Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on 1H-NMR Spectroscopy and HPLC-DAD Analysis
Abstract
1. Introduction
2. Results
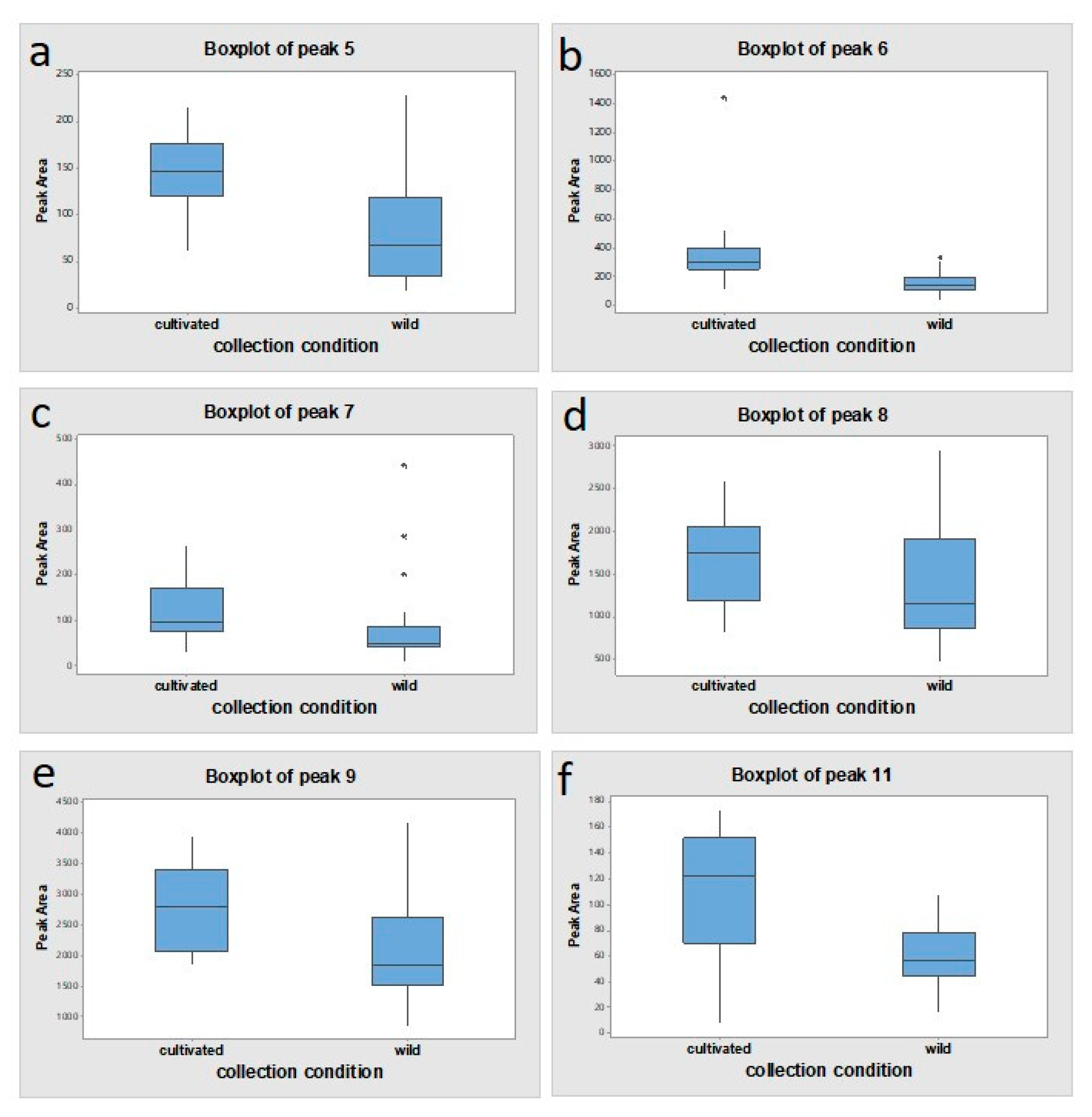
2.1. HPLC Analysis and Peak Identification
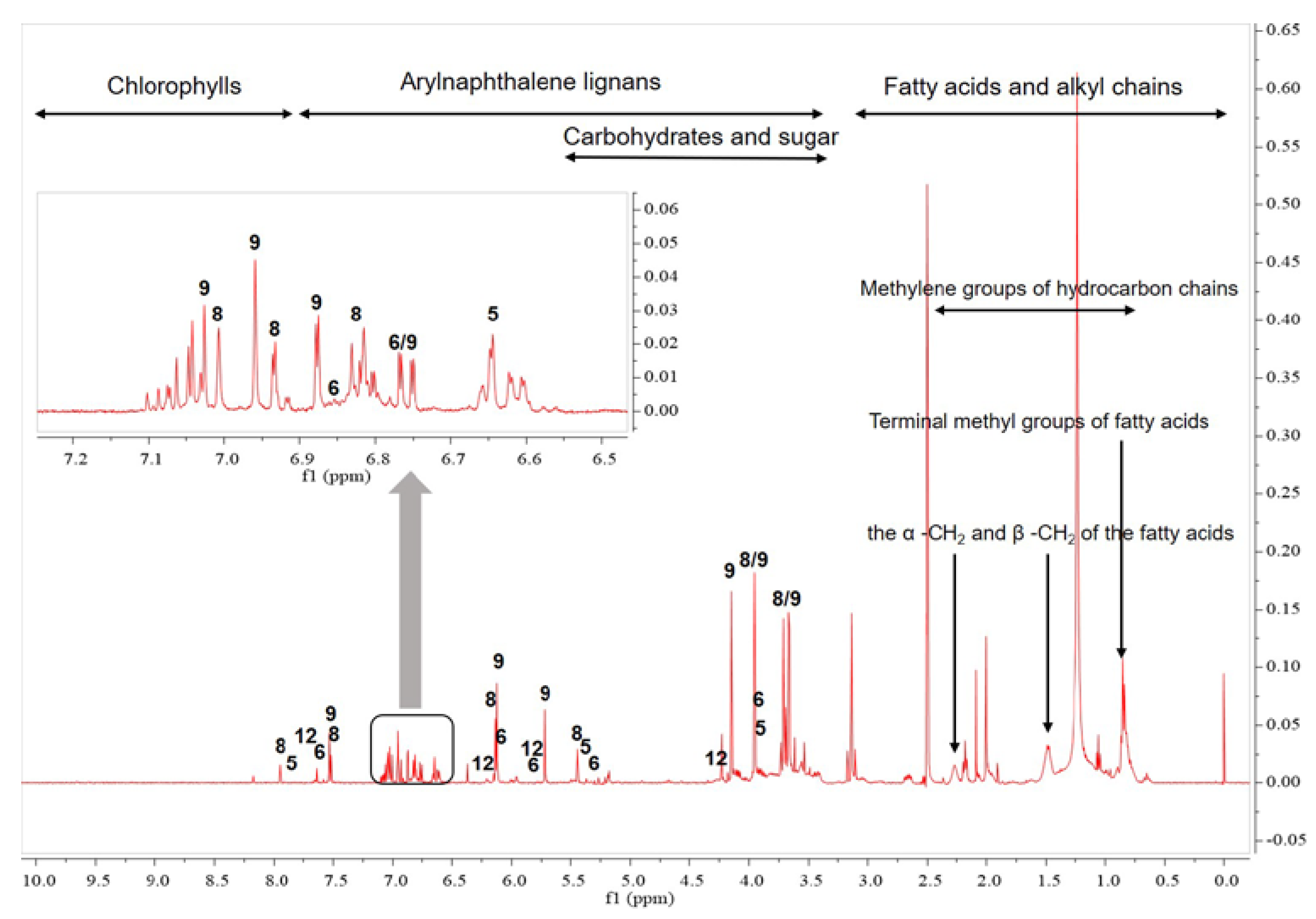
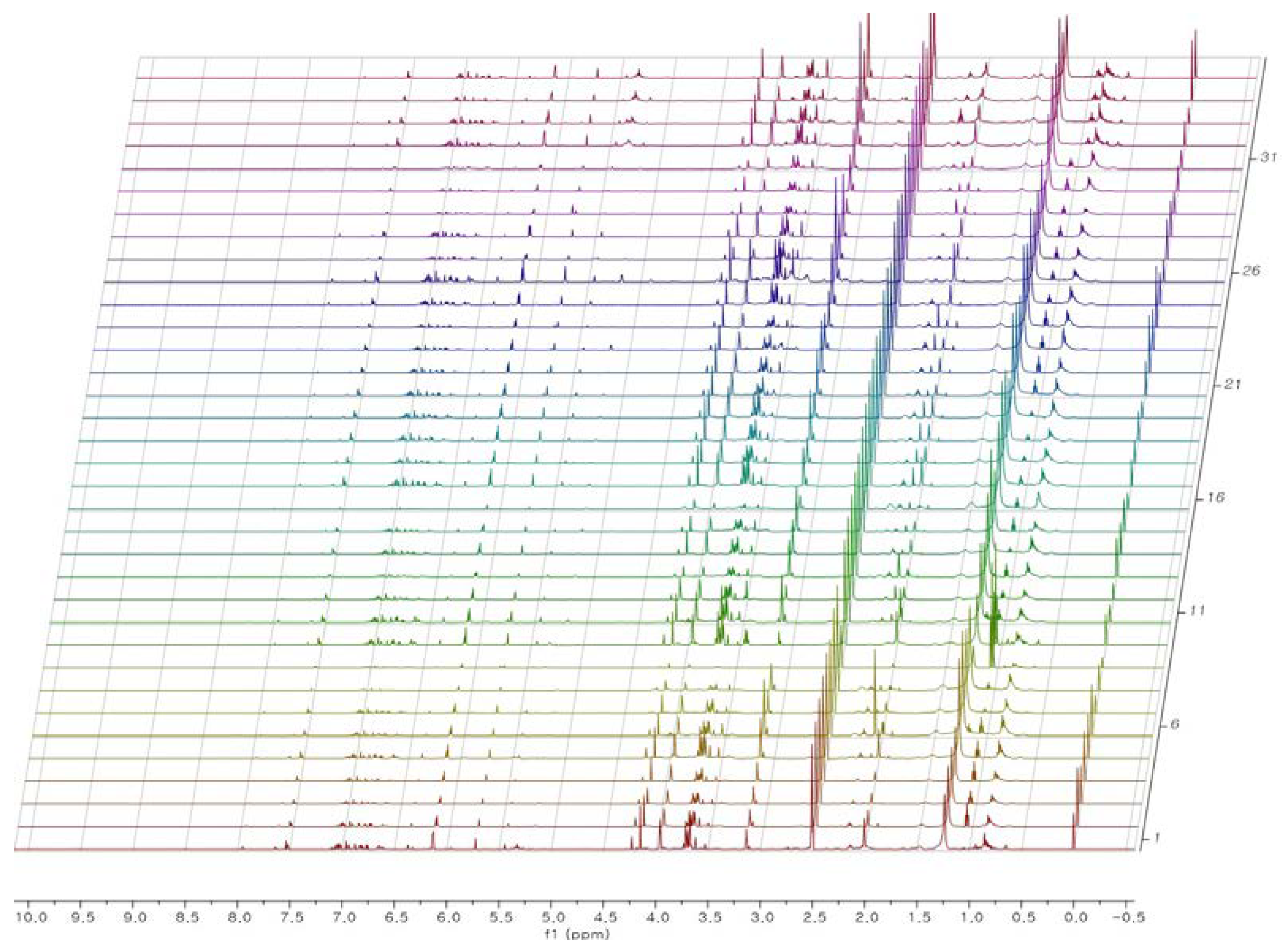
2.2. 1H-NMR Analysis
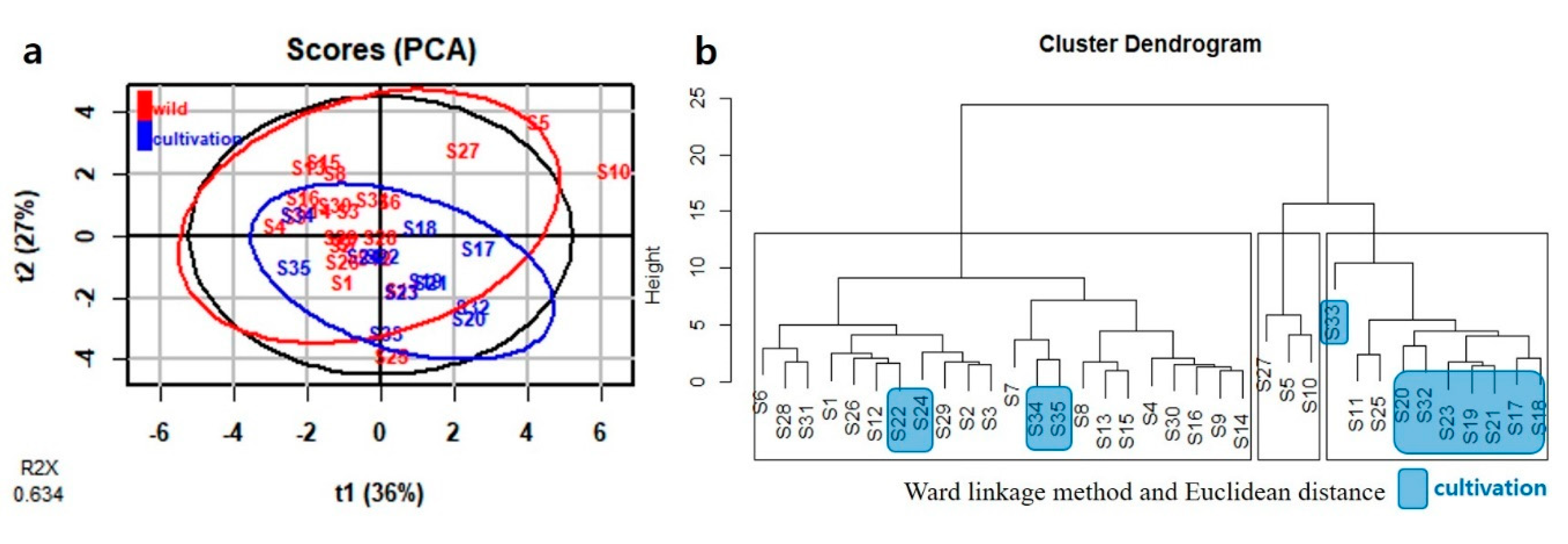
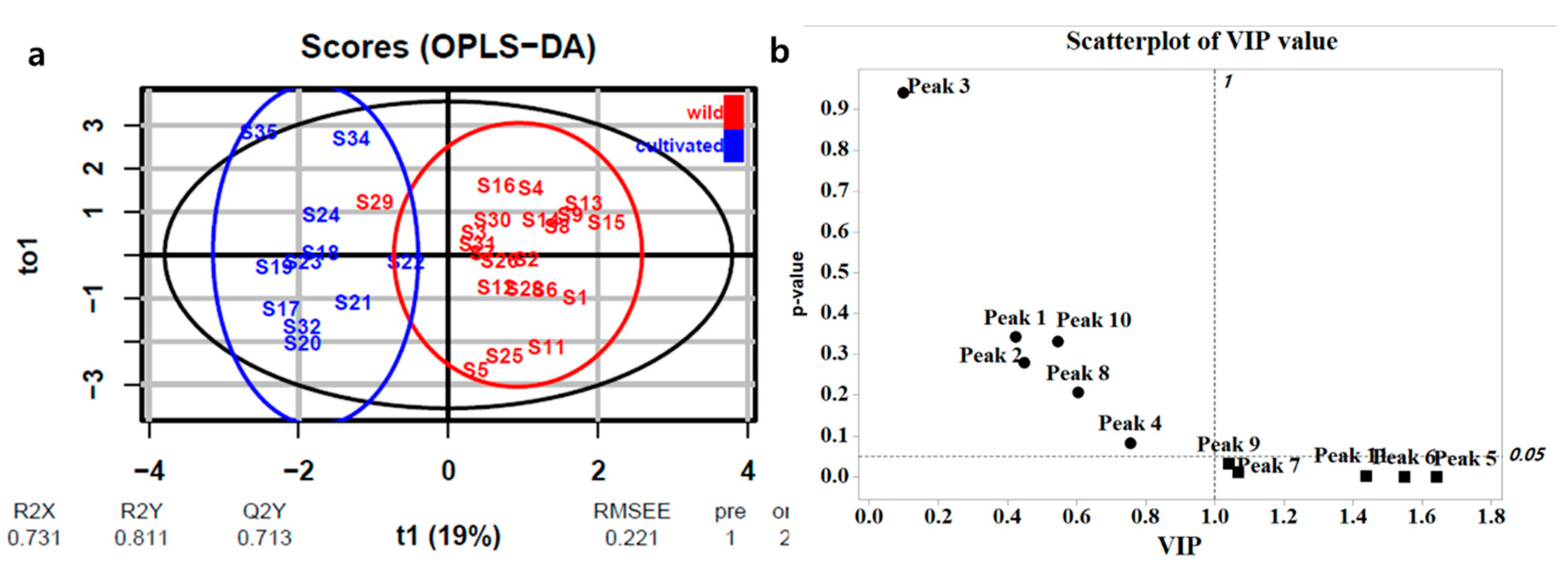
2.3. Multivariate Analysis
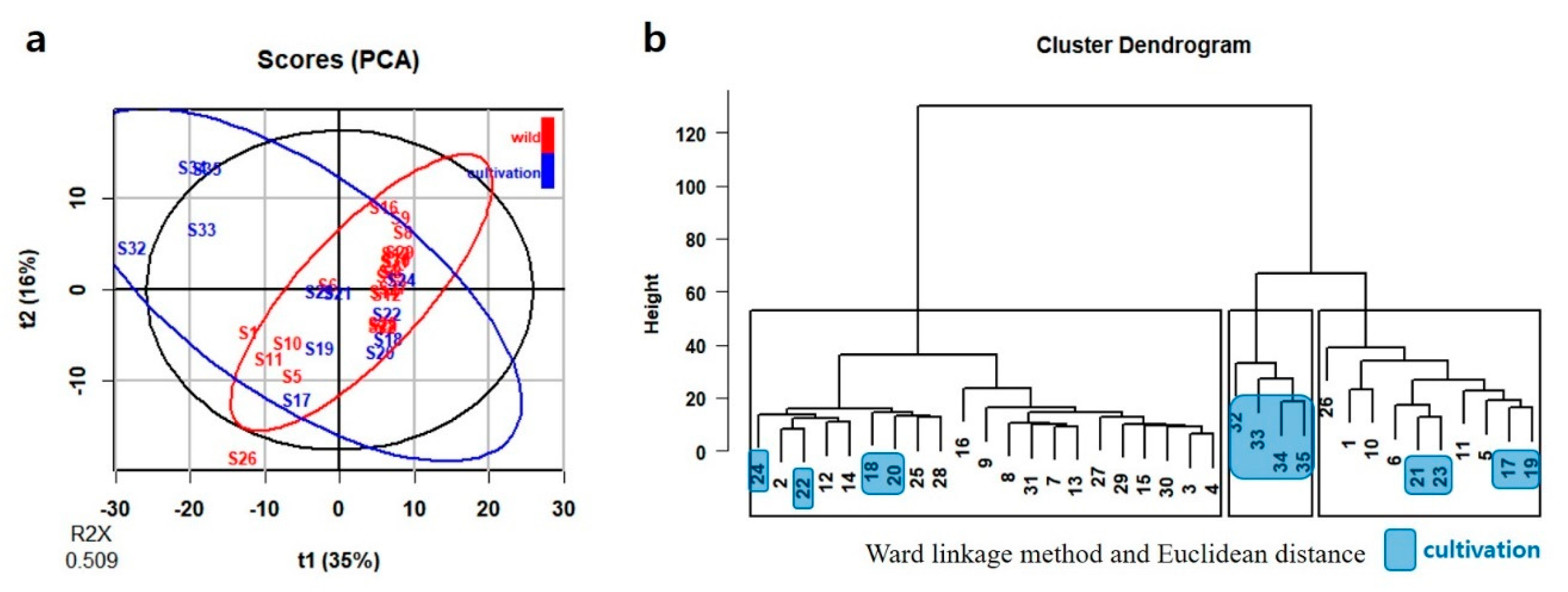
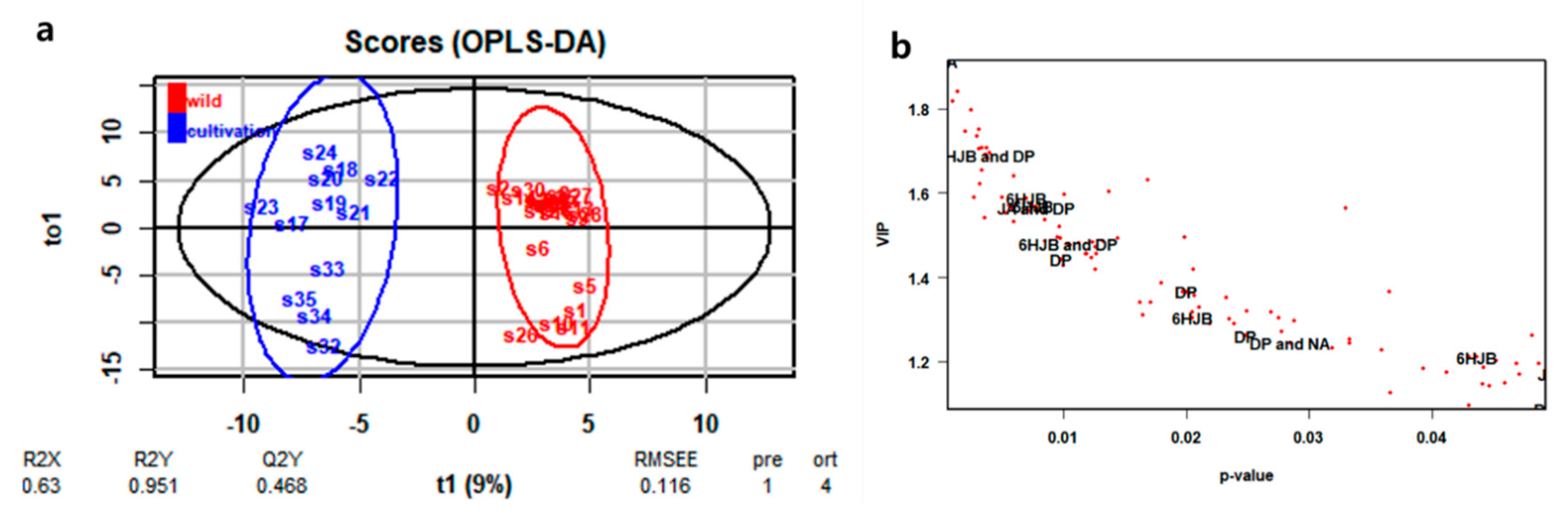
2.3.1. Multivariate Analysis of HPLC-DAD Data
2.3.2. Multivariate Analysis of 1H-NMR Data
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Cultivation
4.2. Sample Preparation
4.3. HPLC-DAD Analysis and 1H NMR Analysis
4.3.1. HPLC-DAD Analysis
4.3.2. 1H-NMR Spectra Acquisition
4.4. Multivariate Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Chang, K.S. English Names for Korean Native Plants; Korea National Arboretum: Gyeonggi-do, Korea, 2015. [Google Scholar]
- Savithramma, N.; Sulochana, C.; Rao, K.N. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J. Ethnopharmacol. 2007, 113, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Korean Food Code, The Ministry of Food and Drug Safety (MFDS). Available online: https://www.foodsafetykorea.go.kr/ (accessed on 7 June 2019).
- Youm, J.; Lee, H.; Choi, Y.; Yoon, J. DW2008S and its major constituents from Justicia procumbens exert anti-asthmatic effect via multitargeting activity. J. Cell Mol. Med. 2018, 22, 2680–2691. [Google Scholar] [CrossRef] [PubMed]
- Youm, J.; Lee, H.; Chang, H.B.; Jeon, J.; Yoon, M.H.; Woo, J.Y.; Choi, M.-S.; Hwang, Y.; Seong, S.; Na, K.; et al. Justicia procumbens extract (DW2008) selectively suppresses th2 cytokines in splenocytes and ameliorates ovalbumin-induced airway inflammation in a mouse model of asthma. Biol. Pharm. Bull. 2017, 40, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Day, S.H.; Lin, Y.-C.; Tsai, M.-L.; Tsao, L.-T.; Ko, H.-H.; Chung, M.-I.; Lee, J.-C.; Wang, J.-P.; Won, S.-J.; Lin, C.-N. Potent cytotoxic lignans from Justicia procumbens and their effects on nitric oxide and tumor necrosis factor-r production in mouse macrophages. J. Nat. Prod. 2002, 65, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Asano, J.; Chiba, K.; Tada, M.; Yoshii, T. Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 1996, 42, 713–717. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsin, W.-C.; Ko, F.-N.; Huang, Y.-L.; Ou, J.-C.; Teng, C.-M. Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 1996, 59, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yin, H.-L.; Liu, S.-J.; Chen, L.; Tian, Y.; Li, B.; Wang, Q.; Dong, J.-X. Cytotoxic activity of lignans from Justicia procumbens. Fitoterapia 2014, 94, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Lee, C.-H.; Su, C.-L.; Hung, C.-W.; Liu, H.-S.; Lin, C.-N.; Won, S.-J. Justicidin A decreases the level of cytosolic Ku70 leading to apoptosis in human colorectal cancer cells. Carcinogenesis 2005, 26, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus. J. Ethnopharmacol. 2006, 103, 181–186. [Google Scholar] [CrossRef]
- Hemmati, S.; Seradj, H. Justicidin B: A promising bioactive lignan. Molecules 2016, 21, 820. [Google Scholar] [CrossRef]
- Tsao, L.-T.; Lin, C.-N.; Wang, J.-P. Justicidin A inhibits the transport of tumor necrosis factor-alpha to cell surface in lipopolysaccharide-stimulated RAW 264.7 macrophages. Mol. Pharmacol. 2004, 65, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dong, H.; Wang, T.; Zhao, R.; Mu, Y.; Geng, Y.; Zheng, Z.; Wang, X. A Strategy for preparative separation of 10 lignans from Justicia procumbens L. by high-speed counter-current chromatography. Molecules 2017, 22, 2024. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, W.; Qiu, F.; Yang, M.; Li, Q.; Wei, R.; Yang, X.; Qin, J. Simultaneous determination of seven lignans in Justicia procumbens by high performance liquid chromatography-photodiode array detection using relative response factors. J. Sep. Sci. 2013, 36, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, J.; Yang, M.; Wu, J.; Yang, J. Chromatographic fingerprint analysis and simultaneous determination of eight lignans in Justicia procumbens and its compound preparation by HPLC-DAD. J. Sep. Sci. 2011, 34, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, J.; Xu, X.; Jin, Y.; Guo, Y.; Chen, J. A new lignan from the Jian-er syrup and its content determination by RP-HPLC. J. Pharm. Biomed. Anal. 2006, 41, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Srivastava, S.; Khalid, A.; Singh, N.; Sangwan, R.S.; Sidhu, O.P.; Roy, R.; Khetrapal, C.L.; Tuli, R. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry 2010, 71, 1085–1094. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concept Magn. Reson. A 2003, 26, 1–19. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, Q.; Wang, Y.; Wang, Y.; Hao, J.; Jiang, M. Quantitative 1H-NMR spectroscopy for profiling primary metabolites in mulberry leaves. Molecules 2018, 23, 554. [Google Scholar] [CrossRef]
- Nafees, M.; Jaskani, M.J.; Ahmad, I.; Maryam; Ashraf, I.; Maqsood, A.; Ahmar, S.; Azam, M.; Hanif, A.; Chen, J.-T. Biochemical analysis of organic acids and soluble sugars in wild and cultivated pomegranate germplasm based in Pakistan. Plants 2020, 9, 493. [Google Scholar] [CrossRef]
- Pariyani, R.; Kortesniemi, M.; Liimatainen, J.; Sinkkonen, J.; Yang, B. Untargeted metabolic fingerprinting reveals impact of growth stage and location on composition of sea buckthorn (Hippopha¨erhamnoides) leaves. J. Food Sci. 2020, 85, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; Abu-Zarga, M.; Sabri, S.; Atta-Ur-Rahman; Voelter, W. An arylnaphthalene lignan from Haplophyllum buxbaumii. Phytochemistry 1998, 49, 1779–1781. [Google Scholar] [CrossRef]
- Day, S.-H.; Chiu, N.-Y.; Tsao, L.-T.; Wang, J.-P.; Lin, C.-N. New lignan glycosides with potent antiinflammatory effect, isolated from Justicia ciliata. J. Nat. Prod. 2000, 63, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Sheriha, G.M.; Abou Amer, K.M. Lignans of Haplophyllum tuberculatum. Phytochemistry 1984, 23, 151–153. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Cheng, F.; Zhou, Y. Complete assignments of 1H and 13C NMR data for seven arylnaphthalide lignans from Justicia procumbens. Magn. Reson. Chem. 2006, 44, 727–730. [Google Scholar] [CrossRef]
- Suman, T.; Elangomathavan, R.; Kasipandi, M.; Chakkaravarthi, K.; Tamilvendan, D.; Perimelazhagan, T. Diphyllin: An effective anticandidal agent isolated from Cleistanthus collinus leaf extract. EJBAS 2018, 5, 130–137. [Google Scholar] [CrossRef]
- Okigawa, M.; Maeda, T.; Kawano, N. The isolation and structure of three new lignans from Justicia procumbens Linn. var. leucantha honda. Tetrahedron 1970, 26, 4301–4305. [Google Scholar] [CrossRef]
- Patel, N.G.; Wang, C.-L.J. Justicidin Insecticidal and Antiviral compounds. U.S. Patent 4486445, 4 December 1984. [Google Scholar]
- Zhou, P.; Luo, Q.; Ding, L.; Fang, F.; Yuna, Y.; Chen, J.; Zhang, J.; Jin, H.; He, S. Preparative isolation and purification of lignans from Justicia procumbens using high-speed counter-current chromatography in stepwise elution mode. Molecules 2015, 20, 7048–7058. [Google Scholar] [CrossRef]
- Fukamiya, N.; Lee, K.-H. Antitumor agents, 81. 1Justicidin-A and diphyllin, two cytotoxic principles from Justicia procumbens. J. Nat. Prod. 1986, 49, 348–350. [Google Scholar] [CrossRef]
- Ohta, K.; Munakata, K. Justicidin C and D, the 1-methoxy-2,3-naphthalide lignans, isolated from Justicia procumbens L. Tetrahedron Lett. 1970, 12, 923–925. [Google Scholar] [CrossRef]
- Lin, M.-T.; Lee, S.-S.; Chen Liu, K.C.S. Phyllamyricins A-C, three novel lignans from Phyllanthus myrtifolius. J. Nat. Prod. 1995, 58, 244–249. [Google Scholar] [CrossRef]
- Munakata, K.; Marumo, S.; Ohta, K. The synthesis of justicidin B and related compounds. Tetrahedron Lett. 1967, 39, 3821–3825. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Bradley, M.; Castro, J.L.; Flanagan, S.R. Total syntheses of justicidin B and retrojusticidin B using a tandem Horner–Emmons–Claisen condensation sequence. Tetrahedron Lett. 2001, 42, 6973–6975. [Google Scholar] [CrossRef]
- Jubert, C.; Bailey, G. Isolation of chlorophylls a and b from spinach by counter-current chromatography. J. Chromatogr. A 2007, 1140, 95–100. [Google Scholar] [CrossRef]
- Katz, J.J.; Brown, C.E. Nuclear magnetic resonance spectroscopy of chlorophylls and corrins. Bull. Magn. Reson. 1983, 5, 3–49. [Google Scholar]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Stanius, Ž. Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Botanica 2012, 18, 101–108. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis; Springer-Verlag New York: New York, NY, USA, 2002. [Google Scholar]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]
- Qi, X.; Chen, X.; Wang, Y. Plant Metabolomics Methods and Applications; Chemical Industry Press: Beijing, China, 2015; pp. 124–126. [Google Scholar]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]

| Sample No. | Content (mg/g, n = 3) | Sample No. | Content (mg/g, n = 3) | Sample No. | Content (mg/g, n = 3) |
|---|---|---|---|---|---|
| S1 | 1.00 ± 0.04 | S13 | 0.38 ± 0.03 | S25 | 1.38 ± 0.08 |
| S2 | 0.67 ± 0.00 | S14 | 0.45 ± 0.10 | S26 | 0.89 ± 0.09 |
| S3 | 0.49 ± 0.01 | S15 | 0.33 ± 0.09 | S27 | 0.40 ± 0.02 |
| S4 | 0.40 ± 0.01 | S16 | 0.28 ± 0.02 | S28 | 0.97 ± 0.23 |
| S5 | 0.54 ± 0.12 | S17 # | 0.86 ± 0.11 | S29 | 0.46 ± 0.04 |
| S6 | 0.58 ± 0.04 | S18 # | 0.54 ± 0.07 | S30 | 0.33 ± 0.02 |
| S7 | 0.95 ± 0.04 | S19 # | 0.79 ± 0.08 | S31 | 0.59 ± 0.03 |
| S8 | 0.22 ± 0.04 | S20 # | 1.21 ± 0.04 | S32 # | 1.12 ± 0.02 |
| S9 | 0.51 ± 0.07 | S21 # | 0.88 ± 0.02 | S33 # | 1.03 ± 0.08 |
| S10 | 0.74 ± 0.19 | S22 # | 0.75 ± 0.03 | S34 # | 0.40 ± 0.02 |
| S11 | 1.13 ± 0.33 | S23 # | 0.84 ± 0.02 | S35 # | 0.64 ± 0.12 |
| S12 | 0.69 ± 0.08 | S24 # | 0.53 ± 0.02 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jeon, J.; Yoon, J.; Kim, S.-H.; Choi, H.S.; Kang, J.S.; Lee, Y.S.; Lee, M.; Kim, Y.H.; Chang, H.B. Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on 1H-NMR Spectroscopy and HPLC-DAD Analysis. Plants 2020, 9, 860. https://doi.org/10.3390/plants9070860
Lee H, Jeon J, Yoon J, Kim S-H, Choi HS, Kang JS, Lee YS, Lee M, Kim YH, Chang HB. Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on 1H-NMR Spectroscopy and HPLC-DAD Analysis. Plants. 2020; 9(7):860. https://doi.org/10.3390/plants9070860
Chicago/Turabian StyleLee, Hyunyong, Jihyun Jeon, Joobyoung Yoon, Seung-Hwan Kim, Hyun Sik Choi, Jong Seung Kang, Yong Sup Lee, Mase Lee, Young Ho Kim, and Hwan Bong Chang. 2020. "Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on 1H-NMR Spectroscopy and HPLC-DAD Analysis" Plants 9, no. 7: 860. https://doi.org/10.3390/plants9070860
APA StyleLee, H., Jeon, J., Yoon, J., Kim, S.-H., Choi, H. S., Kang, J. S., Lee, Y. S., Lee, M., Kim, Y. H., & Chang, H. B. (2020). Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on 1H-NMR Spectroscopy and HPLC-DAD Analysis. Plants, 9(7), 860. https://doi.org/10.3390/plants9070860






