar] [CrossRef]
- Cheng, P.W.; Ng, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Experim. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Cinatl, J. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS− Coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, Y.G.; Park, S.; Kim, K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef]
- Kim, H.Y.; Eo, E.Y.; Park, H.; Kim, Y.C.; Park, S.; Shin, H.J.; Kim, K. Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir. Therap. 2010, 15, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Park, C.M.; Kwon, S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chen, C.; Zhang, H.Q.; Guo, H.Y.; Wang, H.; Wang, L.; Li, R.S. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
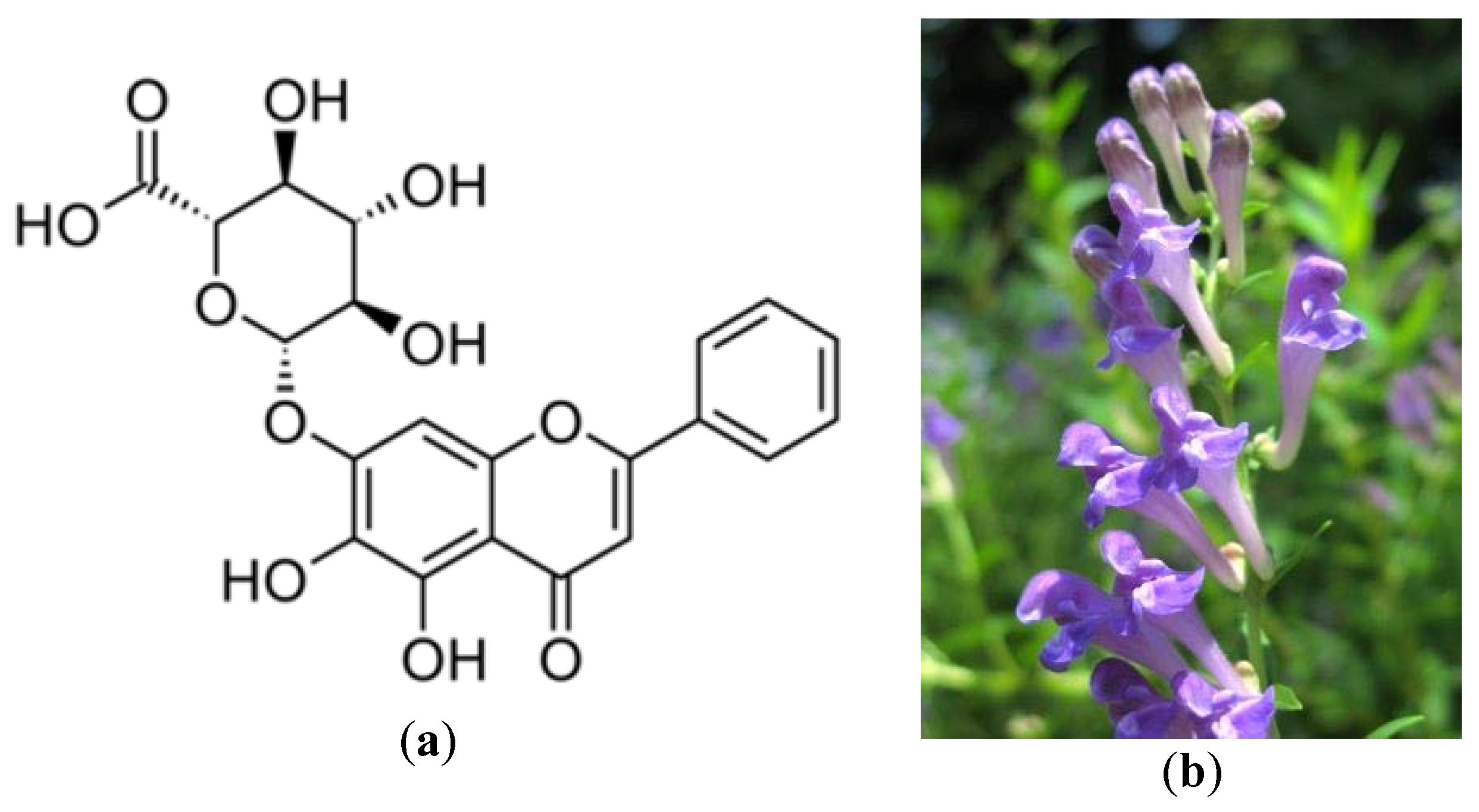
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Song, Y.N.; Wang, S.J.; Rahman, K.; Zhu, J.Y.; Zhang, H. Saikosaponins: A review of pharmacological effects. J. Asian Nat. Prod. Res. 2018, 20, 399–411. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Chao, P.D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Trad. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; He, L.; Li, Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS). Cochr. Database. Syst. Rev. 2012, 10, 1–44. [Google Scholar] [CrossRef]
- McCutcheon, A.R.; Roberts, T.E.; Gibbons, E.; Ellis, S.M.; Babiuk, L.A.; Hancock, R.E.W.; Towers, G.H.N. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, C.L.; Yen, H.R.; Chang, Y.S.; Lin, Y.P.; Huang, S.H.; Lin, C.W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 366. [Google Scholar] [CrossRef]
- Wu, C.Y.; Jan, J.T.; Ma, S.H.; Kuo, C.J.; Juan, H.F.; Cheng, Y.S.E.; Liang, F.S. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Nat. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N. Effective Antiviral Activity of Essential Oils and their Characteristics Terpenes against Coronaviruses: An Update. J. Pharmacol. Clin. Toxicol. 2020, 8, 1138. [Google Scholar]
- Boukhatem, M.N. Novel Coronavirus Disease 2019 (COVID-19) Outbreak in Algeria: A New Challenge for Prevention. J. Community Med. Health Care 2020, 5, 1035. [Google Scholar]
- World Health Organization (WHO). Statement on the Second Meeting of the International Health Regulations Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Available online: www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 2 February 2020).
- COVID-19 Coronavirus Pandemic. Available online: www.worldometers.info/coronavirus/ (accessed on 23 May 2020).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Du, B. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020, 2, 113–122. [Google Scholar]
- Liu, Y.; Yan, L.M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet. Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef]
- Dalton, C.; Corbett, S.; Katelaris, A. Pre-emptive low cost social distancing and enhanced hygiene implemented before local COVID-19 transmission could decrease the number and severity of cases. Med. J. Aust. 2020, 212, 1. [Google Scholar] [CrossRef]
- CDC SARS Response Timeline. Available online: www.cdc.gov/about/history/sars/timeline.htm (accessed on 18 March 2020).
- Yang, Q.Y.; Tian, X.Y.; Fang, W.S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65. [Google Scholar] [CrossRef]
- Wen, C.C.; Shyur, L.F.; Jan, J.T.; Liang, P.H.; Kuo, C.J.; Arulselvan, P.; Yang, N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011, 1, 41–50. [Google Scholar] [CrossRef]
- Thabti, I.; Albert, Q.; Philippot, S.; Dupire, F.; Westerhuis, B.; Fontanay, S.; Varbanov, M. Advances on Antiviral Activity of Morus spp. Plant Extracts: Human Coronavirus and Virus-Related Respiratory Tract Infections in the Spotlight. Molecules 2020, 25, 1876. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Wang, L.T.; Khalil, A.T.; Chiang, L.C.; Cheng, P.W. Bioactive pyranoxanthones from the roots of Calophyllum blancoi. Chem. Pharm. Bull. 2005, 53, 244–247. [Google Scholar] [CrossRef]
- Michaelis, M.; Doerr, H.W.; Cinatl, J., Jr. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 2011, 18, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Chen, L. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodiv. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Jiang, H.; Suzuki, Y.; Li, X.; Xiao, P.; Tanaka, T.; Qin, C. Procyanidins and butanol extract of Cinnamomi Cortex inhibit SARS-CoV infection. Antivir. Res. 2009, 82, 73–81. [Google Scholar] [CrossRef]
- Yu, M.S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.W.; Jee, J.G.; Jeong, Y.J. Identification of marketing and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Luo, W.; Su, X.; Gong, S.; Qin, Y.; Liu, W.; Li, J.; Xu, Q. Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts. Biosci. Trends. 2009, 3, 124–126. [Google Scholar]
- Lau, K.M.; Lee, K.M.; Koon, C.M.; Cheung, C.S.F.; Lau, C.P.; Ho, H.M.; Tsui, S.K.W. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Ryu, Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012, 20, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.Y.; Kim, D.; Rho, M.C. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Seo, K.H.; Curtis-Long, M.J.; Oh, K.Y.; Oh, J.W.; Cho, J.K.; Park, K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Lin, C.S.; Lai, H.C.; Lin, Y.P.; Wang, C.Y.; Tsai, Y.C.; Lin, C.W. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus. Res. 2019, 273, 197767. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.; McMahon, J.B.; McCray, P.B. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef]
- Pyrrosia Lingua. Available online: https://www.flickr.com/photos/harumkoh/17118611672/ (accessed on 16 June 2020).
- Artemisia annua. Available online: https://www.flickr.com/photos/47108884@N07/4738072658 (accessed on 16 June 2020).
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhetinic acid. In Sweeteners, 1st ed.; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Gewerbestrasse, Switzerland, 2018; pp. 245–261. [Google Scholar]
- Glycyrrhiza Glabra Linn. Available online: https://www.flickr.com/photos/valdelobos/4657830744 (accessed on 16 June 2020).
- Kitamura, K.; Honda, M.; Yoshizaki, H.; Yamamoto, S.; Nakane, H.; Fukushima, M.; Tokunaga, T. Baicalin, an inhibitor of HIV-1 production in vitro. Antivir. Res. 1998, 37, 131–140. [Google Scholar] [CrossRef]
- Scutellaria Baicalensis. Available online: https://www.flickr.com/photos/tanaka_juuyoh/2718717267 (accessed on 16 June 2020).
- Renard-Nozaki, J.; Kim, T.; Imakura, Y.; Kihara, M.; Kobayashi, S. Effect of alkaloids isolated from Amaryllidaceae on herpes simplex virus. Res. Virol. 1989, 140, 115–128. [Google Scholar] [CrossRef]
- Ieven, M.; Vlietinick, A.J.; Berghe, D.V.; Totte, J.; Dommisse, R.; Esmans, E.; Alderweireldt, F. Plant antiviral agents. III. Isolation of alkaloids from Clivia miniata Regel (Amaryl-lidaceae). J. Nat. Prod. 1982, 45, 564–573. [Google Scholar] [CrossRef]
- Çitoğlu, G.S.; Acıkara, Ö.B.; Yılmaz, B.S.; Özbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lonicera japonica ‘Japanese Honeysuckle’. Available online: https://www.flickr.com/photos/89906643@N06/9892035994/ (accessed on 27 April 2020).
- Ginseng (Panax ginseng). Available online: https://www.flickr.com/photos/eekim/4145898809 (accessed on 16 June 2020).
- Wild Rose-Rosa nutkana. Available online: https://www.flickr.com/photos/nordique/7188593733 (accessed on 22 April 2020).
- Potentilla arguta. Available online: https://www.flickr.com/photos/glaciernps/23703091762 (accessed on 16 June 2020).
- Red elderberry. Available online: https://www.flickr.com/photos/brewbooks/217464248 (accessed on 16 June 2020).
- Tsuchiya, Y.; Shimizu, M.; Hiyama, Y.; Itoh, K.; Hashimoto, Y.; Nakayama, M.; Morita, N. Antiviral activity of natural occurring flavonoids in vitro. Chem. Pharmac. Bull. 1985, 33, 3881–3886. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.J.; Grant, P.G.; Sarr, A.B.; Belakere, J.R.; Swaggerty, C.L.; Phillips, T.D.; Woode, G.N. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Veterin. Microbiol. 1998, 63, 147–157. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liu, Z.; Seril, D.N.; Liao, J.; Ding, W.; Kim, S.; Yang, C.S. Black tea constituents, theaflavins, inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Carcinogenesis 1997, 18, 2361–2365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parthasarathy, K.; Ng, L.; Lin, X.; Liu, D.X.; Pervushin, K.; Gong, X.; Torres, J. Structural flexibility of the pentameric SARS coronavirus envelope protein ion channel. Biophys. J. 2008, 95, L39–L41. [Google Scholar] [CrossRef] [PubMed]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Reichling, J.; Koch, C.; Stahl-Biskup, E.; Sojka, C.; Schnitzler, P. Virucidal activity of a β-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med. 2005, 71, 1123–1127. [Google Scholar] [CrossRef]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents. Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, S.H.; Jung, K.; Yoo, H.; Park, G. Inhibitory Effects of Myricetin on Lipo- polysaccharide-Induced Neuroinflammation. Brain Sci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, N.; Li, S.; Chen, M.; Pu, P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed. Pharmacother. 2019, 117, 109042. [Google Scholar] [CrossRef] [PubMed]
- Woad Root (Ban Lan Gen), Isatis Indigotica-Radix Isatidis. Available online: https://www.flickr.com/photos/nhq9801/9216111022/in/photostream/ (accessed on 16 June 2020).
- Hughes, K. A Plant a Day: Japanese Nutmeg-Yew (Torreya nucifera, T. spp.). Available online: https://www.flickr.com/photos/138014579@N08/35706702426 (accessed on 16 June 2020).
- Houttuynia cordata. Available online: https://www.flickr.com/photos/dakiny/34949957600 (accessed on 16 June 2020).
- Zhu, H.; Zhang, Y.; Ye, G.; Li, Z.; Zhou, P.; Huang, C. In vivo and in vitro antiviral activities of calycosin-7-O-beta-D-glucopyranoside against coxsackie virus B3. Biol. Pharm. Bull. 2009, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Lin, C.P.; Huang, K.K.; Chen, W.C.; Hsieh, H.P.; Liang, P.H.; Hsu, J.T.A. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3, 3′-digallate (TF3). Evid Based Complement Alternat Med. 2005, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.; Piao, X.S.; Li, D.F.; Kim, S.W.; Lee, H.S.; Guo, P.F. Effects of dietary Astragalus polysaccharide on growth performance and immune function in weaned pigs. Anim. Sci. 2006, 82, 501–507. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Hou, C.C. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef]
- Ho, T.Y.; Wu, S.L.; Chen, J.C.; Li, C.C.; Hsiang, C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101. [Google Scholar] [CrossRef]
- Kumar, V.; Tan, K.P.; Wang, Y.M.; Lin, S.W.; Liang, P.H. Identification, synthesis and evaluation of SARS-CoV and MERS-CoV 3C-like protease inhibitors. Bioorg. Med. Chem. 2016, 24, 3035–3042. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; Wei, M.; Yang, X.; Cao, Q.; Guan, M.; Deng, X. Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J. Agr. Food. Chem. 2012, 60, 3947–3954. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, L.; Gao, M.; Jiang, W.; Shao, F.; Li, J.; Yu, B. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: Involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int. Immunopharmacol. 2012, 12, 88–93. [Google Scholar] [CrossRef]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Veter. Res. 2014, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Abbas, G. Antiviral Activity Against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Niu, J.; Wang, C.; Huang, B.; Wang, W.; Zhu, N.; Tan, W. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019, 93, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- Maxmen, A. More than 80 clinical trials launch to test coronavirus treatments. Nature 2020, 578, 347. [Google Scholar] [CrossRef]
- Vellingiri, B.; Jayaramayya, K.; Iyer, M.; Narayanasamy, A.; Govindasamy, V.; Giridharan, B.; Rajagopalan, K. COVID-19: A promising cure for the global panic. Sci. Total. Environ. 2020, 138277. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.L.; Shang, Y.X.; Liang, S.B.; Yang, M.; Robinson, N.; Liu, J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese. J. Integr. Med. 2020, 26, 1–8. [Google Scholar]
- Astragalus Membranaceus. Available online: https://www.flickr.com/photos/jennyhsu47/4539175733 (accessed on 16 June 2020).
- Rhizoma Atractylodes macrocephalae. Available online: https://www.flickr.com/photos/jennyhsu47/4539818092 (accessed on 16 June 2020).
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Huang, X. Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing. J. Med. Virol. 2020, 7, 797–806. [Google Scholar] [CrossRef]
- McMahon, M.A.; Siliciano, J.D.; Lai, J.; Liu, J.O.; Stivers, J.T.; Siliciano, R.F.; Kohli, R.M. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J. Biol. Chem. 2008, 283, 31289–31293. [Google Scholar] [CrossRef]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Russell, R.J.; Walker, P.A.; Gamblin, S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef]
- Delaney, W.E., IV; Borroto-Esoda, K. Therapy of chronic hepatitis B: Trends and developments. Curr. Opinion. Pharmacol. 2008, 8, 532–540. [Google Scholar] [CrossRef]
- Asres, K.; Bucar, F.; Kartnig, T.; Witvrouw, M.; Pannecouque, C.; De Clercq, E. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother. Res. 2001, 15, 62–69. [Google Scholar] [CrossRef]
- Hudson, J.B. Antiviral Compounds from Plants; CRC Press: Boston, MA, USA, 1990. [Google Scholar]



















| Coronavirus Strains | Plant Species or Isolated Compound | References |
|---|---|---|
| SARS-CoV | Lycoris radiata | Li et al. [23] |
| Artemisia annua Pyrrosia lingua Lindera aggregata Isatis indigotica | Lin et al. [26] | |
| Boenninghausenia sessilicarpa | Yang et al. [43] | |
| Lonicera japonica Eucalyptus spp. Panax ginseng | Wu et al. [31] | |
| Bovine coronavirus (BCV) | Amelanchier alnifolia Cardamine angulata Rosa nutkana Verbascum Thapsus | McCutcheon et al. [29] |
| SARS-CoV (Hong Kong strain) | Dioscorea batatas Cassia tora Taxillus chinensis | Wen et al. [44] |
| 10 strains of SARS-CoV in fRhK4 cell line | Glycyrrhizin (Glycyrrhiza uralensis) Baicalin (Scutellaria baicalensis) | Chen et al. [15] |
| HCoV-229E | Mulberry (Morus alba var. alba, Morus alba var. rosa, and Morus rubra) | Thabti et al. [45] |
| Calophyllum blancoi | Shen et al. [46] | |
| Pelargonium sidoides | Michaelis et al. [47] | |
| Saikosaponins (Bupleurum spp., Heteromorpha spp., Scrophularia scorodonia) | Cheng et al. [17] | |
| SARS-CoV BJ01 | Galla chinensis | Yi et al. [48] |
| SARS-CoV FFM1 | Glycyrrhizin and glycyrrhetinic acid found in: Glycyrrhiza radix | Hoever et al. [18] |
| Laurus nobilis Essential oil fGentiana scabra | Loizzo et al. [49] | |
| SARS-CoV PUMC01 F5 | Cinnamomum sp. | Zhuang et al. [50] |
| SARS-CoV helicase non-structural protein 13 (nsP13) | Scutettaria baicalensis | Yu et al. [51] |
| SARS-CoV 3CLpro | Rheum palmatum | Luo et al. [52] |
| Houttuynia cordata | Lau et al. [53] | |
| SARS-CoV CLpro | Salvia miltiorrhiza | Park et al. [54] |
| Torreya nucifera | Ryu et al. [55] | |
| SARS-CoV PLpro | Broussonetia papyrifera | Park et al. [56] |
| Psoralea corylifolia | Kim et al. [57] | |
| HCoV-NL63 | Strobilanthes cusia leaf | Tsai et al. [30] |
| Sambucus formosana | Weng et al. [58] | |
| HCoV-OC43 HCoV-299E HCoV-NL63 | Griffithsin (Griffithsia sp.) | O’Keefe et al. [59] |
| Medicinal Plants (Phytochemicals or Compounds) | Common Name | Antiviral Mechanism | IC50 or EC50 Value | References |
|---|---|---|---|---|
| Rosa nutkana | Nootka Rose or wild Rose | Inhibition or reduction of the activity of enteric coronavirus—unidentified mechanisms. | - | McCutcheon et al. [29] |
| Amelanchier alnifolia | Saskatoon or pacific serviceberry or western serviceberry | - | ||
| Luteolin | Blocking the viral entry of HIV-luc/SARS pseudo-type virus. | 9.02 μM | Yi et al. [48] | |
| Lycoris radiata | Red spider lily | Inhibition or reduction of viral attachment and penetration. | 2.4 ± 0.2 μg/mL | Li et al. [23] |
| Artemisia annua | Sweet wormwood | 34.5 ± 2.6 μg/mL | ||
| Pyrrosia lingua | Tongue Fern | 43.2 ± 14.1 μg/mL | ||
| Lindera aggregata | Spicewood | 88.2 ± 7.7 μg/mL | ||
| Isatis indigotica (Beta-sitosterol) | Chinese Woad or dyer’s woad | Inhibition of nsP13 helicase and 3CL-like protease. | 1.210 μM | Lin et al. [26] |
| Black tea (Theaflavin) | Inhibition of 3C-like protease of SARS-CoV. | 9.5 μM | Chen et al. [89] | |
| Bupleurum marginatum | Margined Chinese Thoroughwax | Interfering with early stages of viral replication, such as the penetration of the virus into the target cells. Some flavonoids are metabolized within the body into phenolate ions, inhibiting viral polymerase function, and connecting with viral nucleic acid or viral cuspid proteins. That tends to lead to viral replication being inhibited or reduced. | - | Cheng et al. [17] |
| Astragalus membranaceus | Mongolian milkvetch or Chinese astragalus | Immunomodulatory effects by increasing the number of lymphocytes and the proportion of CD4+ lymphocytes. | - | Yuan et al. [90] |
| Saikosaponins B2 | Inhibition of viral attachment and penetration steps of HCoV-22E9. | 1.7 ± 0.1 μM/L | Cheng et al. [17] | |
| Curcumin | Inhibition of 3CL protease. | 40 μM | Wen et al. [91] | |
| Rheum officinale | Chinese rhubarb | Inhibition of the interaction between SARS-CoV S protein and angiotensin-converting enzyme 2 (ACE2). | 1 to 10 μg/mL | Ho et al. [92] |
| Polygonum multiflorum | Tuber fleeceflower | |||
| Houttuynia cordata | Fish mint or Chameleon-plant | Inhibition of 3CL-like protease and viral polymerase, and RNA-dependent RNA polymerase (RdRp) which are key enzymes involved with virus functions. Stimulate the proliferation of splenic lymphocytes which are necessary immune cells for fighting infection. Increase the proportion of CD4+ and CD8+ T cells necessary to fight viral infection. | - | Lau et al. [53] Kumar et al. [93] |
| Torreya nucifera (Amentoflavone) | Japanese nutmeg-yew or Japanese torreya | Inhibition of nsP13 helicase and 3CL protease. | 8.3 μM | Ryu et al. [55] |
| Verbascum Thapsus (Verbascoside) | Great Mullein or Common mullein | Active ingredients decrease inflammation during respiratory infection. | - | Speranza et al. [94] |
| Herbal extracts (Gentiana scabra, Dioscorea batatas, Cassia tora, Taxillus chinensis, Cibotium barometz) | Inhibition of 3CL-like protease. | 39 μg/mL and 44 μg/mL (two extracts of Cibotium barometz) | Wen et al. [44] | |
| Glycyrrhiza glabra (Licorice Root) | Liquorice or Sweetwood | In vivo anti-inflammatory effect in the lungs by a glycoside known as LicoA. | - | Chu et al. [95] |
| Ruscus aculeatus | Butcher’s broom, knee holly or piaranthus | In vivo protection of lungs from inflammatory injury by the active ingredient (Ruscogenin, steroid sapogenin). Decreases of cerebral ischemia-induced blood–brain barrier dysfunction. Anti-inflammatory and anti-thrombotic properties. | - | Sun et al. [96] |
| Myricetin | 3CL protease inhibition of SARS-CoV. | - | Yu et al. [51] | |
| Sambucus nigra | Blue elder, common elder or Elderberry | Inhibition of chicken coronavirus strain if given at an early stage of infection. | - | Chen et al. [97] |
| Psoralea corylifolia (Bavachinin) | Babchi | Inhibitions of papain-like protease (PLpro). | 38.4 ± 2.4 μM | Kim et al. [57] |
| Hypericum perforatum | Perforate St John’s wort or common Saint John’s wort | Inhibition of mRNA expression in Avian coronavirus infectious bronchitis virus (IBV). | Chen et al. [98] | |
| Sambucus formosana | Blue elder, common elder or elderberry | Inhibition of chicken coronavirus strain and coronavirus NL63 by interfering with the viral envelopes, rendering them non-infectious. | - | Weng et al. [58] |
| Lycorine | Inhibition of cell division of different strains of coronaviruses (HCoV-OC43, HCoV-NL63, MERS-CoV, and MHV-A59). | 0.15–0.31 μM. | Shen et al. [99] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhatem, M.N.; Setzer, W.N. Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants 2020, 9, 800. https://doi.org/10.3390/plants9060800
Boukhatem MN, Setzer WN. Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants. 2020; 9(6):800. https://doi.org/10.3390/plants9060800
Chicago/Turabian StyleBoukhatem, Mohamed Nadjib, and William N. Setzer. 2020. "Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives" Plants 9, no. 6: 800. https://doi.org/10.3390/plants9060800
APA StyleBoukhatem, M. N., & Setzer, W. N. (2020). Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants, 9(6), 800. https://doi.org/10.3390/plants9060800