Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample and Chemicals
2.2. Extraction
2.3. Working Protocol
- -
- the lavender (Lavandula angustifolia Mill.) seeds were sterilized (immersion in a 20% HClO solution for 2 min and well washed with water).
- -
- the seeds were carefully selected and then immersed (first application) in the tested extracts (BBE500, BBE1000, SBE500, SBE1000) for 12 h, at a constant temperature of 25 °C.
- -
- the seeds (3 seeds/pot and 3x60 seeds/experimental variant) were sown manually into pots (60 pots/experimental variant).
- -
- the pots were wetted with 20 mL of tested extracts/pot (second application).
- -
- at vegetative stage, after 30 days from germination, the plants were wetted with 20 mL/of tested extracts/pot (third application—at radicular level).
- -
- after 60 days from the beginning of the experiments, the lavender plants were transferred in the field.
- -
- after 1 year, at vegetative stage, the plants were wetted with 10 mL of tested extracts/plant (fourth application—at foliar level by spraying).
- -
- during the flowering stage, the aerial part of the plant was harvested, separating the plant organs into inflorescences (flos) and stems with leaves (herba). The plant material was dried in the open air, being prepared for hydrodistillation.
2.4. Plant Growth and Development Analysis
2.5. Histo-Anatomical Analysis
2.6. GC-MS Qualitative Analysis of Volatile Compounds
2.7. Statistical Analysis
3. Results
3.1. Seed Germination
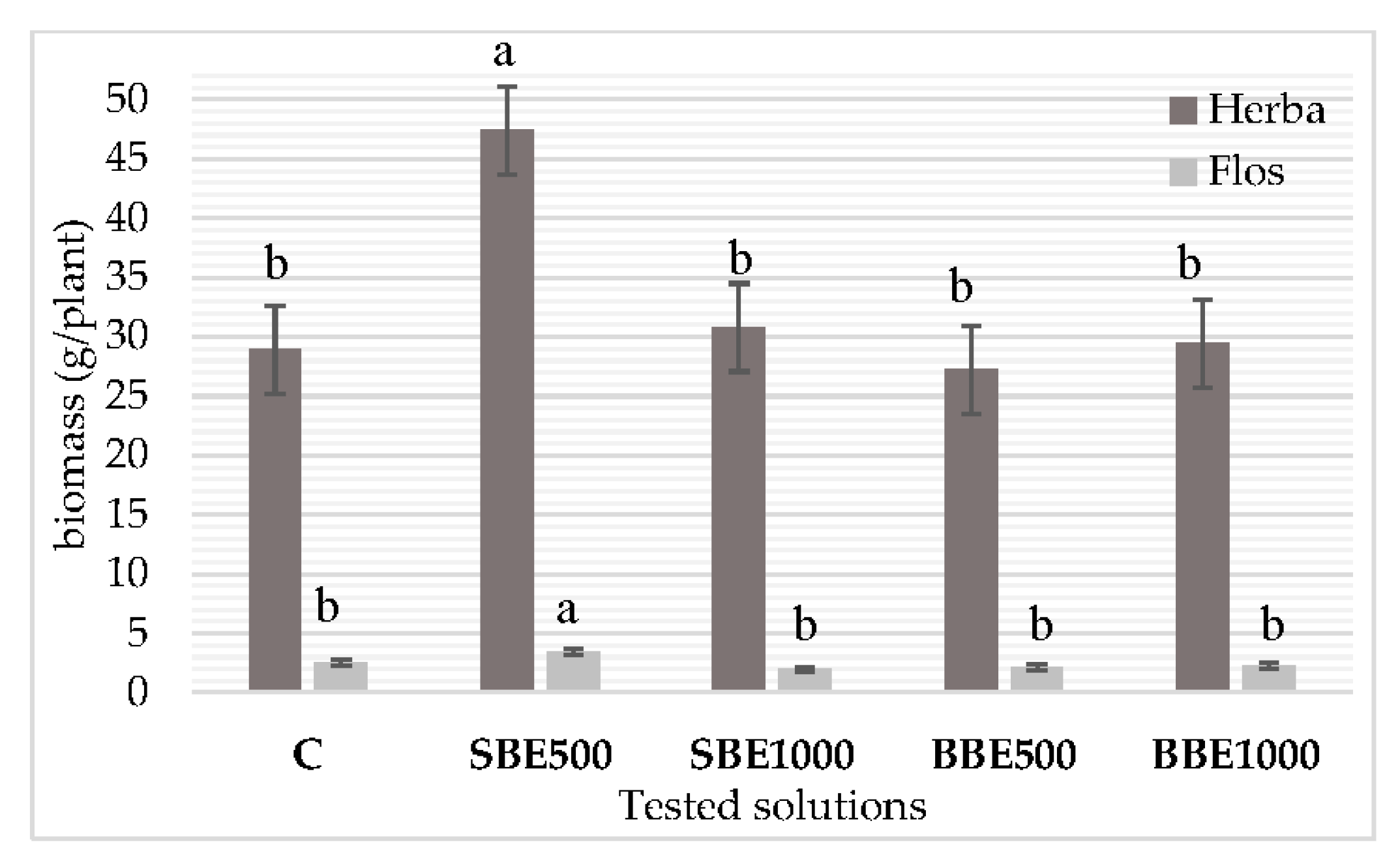
3.2. Biomass Accumulation
3.3. Photo-Assimilating Pigment Content in Lavender Leaves
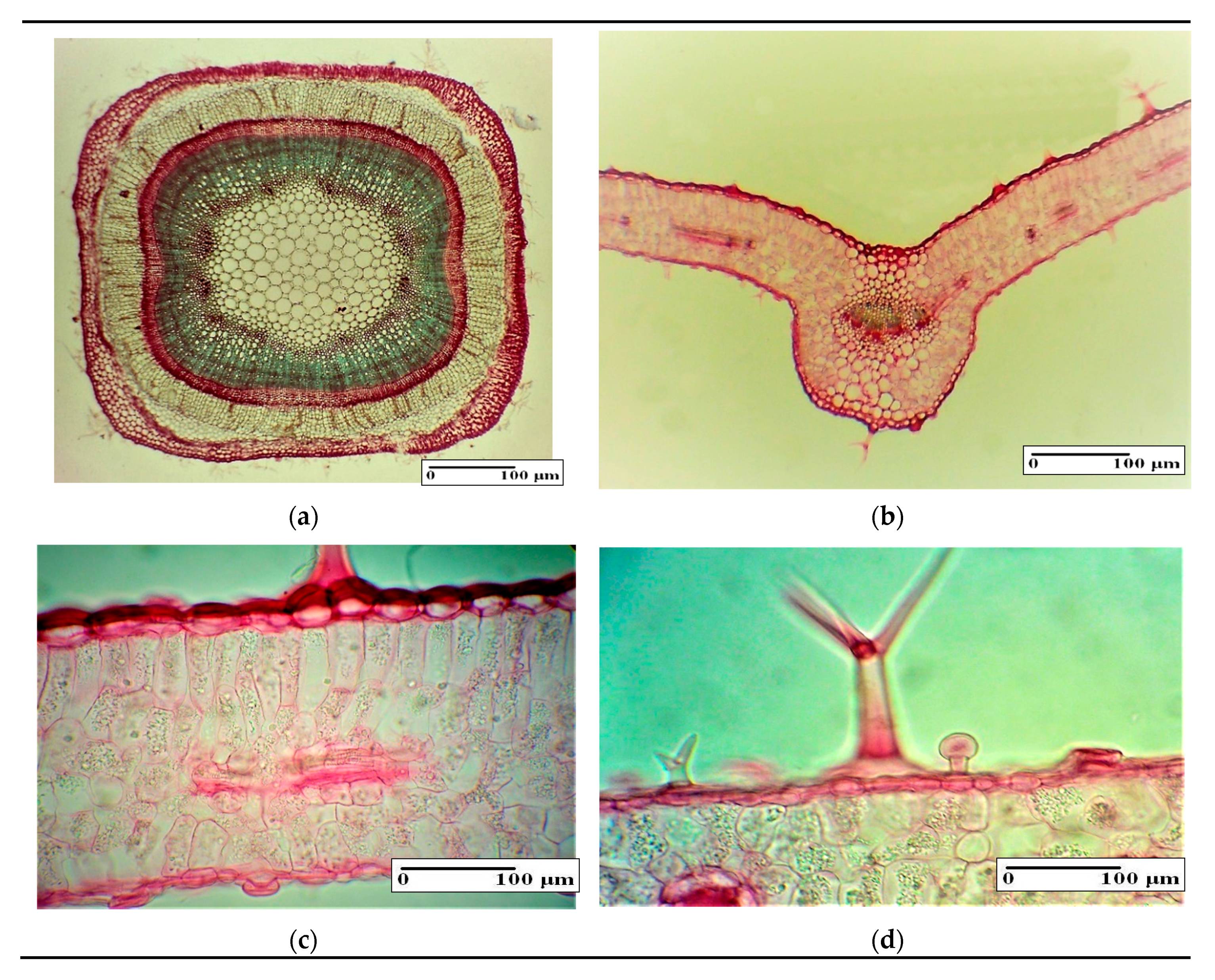
3.4. Histo-Anatomical Aspects of the Lavender Stem and Leaves
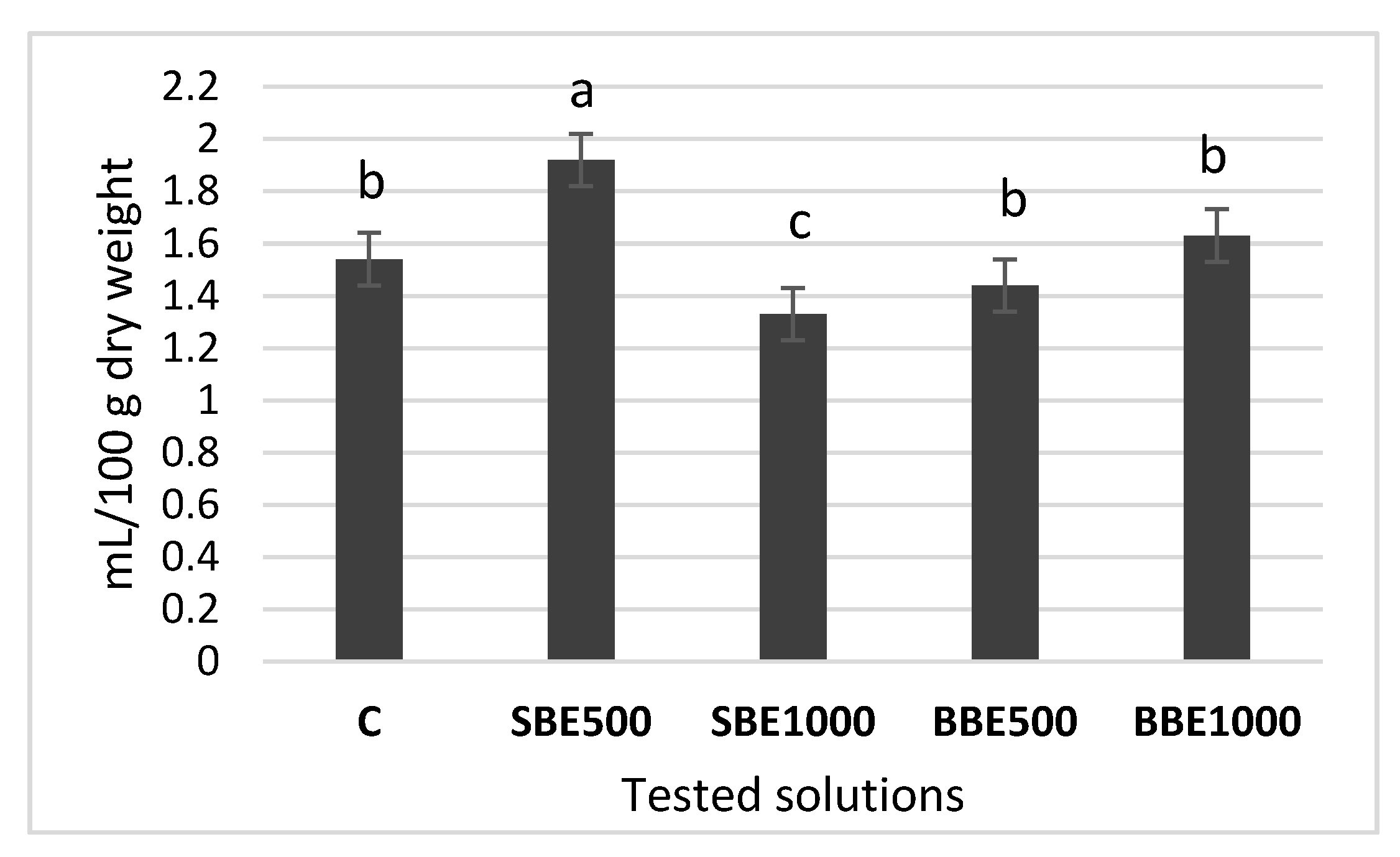
3.5. Volatile oil Content Analysis
3.5.1. Quantitative Analysis of Volatile oil from Lavandulae Flos
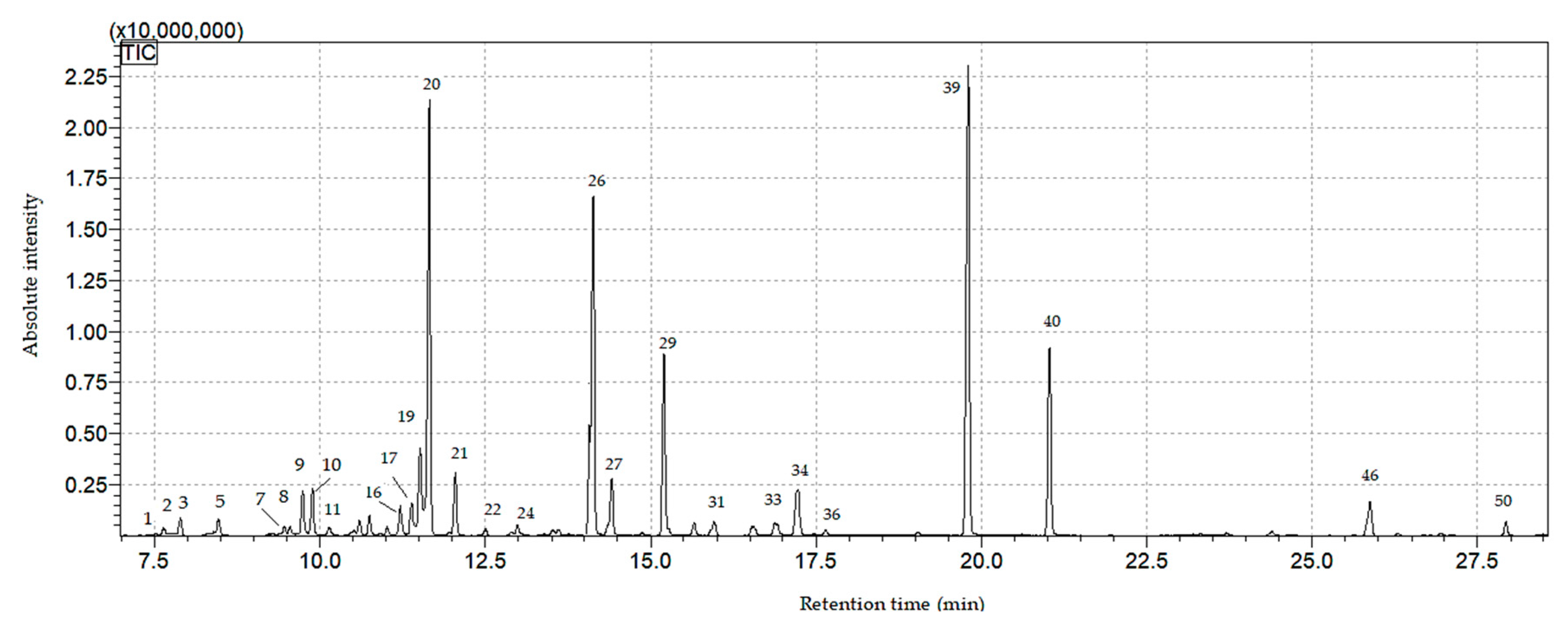
3.5.2. ITEX/GC-MS Analysis of Volatile Compounds from Lavandulae Flos
3.5.3. ITEX/GC-MS Analysis of Volatile Compound from Lavandulae Folium
4. Discussions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2018, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Müller, W.E.; Volz, H.P.; Möller, H.J.; Schläfke, S.; Dienel, A. Lavender oil preparation Silexan is effective in generalized anxiety disorder—A randomized, double-blind comparison to placebo and paroxetine. Int. J. Neuropsychopharmacol. 2014, 17, 859–869. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 2nd ed.; Council of Europe: Strasbourg, France, 2019; ISBN 92-871-8505-0. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Biostimulants Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Mol. Basel Switz. 2019, 24, 1182. [Google Scholar] [CrossRef]
- Tanase, C.; Ștefănescu, R.; Gheorghieș, D.G.; Dandu, L.; Nisca, A.; Darkó, B.; Ancuța Socaci, S. Effects of Beech Bark Extract in the Sage (Salvia officinalis L.) Plant Growth and Volatile Oil Profile. Agronomy 2020, 10, 676. [Google Scholar] [CrossRef]
- Cosarca, S.-L.; Moaca, E.A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.M.; Vodnar, C.D.; Muntean, D.L.; Crișan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Bargali, K.; Bargali, S.S. Germination capacity of seeds of leguminous plants under water deficit conditions: Implication for restoration of degraded lands in Kumaun Himalaya. Trop. Ecol. 2016, 57, 445–453. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Popa, V.I. Histo-Anatomic Aspects on Zea mays L. Influenced by Spruce Bark Polyphenolic Extract. Rom. Biotechnol. Lett. 2016, 21, 11238–11245. [Google Scholar]
- Socaci, S.A.; Socaciu, C.; Tofană, M.; Raţi, I.V.; Pintea, A. In-tube Extraction and GC–MS Analysis of Volatile Components from Wild and Cultivated sea buckthorn (Hippophae rhamnoides L. ssp. carpatica) Berry Varieties and Juice. Phytochem. Anal. 2013, 24, 319–328. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Cosarca, A.; Man, A.; Miklos, A.; Imre, S. Antibacterial activities of spruce bark (Picea abies L.) extract and its components against human pathogens. Rev. Chim. 2018, 69, 1462–1467. [Google Scholar] [CrossRef]
- Stingu, A.; Stanescu, I.; Volf, I.; Popa, V.I. Hyperaccumulation of cadmium in maize plant (Zea mays). Cellul. Chem. Technol. 2011, 45, 287–290. [Google Scholar]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Slimani, C.; Sqalli, H.; Rais, C.; Wafae, S.; Lazraq, A.; Ghadraoui, L.E.; Belmalha, S.; Echchgadda, G. Improvement of germination rate and in vitro multiplication of Lavandula angustifolia. J. Appl. Biol. Biotech. 2020, 8, 52–57. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.C.; Popa, V.I. Chapter 3—Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. ISBN 978-0-12-813768-0. [Google Scholar]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Essential Oil Quantity and Composition from 10 Cultivars of Organically Grown Lavender and Lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N. Lignin and Polyphenols from Vegetal Wastes as Key Modulators of Metabolic Pathways During Plant Development. Cellul. Chem. Technol. 2016, 50, 967–971. [Google Scholar]
- Peter, K.V. Handbook of Herbs and Spices, 2nd ed.; Elsevier Science & Technology: Sawston, UK, 2004; Volume 2, ISBN 0-85709-040-2. [Google Scholar]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeon, W.K.; Lee, K.W.; Park, Y.H.; Han, J.S. Ameliorating Effects of Ethanol Extract of Fructus mume on Scopolamine-Induced Memory Impairment in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 102734. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.L.D.; Jayaweera, S.A.; Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and Molecular Mechanism of Eugenol-Induced Apoptosis in Cancer Cells. Molecules 2012, 17, 6290. [Google Scholar] [CrossRef]
- Leite, A.M.; de Lima, E.O.; de Souza, E.L.; de Diniz, M.F.F.M.; Trajano, V.N.; Medeiros, I.A. de Inhibitory effect of b-pinene, a-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Ciênc. Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
| Tested Solutions | Total Seeds Number | GC (%) | SD (±) | SCC (%) |
|---|---|---|---|---|
| C | 198 | 60.10 b | 7.00 | - |
| SBE500 | 198 | 70.20 a | 6.31 | 16.81 |
| SBE1000 | 198 | 64.65 b | 7.29 | 7.56 |
| BBE500 | 198 | 66.16 b | 7.32 | 10.08 |
| BBE1000 | 198 | 80.81 a | 6.25 | 34.45 |
| Experimental Variant | Chl a | Chl b | Chl a + Chl b | Chl a/Chl b | Carotens |
|---|---|---|---|---|---|
| C | 64.85 ± 5.08 b | 11.71 ± 1.82 | 76.57 | 5.54 | 2.04 ± 0.01 |
| SBE500 | 102.86 ± 8.00 a | 19.44 ± 1.09 | 122.29 | 5.29 | 2.83 ± 0.02 |
| SBE1000 | 84.66 ± 7.57 b | 15.11 ± 1.09 | 99.77 | 5.6 | 2.54 ± 0.02 |
| BBE500 | 101.21 ± 6.87 a | 20.41 ± 1.09 | 121.62 | 4.96 | 2.48 ± 0.02 |
| BBE1000 | 97.22 ± 11.06 a | 19.53 ± 2.03 | 116.74 | 4.98 | 2.56 ± 0.05 |
| Vegetative Organs | Microscopic Characteristics | Control (Mean ± SD) | Experimental Variants (Mean ± SD) | |||
|---|---|---|---|---|---|---|
| SBE500 1 | SBE10001 | BBE500 1 | BBE1000 1 | |||
| n = 25 | n = 25 | n = 25 | n = 25 | n = 25 | ||
| Stem | Epiderm and cortex area (%) | 37.71 ± 2.41 ab | 38.89 ± 2.16 a | 36.27 ± 2.14 bc | 35.05 ± 1.14 c | 36.07 ± 1.87 c |
| Floem area (%) | 13.85 ± 1.49 a | 14.11 ± 1.25a | 13.35 ± 1.31 ab | 12.35 ± 1.51 b | 13.37 ± 1.22 ab | |
| Xylem area (%) | 28.99 ± 1.97 ab | 27.46 ± 2.77 a | 30.28 ± 1.87 bc | 29.87 ± 1.98 bc | 30.91 ± 2.01 c | |
| Pith area (%) | 15.87 ± 1.29 a | 15.82 ± 1.43 a | 16.38 ± 2.17 a | 18.44 ± 2.37 b | 16.16 ± 1.37 a | |
| Colenchim area (%) | 3.58 ± 0.87 a | 3.72 ± 0.84 ab | 3.09± 0.91 a | 4.29 ± 0.83 b | 3.49 ± 0.82 a | |
| Leaf | Leaf lamina thickness (mm) | 0.052 ± 0.006 b | 0.068 ± 0.009 a | 0.054 ± 0.004 b | 0.054 ± 0.005 b | 0.053 ± 0.005 b |
| Mesophyll thickness (mm) | 0.043 ± 0.004 b | 0.059 ± 0.006 a | 0.041 ± 0.004 b | 0.041 ± 0.004 b | 0.044 ± 0.004 b | |
| Vascular bundles area in the main string (%) | 18.55 ± 1.44 a | 20.42 ± 2.03 b | 18.02 ± 2.11 a | 17.11 ± 3.02 a | 18.75 ± 2.02 a | |
| No | Compounds | Retention Time | Concentration (% of Total Surface Area of Peaks) | ||||
|---|---|---|---|---|---|---|---|
| C 1 | SBE500 1 | SBE1000 1 | BBE500 1 | BBE1000 1 | |||
| 1 | Cyclene | 7.522 | - | - | 0.06 ± 0.01 | - | 0.13 ± 0.05 |
| 2 | α- Thujene | 7.634 | 0.5 ± 0.05 | 0.25 ± 0.04 | 0.23 ± 0.05 | 0.4 ± 0.02 | 0.21 ± 0.04 |
| 3 | α- Pinene | 7.889 | 0.71 ± 0.06 a | 0.43 ± 0.05 a | 0.62 ± 0.03 a | 1.91 ± 0.11 b | 0.43 ± 0.03 a |
| 4 | Dimethylcrotonolactone* | 8.407 | 0.09 ± 0.02 | ||||
| 5 | Camphene | 8.467 | 0.11 ± 0.03 a | 0.13 ± 0.02 a | 0.69 ± 0.06 abc | 0.41 ± 0.05 b | 0.83 ± 0.05 c |
| 6 | Sabinen | 9.288 | 0.18 ± 0.02 | 0.49 ± 0.03 | |||
| 7 | β—Pinene | 9.459 | 0.43 ± 0.02 a | 0.24 ± 0.02 a | 0.29 ± 0.02 a | 3.07 ± 0.08 b | 0.17 ± 0.02 a |
| 8 | 1-Octen-3-ol | 9.547 | 0.39 ± 0.02 | 0.58 ± 0.07 | 0.27 ± 0.03 | 0.33 ± 0.03 | 0.21 ± 0.03 |
| 9 | 3-Octanone | 9.739 | 1.29 ± 0.11 a | 2.82 ± 0.09 b | 1.73 ± 0.08 b | 0.86 ± 0.03 d | 5.36 ± 0.12 e |
| 10 | β—Myrcene | 9.885 | 2.15 ± 0.08 ab | 2.24 ± 0.05 a | 1.83 ± 0.06 bc | 1.59 ± 0.06 c | 2.72 ± 0.09 d |
| 11 | Butanoic acid | 10.137 | 0.25 ± 0.04 | 0.29 ± 0.03 | 0.33 ± 0.05 | 0.37 ± 0.03 | 0.19 ± 0.02 |
| 12 | α- Phellandrene | 10.517 | 0.29 ± 0.01 | 0.14 ± 0.01 | |||
| 13 | 3-Carene* | 10.596 | 0.66 ± 0.07 | 0.6 ± 0.09 | 0.57 ± 0.08 | 0.35 ± 0.02 | 0.49 ± 0.07 |
| 14 | Acetic acid | 10.748 | 0.06 ± 0.01 a | 1.26 ± 0.11 d | 0.74 ± 0.06 b | 0.32 ± 0.05 ac | 0.49 ± 0.05 bc |
| 15 | 4-Carene* | 11.018 | 0.23 ± 0.02 | ||||
| 16 | p-Cymene | 11.22 | 1.24 ± 0.05 a | 1.15 ± 0.06 a | 1.03 ± 0.07 a | 6.28 ± 0.12 b | 1.09 ± 0.04 a |
| 17 | D-Limonene | 11.386 | 2.99 ± 0.07 a | 2.05 ± 0.05 c | 1.32 ± 0.03 b | 1.33 ± 0.04 b | 0.84 ± 0.02b |
| 18 | β- Phellandrene | 11.453 | 0.81 ± 0.07 | ||||
| 19 | Eucalyptol | 11.52 | 2.76 ± 0.09 a | 1.22 ± 0.07 b | 4.53 ± 0.10 c | 8.18 ± 0.11 d | 0 |
| 20 | β—trans-Ocimene | 11.647 | 11.14 ± 0.37 a | 16.42 ± 0.44 b | 18.17 ± 0.54 c | 7.29 ± 0.44 d | 15.21 ± 0.23 e |
| 21 | β—cis-Ocimene | 12.047 | 7.9 ± 0.15 a | 3.88 ± 0.09 b | 2.58 ± 0.11 c | 1.68 ± 0.08 d | 8.82 ± 0.27 e |
| 22 | γ—Terpinene | 12.501 | 0.34 ± 0.04 | 0.19 ± 0.03 | 0.26 ± 0.07 | 0.11 ± 0.03 | 0.19 ± 0.04 |
| 23 | cis-Sabinenhydrate | 12.897 | 0.21 ± 0.07 | ||||
| 24 | cis-Linalool oxide | 12.99 | 0.37 ± 0.06 a | 0.62 ± 0.07 a | 0.47 ± 0.05 a | 2.79 ± 0.09 b | |
| 25 | 1,2-Oxolinalool | 13.61 | 0.16 ± 0.06 a | 0.38 ± 0.07 a | 0.27 ± 0.04 a | 1.92 ± 0.11 b | |
| 26 | β—Linalool | 14.127 | 20.02 ± 0.45 a | 26.98 ± 0.76 b | 14.75 ± 0.32 c | 17.43 ± 0.48 d | 15.34 ± 0.51 e |
| 27 | 1-Octenyl acetate | 14.407 | 0.65 ± 0.03 a | 0.87 ± 0.04 a | 2.35 ± 0.08 b | 0.56 ± 0.07 a | 1.45 ± 0.12 b |
| 28 | 3-Octyl acetate | 14.87 | 0.08 ± 0.02 | 0.25 ± 0.04 | 0.12 ± 0.04 | 0.25 ± 0.03 | |
| 29 | allo-Ocimene | 15.195 | 5.59 ± 0.44 a | 6.37 ± 0.22 b | 7.88 ± 0.15 c | 2.33 ± 0.04 d | 5.45 ± 0.11 a |
| 30 | n.i. | 15.655 | 0.59 ± 0.21 | ||||
| 31 | Camphor | 15.96 | 0.19 ± 0.03 b | 0.84 ± 0.04 a | 0.54 ± 0.07 b | 0.32 ± 0.05 ab | |
| 32 | Lavandulol | 16.563 | 0.94 ± 0.06 | 1.00 ± 0.12 | 1.24 ± 0.14 | ||
| 33 | Borneol | 16.9 | 0.85 ± 0.13 a | 0.66 ± 0.07 b | 0.4 ± 0.11 ab | ||
| 34 | 1-Terpinen-4-ol | 17.221 | 4.66 ± 0.12 a | 2.06 ± 0.11 b | 1.71 ± 0.08 bc | 1.42 ± 0.11 c | 1.74 ± 0.09 bc |
| 35 | Cryptone | 1.08 ± 0.09 | |||||
| 36 | Butyric acid | 17.65 | 0.18 ± 0.05 | 0.46 ± 0.07 | 0.27 ± 0.02 | 0.44 ± 0.12 | 0.28 ± 0.05 |
| 37 | n.i. | 18.601 | 0.14 ± 0.03 | ||||
| 38 | Isoborneol | 19.041 | 0.18 ± 0.02 | ||||
| 39 | Linalyl acetate | 19.803 | 24.02 ± 0.27 a | 18.52 ± 0.42 b | 22.44 ± 0.54 c | 11.35 ± 0.88 d | 33.39 ± 1.26e |
| 40 | Lavandulyl Acetate | 21.03 | 5.05 ± 0.61 a | 5.19 ± 0.07 a | 8.65 ± 0.97 b | 8.21 ± 0.74 b | 1.28 ± 0.06 b |
| 41 | n.i. | 23.202 | 0.25 ± 0.06 | ||||
| 42 | n.i. | 23.3 | 0.27 ± 0.05 | ||||
| 43 | trans-Geraniol* | 23.71 | 0.15 ± 0.02 | 0.21 ± 0.02 | 0.12 ± 0.03 | 0.18 ± 0.06 | 0.21 ± 0.04 |
| 44 | cis-Geraniol | 24.391 | 0.31 ± 0.09 | 0.36 ± 0.02 | 0.27 ± 0.03 | 0.39 ± 0.05 | 0.27 ± 0.05 |
| 45 | Santalen | 25.839 | 1.46 ± 0.07 | ||||
| 46 | Caryophyllene | 25.868 | 1.62 ± 0.10 a | 1.81 ± 0.08 ab | 2.12 ± 0.07 b | 1.55 ± 0.10 a | |
| 47 | α—trans-Bergamotene | 26.299 | 0.13 ± 0.08 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.12 ± 0.03 | |
| 48 | β – cis-Farnesene * | 26.928 | 0.33 ± 0.10 | 0.13 ± 0.05 | 0.32 ± 0.06 | ||
| 49 | n.i. | 27.922 | 0.37 ± 0.08 | 0.12 | |||
| 50 | n.i. | 27.931 | 0.59 ± 0.06 | 0.46 ± 0.07 | |||
| 51 | Caryophyllene oxide | 30.894 | 0.37 ± 0.05 | ||||
| Total % of identified compounds | 99.63 | 99.41 | 98.95 | 99.34 | 99.88 | ||
| Compounds | Retention Time | Concentration (% of Total Surface Area of Peaks) | ||||
|---|---|---|---|---|---|---|
| C 1 | SBE500 1 | SBE1000 1 | BBE500 1 | BBE1000 1 | ||
| Tricyclene | 7.523 | 0.79 ± 0.05 | 0.22 ± 0.05 | 0.27 ± 0.03 | ||
| α- Thujene | 7.638 | 0.82 ± 0.02 | 0.95 ± 0.08 | 1.09 ± 0.11 | 0.92 ± 0.08 | |
| α- Pinene | 7.891 | 1.97 ± 0.07 a | 4.9 ± 0.25 b | 4.17 ± 0.46 c | 3.19 ± 0.54 d | 1.82 ± 0.11 a |
| Camphene | 8.472 | 3.09 ± 0.51 ac | 11.15 ± 1.07 b | 3.49 ± 0.24 a | 3.04 ± 0.44 ac | 2.73 ± 0.28 c |
| Sabinene | 9.223 | 1.98 ± 0.5 ab | 3.2 ± 0.33 a | 1.34 ± 0.03 b | 1.42 ± 0.41 b | |
| Β—Terpinene * | 9.276 | 1.33 ± 0.51 | 1.12 ± 0.11 | |||
| β—Pinene | 9.463 | 2.99 ± 0.11 ab | 2.45 ± 0.12 b | 3.17 ± 0.08 ac | 3.13 ± 0.09 b | 1.09 ± 0.06 c |
| 1-Octen-3-ol | 9.544 | 2.26 ± 0.11 a | 0.5 ± 0.04 b | 1.92 ± 0.07 a | 0.83 ± 0.04 b | |
| 3-Octanone | 9.743 | 2.09 ± 0.12 a | 0.32 ± 0.11 b | 0.71 ± 0.08 b | 1.07 ± 0.04 c | |
| β—Myrcene | 9.894 | 0.46 ± 0.07 a | 0.28 ± 0.03 a | 1.41 ± 0.05 b | 0.83 ± 0.06 ab | |
| 3-Carene* | 10.594 | 5.79 ± 0.12 a | 4.23 ± 0.22 b | 5.96 ± 0.23 a | 2.97 ± 0.31 b | |
| p-Cymene | 11.213 | 24.95 ± 1.22 a | 19.35 ± 1.08 b | 24.94 ± 1.05 a | 15.84 ± 1.13 c | 17.03 ± 1.27 d |
| D-Limonene | 11.386 | 12.54 ± 0.97 a | 12.49 ± 0.86 a | 25.64 ± 1.02 b | 27.15 ± 1.11 c | 32.76 ± 0.98 d |
| Eucalyptol | 11.508 | 25.12 ± 0.75 a | 20.55 ± 0.84 b | 3.56 ± 0.12 c | 11.98 ± 0.29 d | 24.46 ± 0.63 a |
| 1,2-Oxolinalool | 12.983 | 0.92 ± 0.07 | 0.68 ± 0.06 | 0.4 ± 0.04 | 0.34 ± 0.07 | |
| n.i. | 13.749 | 0.38 ± 0.07 | 0.24± 0.04 | |||
| β—Linalool | 14.122 | 1.51 ± 0.11 a | 0.73 ± 0.06 b | 0.26 ± 0.04 b | ||
| 1-Octenyl acetate | 14.413 | 3.32 ± 0.12 a | 0.37 ± 0.08 b | 0.28 ± 0.02 b | 0.79 ± 0.08 b | |
| n.i. | 15.387 | 0.46 ± 0.03 | ||||
| Camphor | 15.957 | 3.17 ± 0.12 ab | 3.43 ± 0.10 a | 2.57 ± 0.09 b | 5.78 ± 0.14 c | 0.56 ± 0.07 d |
| Borneol | 16.907 | 9.6 ± 0.47 a | 9.36 ± 0.15 a | 6.25 ± 0.17 b | 13.9 ± 0.33 c | 1.4 ± 0.11 d |
| n.i. | 17.229 | 0.82 ± 0.07 | ||||
| Cryptone | 17.459 | 2.54 ± 0.12 | 1.79 ± 0.28 | 3.1 ± 0.11 | ||
| Isobornyl formate * | 19.047 | 0.97 ± 0.09 | 0.86 ± 0.07 | |||
| Linalyl acetate | 19.794 | 2.47 ± 0.22 a | 0.28 ± 0.04 b | 0.13 ± 0.02 b | ||
| Lavandulol | 21.025 | 1.21 ± 0.07 a | 2.16 ± 0.17 b | 1.28 ± 0.11 a | 0.9 ± 0.07 a | |
| Isobornyl acetate | 21.091 | 0.5 ± 0.06 | ||||
| n.i. | 21.944 | 0.45 ± 0.04 | 0.2 ± 0.04 | |||
| n.i. | 23.706 | 0.19 ± 0.07 | 0.2 ± 0.05 | |||
| cis-Geraniol * | 24.393 | 2.79 ± 0.22 a | 0.4 ± 0.07 b | 1.44 ± 0.08 c | 1.07 ± 0.11 bc | |
| α- Santalene | 25.833 | 1.97 ± 0.11 a | 2.77 ± 0.12 b | 1.61 ± 0.09 ac | 0.92 ± 0.07 c | |
| n.i. | 26.301 | 0.24 ± 0.07 | ||||
| γ-Cadinene | 28.958 | 0.96 ± 0.08 | 0.15 ± 0.04 | |||
| n.i. | 28.968 | 0.22 ± 0.07 | ||||
| n.i. | 30.902 | 0.31 ± 0.08 | 0.18 ± 0.06 | 0.2 ± 0.05 | ||
| n.i. | 32.052 | 0.21 ± 0.04 | ||||
| Total % of identified compounds | 100 | 99.09 | 97.63 | 99.82 | 99.16 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, C.; Ștefănescu, R.; Darkó, B.; Muntean, D.L.; Fărcaş, A.C.; Socaci, S.A. Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application. Plants 2020, 9, 859. https://doi.org/10.3390/plants9070859
Tanase C, Ștefănescu R, Darkó B, Muntean DL, Fărcaş AC, Socaci SA. Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application. Plants. 2020; 9(7):859. https://doi.org/10.3390/plants9070859
Chicago/Turabian StyleTanase, Corneliu, Ruxandra Ștefănescu, Béla Darkó, Daniela Lucia Muntean, Anca Corina Fărcaş, and Sonia Ancuţa Socaci. 2020. "Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application" Plants 9, no. 7: 859. https://doi.org/10.3390/plants9070859
APA StyleTanase, C., Ștefănescu, R., Darkó, B., Muntean, D. L., Fărcaş, A. C., & Socaci, S. A. (2020). Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application. Plants, 9(7), 859. https://doi.org/10.3390/plants9070859










