Feeding Behavior and Virus-transmission Ability of Insect Vectors Exposed to Systemic Insecticides
Abstract
:1. Introduction
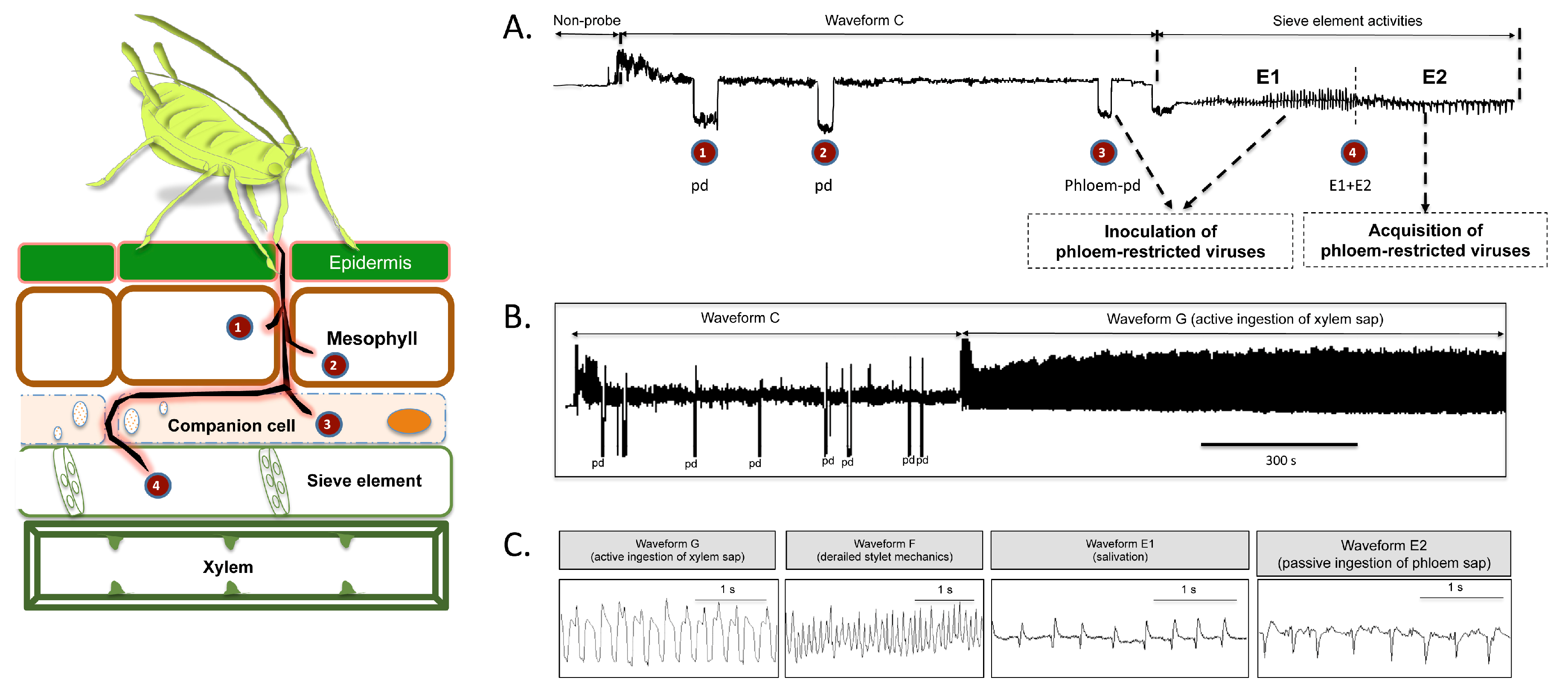
2. Results
2.1. Effect of Different Systemic Insecticides on Probing and Feeding Behavior of Bemisia tabaci
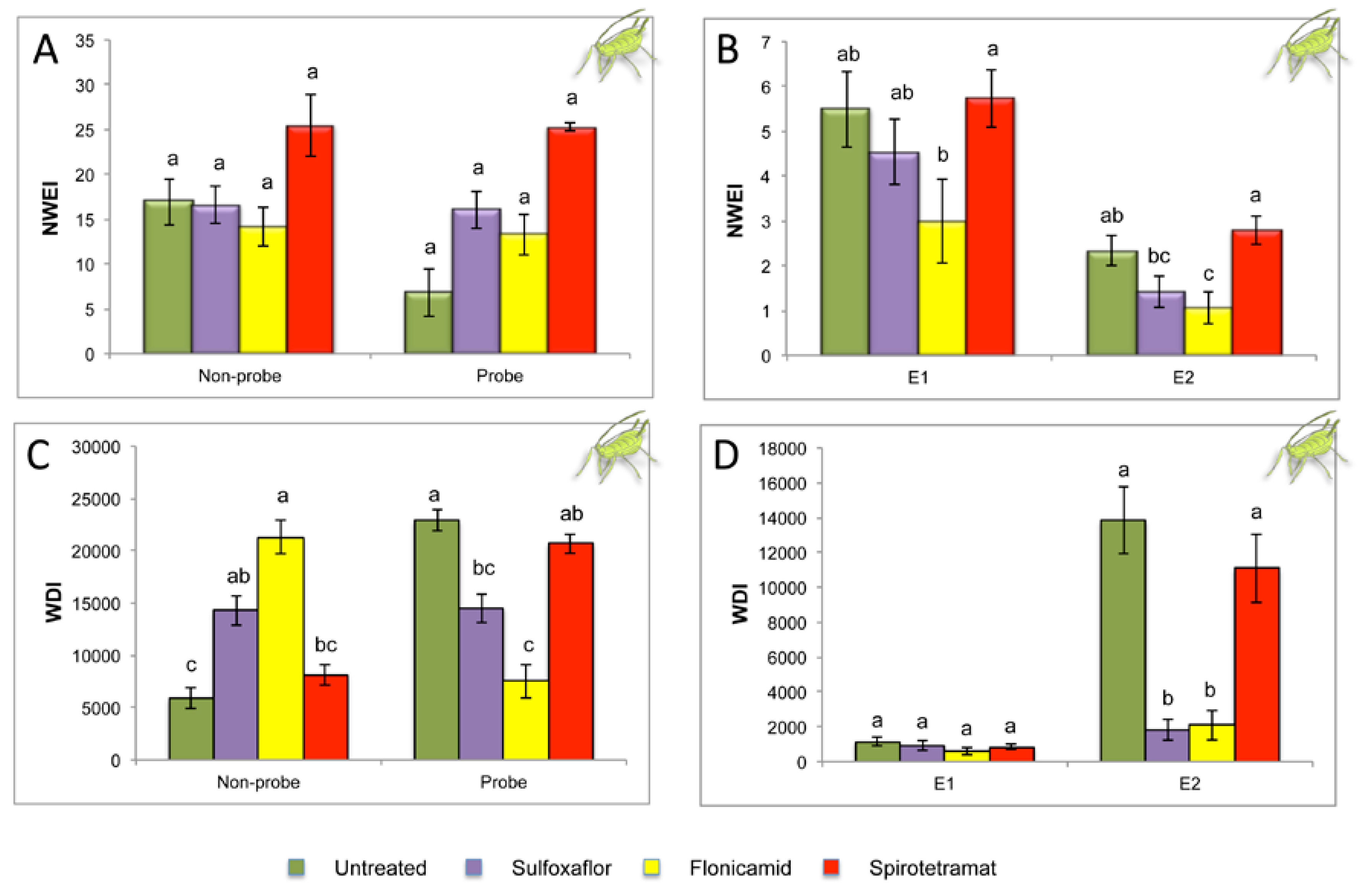
2.2. Effect of Different Systemic Insecticides on Probing and Feeding Behavior of Myzus persicae
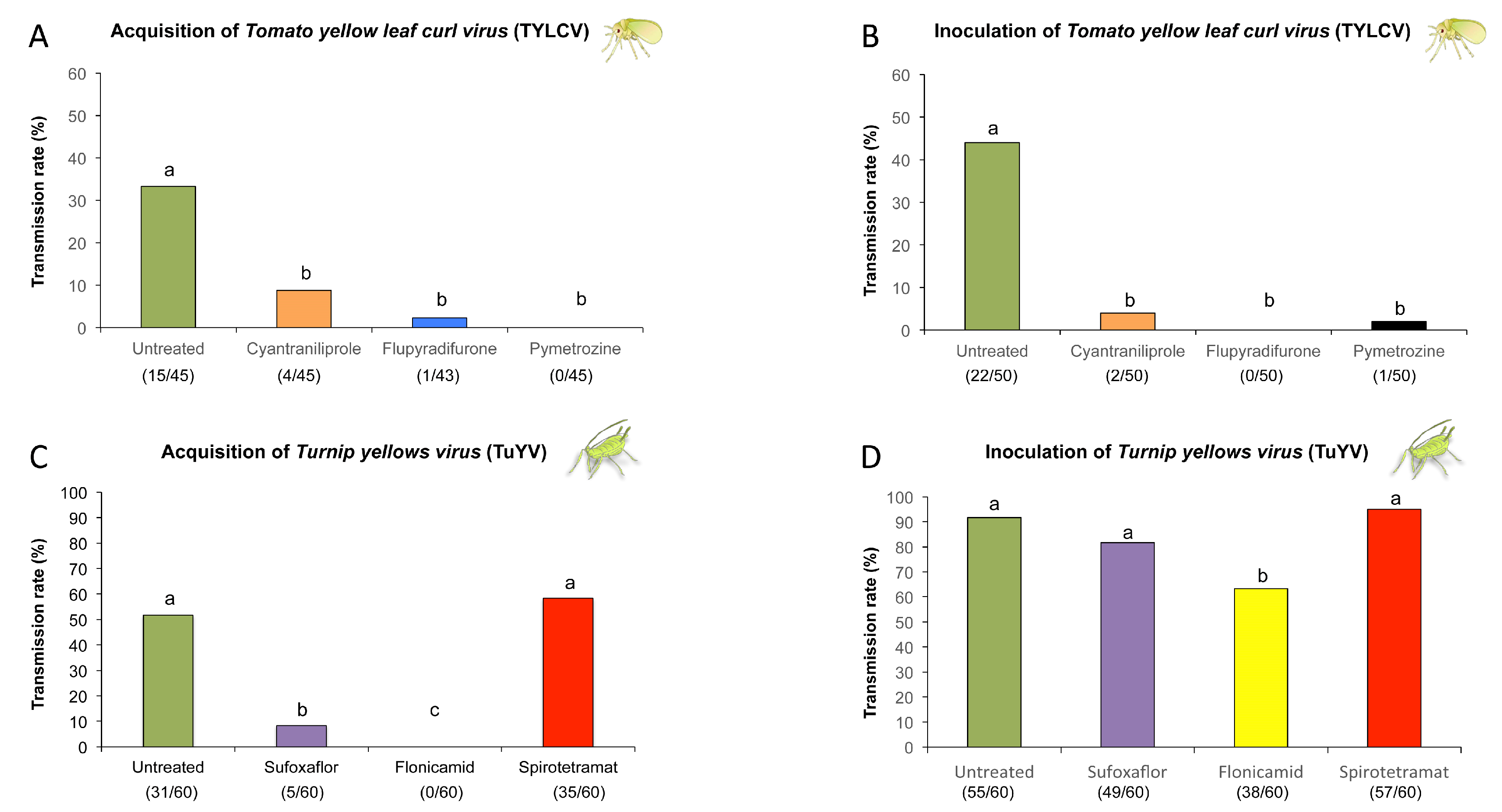
2.3. Evaluation of Systemic Insecticides against Transmission of Circulative Viruses by Bemisia tabaci and Myzus persicae
3. Discussion
4. Material and Methods
4.1. Insects and Plants
4.2. Virus Source Plants
4.3. Insecticide Applications
4.4. Effect of Different Systemic Insecticides on Probing and Feeding Behavior of Bemisia tabaci and Myzus persicae
4.5. Evaluation of Activity of Systemic Insecticides against Transmission of Circulative Viruses by Bemisia tabaci and Myzus persicae
4.5.1. Can Insecticides Deter Acquisition and Subsequent Transmission from Insecticide-Treated Plants?
4.5.2. Can Insecticides Deter Transmission to Insecticide-Treated Plants?
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lwoff, A. The concept of virus. Microbiology 1957, 17, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Fereres, A.; Raccah, B. Plant Virus Transmission by Insects; eLS John Wiley and Sons Ltd.: Chiclester, UK, 2015. [Google Scholar]
- Kennedy, J.S.; Day, M.F.; Eastop, V.F. A Conspectus of Aphids as Vectors of Plant Viruses; Commonwealth Institute of Entomólogy: London, UK, 1962. [Google Scholar]
- Harris, K.F. An ingestion–egestion hypothesis of non-circulative virus transmission. In Aphids as Virus Vectors; Harris, K.F., Maramorosch, K., Eds.; Academic Press: New York, NY, USA, 1977; pp. 165–220. [Google Scholar]
- Sylvester, E.S. Aphid transmission of non-persistent plant viruses with special reference to the Brassica nigra Virus. Hilgardia 1954, 23, 53–98. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, E.S. Mechanisms of Plant. Virus Transmission by Aphids: Symposium on Biological Transmission of Disease Agents; Maramorosch, K., Ed.; Academic Press: New York, NY, USA, 1962; pp. 11–31. [Google Scholar]
- Powell, G.; Pirone, T.; Hardie, J. Aphid stylet activities during potyvirus acquisition from plants and an in vitro system that correlate with subsequent transmission. Eur. J. Plant Pathol. 1995, 101, 411–420. [Google Scholar] [CrossRef]
- Martin, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005, 86, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Tjallingii, W.F.; Moreno, A.; Fereres, A. Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited beet yellows virus. J. Virol. 2018, 2, e01076-18. [Google Scholar]
- Nault, L.R.; Gyrisco, G.G. Relation of the feeding process of the pea aphid to the inoculation of pea enation mosaic virus. Ann. Entomol. Soc. Am. 1966, 59, 1185–1197. [Google Scholar] [CrossRef]
- Gildow, F.E. Luteovirus transmission and mechanisms regulation vector specificity. In The Luteoviridae; Smith, H.G., Barker, H.O., Eds.; CAB International: Wallingford, UK, 1999; pp. 88–112. [Google Scholar]
- Fereres, A. Insects vectors as drivers of plant virus emergence. Curr. Opin. Virol. 2015, 10, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Tjallingii, W.F. Membrane potentials as an indication for plant cell penetration by aphid stylets. Entomol. Exp. Appl. 1985, 38, 187–193. [Google Scholar] [CrossRef]
- Van Helden, M.; Tjallingii, W.F. Tissue localization of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 1993, 68, 269–278. [Google Scholar] [CrossRef]
- Garzo, E.; Soria, M.L.; Gómez-Guillamon, M.L.; Fereres, A. Feeding behaviour of Aphis gossypii on resistant accessions of different melon genotypes (Cucumis melo). Phytoparasitica 2002, 30, 129–140. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Broglia, V.G.; Alberti D’Amato, A.M.; Wouters, D.; van der Vossen, E.; Garzo, E.; Tjallingii, W.F.; Dicke, M.; Vosman, B. Comparative analysis of Solanum stoloniferum responses to probing by the green peach aphid Myzus persicae and the potato aphid Macrosiphum euphorbiae. Insect Sci. 2012, 20, 207–227. [Google Scholar] [CrossRef]
- Sun, M.; Voorrps, R.E.; Steenhuis-Broers, G.; van’t Westende, W.; Vosman, B. Reduced phloem uptake of Myzus persicae on an aphid resistant pepper accession. BMC Plant. Biol. 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Fereres, A.; Moreno, A. Behavioural aspects influencing plants virus transmission by homopteran insects. Virus Res. 2009, 141, 158–168. [Google Scholar] [CrossRef]
- Moreno, A.; Tjallingii, W.F.; Fernandez-Mata, G.; Fereres, A. Differences in the mechanism of inoculation between a semi-persistent and a non-persistent aphid-transmitted plant virus. J. Gen. Virol. 2012, 93, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.; Garzo, E.; Alba-Tercedor, J.; Moreno, A.; Fereres, A.; Walker, G.P. The phloem-pd: A distinctive brief sieve element stylet puncture prior to sieve element phase of aphid feeding behavior. Arthropod Plant. Interact. 2019, 14, 67–78. [Google Scholar] [CrossRef]
- Jimenez, J.; Arias-Martin, M.; Moreno, A.; Garzo, E.; Fereres, A. Barley yellow dwarf virus can be inoculated during brief intracellular punctures in phloem cells before the sieve element continuous salivation phase. Virology 2020, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Harrewijn, P.; Kayser, H. Pymetrozine, a fast-acting and selective inhibitor of aphid feeding. In situ studies with electronic monitoring of feeding behaviour. Pest Sci. 1997, 49, 130–140. [Google Scholar] [CrossRef]
- Costa, R.R.; Moraes, J.C.; DaCosta, R.R. Feeding behaviour of the greenbug Schizaphis graminum on wheat plants treated with imidacloprid and/or silicon. J. Appl. Entom. 2011, 35, 115–120. [Google Scholar] [CrossRef]
- He, Y.; Chen, L.; Chen, J.; Zhang, J.; Chen, L.; Shen, J.; Zhu, Y.C. Electrical penetration graph evidence that pymetrozine toxicity to the rice brown planthopper is by inhibition of phloem feeding. Pest Manag. Sci. 2011, 67, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.D.; Walker, G.P.; Trumble, J.T. Feeding disruption of potato psyllid, Bactericera cockerelli, by imidacloprid as measured by electrical penetration graphs. Entomol. Exp. Appl. 2012, 142, 247–257. [Google Scholar] [CrossRef]
- Miao, J.; Du, Z.B.; Wu, Y.Q.; Gong, Z.J.; Jiang, Y.L.; Duan, Y.; Li, T.; Lei, C.L. Sub-lethal effects of four neonicotinoid seed treatments on the demography and feeding behaviour of the wheat aphid Sitobion avenae. Pest Manag. Sci. 2013, 70, 55–59. [Google Scholar] [CrossRef]
- Jacobson, A.L.; Kennedy, G.G. Electrical penetration graph studies to investigate the effects of cyantraniliprole on feeding behaviour of Myzus persicae (Hemiptera: Aphididae) on Capsicum annuum. Pest Manag. Sci. 2013, 70, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Civolani, S.; Cassanelli, S.; Chicca, M.; Rison, J.L.; Bassi, A.; Alvarez, J.M.; Annan, I.B.; Parrella, G.; Giorgini, M.; Fano, E.A. An EPG study of the probing behaviour of adult Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) following exposure to cyantraniliprole. J. Econ. Entomol. 2014, 107, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Garzo, E.; Moreno, A.; Hernando, S.; Mariño, V.; Torne, M.; Santamaria, E.; Díaz, I.; Fereres, A. Electrical penetration graph technique as a tool to monitor the early stages of aphid resistance to insecticides. Pest Manag. Sci. 2016, 72, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Sousa, M.; García, R.B.; Wullf, N.A.; Fereres, A.; Pedreira Miranda, M. Drench application of systemic insecticides disrupts probing behaviour of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, F.M.; Tjallingii, W.F. Ultrastructure of sieve element penetration by aphid stylets during electrical recording. Entomol. Exp. Appl. 1985, 39, 135–141. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Hogen Esch, T.H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Aphids activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Chen, J.Q.; Martín, B.; Rahbé, Y.; Fereres, A. Early intracellular punctures by two aphid species on near-isogenic melon lines with and without the virus aphid transmission (Vat) resistance gene. Eur. J. Plant. Pathol. 1997, 103, 521–536. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Gabrys, B. Anomalous stylet punctures of phloem sieve elements by aphids. Entomol. Exp. Appl. 1999, 91, 97–103. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Lei, H.; Collar, J.L.; Martin, B.; Muñoz, M.; Fereres, A. Probing and feeding behaviour of two distinct biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on tomato plants. J. Econ. Entomol. 1999, 92, 357–366. [Google Scholar] [CrossRef]
- Ng, J.; Walker, G.P. Whitefly feeding behavior and transmission of noncirculative plant viruses. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; APS Press: St Paul, MN, USA, 2016; pp. 47–58. [Google Scholar]
- Johnson, D.D.; Walker, G.P. Intracellular punctures by adult whitefly Bemisia argentifoliion DC and AC electronic feeding monitors. Entomol. Exp. Appl. 1999, 92, 257–270. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef]
- Brown, J.K.; Frolich, D.R.; Rosell, R.C. The sweet-potato or silverleaf whiteflies-Biotypes of Bemisia tabaci or a species complex. Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Dalton, R. Whitefly infestations: The Christmas Invasion. Nature 2006, 443, 898–900. [Google Scholar] [CrossRef]
- van Emden, H.F.; Harrington, R. Aphids as Crop Pests; CAB International: Wallingford, UK, 2007. [Google Scholar]
- Blackman, R.L.; Eastop, V.P. Aphids on the World’s Crops An. Identification and Information Guide, 2nd ed.; The Natural History Museum John Wiley and Sons Ltd.: Chichester, UK, 2000; p. 466. [Google Scholar]
- Collar, J.L.; Avilla, C.; Duque, M.; Fereres, A. Behavioral Response and Virus Vector Ability of Myzus persicae (Homoptera:Aphididae) Probing on Pepper Plants Treated with Aphicides. J. Econ. Entomol. 1997, 90, 1628–1634. [Google Scholar] [CrossRef]
- Castle, S.; Palumbo, J.; Prabhaker, N. Newer insecticides for plant virus disease management. Virus Res. 2009, 141, 131–139. [Google Scholar] [CrossRef]
- Butler, C.D.; Byrne, F.J.; Keremane, M.L.; Lee, R.F.; Trumble, J.T. Effects of insecticides on behavior of adult Bactericera cockerelli (Hemiptera: Triozidae) and transmission of Candidatus Liberibacter psyllaurous. J. Econ. Entomol. 2011, 104, 586–594. [Google Scholar] [CrossRef]
- Polston, J.E.; Sherwood, T. Pymetrozine interferes with transmission of Tomato yellow leaf curl virus by the whitefly Bemsia tabaci. Phytoparasitica 2003, 31, 490–498. [Google Scholar] [CrossRef]
- Asjes, C.J. Control of field spread of non-persistent viruses in flower-bulb crops by synthetic pyrethroid and pirimicarb insecticides, and mineral oils. Crop. Prot. 1985, 4, 485–493. [Google Scholar] [CrossRef]
- Irwin, M.E.; Kampmeier, G.E. Vector behaviour, environment stimuli and the dynamics of plants virus epidemics. In Spatial Components of Plant Disease Epidemics; Jeger, M.J., Ed.; Printice Hall: Upper Saddle River, NJ, USA, 1989; pp. 14–39. [Google Scholar]
- Irwin, M.E. Implications of movements in developing and deploying integrated pet management strategies. Agric. For. Meteorol. 1999, 97, 215–248. [Google Scholar] [CrossRef]
- Elbert, A.; Nauen, R. Resistance of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides in southern Spain with special reference to neonicotinoids. Pest Manag. Sci. 2000, 56, 60–64. [Google Scholar] [CrossRef]
- Gorman, K.; Slater, R.; Blande, J.D.; Clarke, A.; Wren, J.; McCaffrey, A.; Denholm, I. Cross-resistance relationships between neonicotinoids and pymetrozine in Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Fiel, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus Persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Caballero, R.; Schuster, D.J.; Feres, N.A.; Mangandi, J.; Hasing, T.; Trexler, F.; Kalb, S.K.; Portillo, H.E.; Marçon, P.C.; Annan, I.B. Effectiveness of Cyantraniliprole for managing Bemisia tabaci (Hemiptera: Aleyrodidae) and interfering with transmission of tomato yellow leaf curl virus on tomato. J. Econ. Entomol. 2015, 108, 894–903. [Google Scholar] [CrossRef]
- Rodasky, E.; Stavrakaki, M.; Grispou, M.; Achimastou, A.; Waetermeulen, X.V.; Nauen, R.; Tsagkarakou, A. Flupyradifurone effective manages whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) and tomato yellow leaf curl virus in tomato. Pest Manag. Sci. 2017, 73, 1574–1584. [Google Scholar]
- Cameron, R.; Lang, E.B.; Annan, I.B.; Portillo, H.E.; Alvarez, J.M. Use of fluorescence, a novel technique to determine reduction in Bemisia tabaci (Hemiptera: Aleyrodidae) nymph feeding when exposed to Benevia and other insecticides. J. Econ. Entomol. 2013, 106, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Ueda, T.; Yoneda, T.; Koyanagi, T.; Haga, T. Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest Manag. Sci. 2007, 63, 969–973. [Google Scholar] [CrossRef]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thierlert, W. Biological profile of spirotetramat (Movento®)-a new two-way systemic (ambiomobile) insecticide against sucking pest species. Bayer Crop Sci. J. 2008, 61, 245–278. [Google Scholar]
- Viñuela, E.; Adan, A.; Smagghe, G.; Gonzalez, M.; Medina, M.P.; Budia, F.; Vogt, H.; Del Estal, P. Laboratory effects of ingestion of azadirachtin by two pests (Ceratitis capitata and Spodoptera exigua) and three natural enemies (Chrysoperla carnea, Opius concolor and Podisus maculiventris). Biocontrol Sci. Technol. 2000, 10, 65–177. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Wanumen, A.C.; Sánchez-Ramos, I.; Viñuela, E.; Medina, P.; Adán, A. Impact of feeding on contaminated prey on the life parameters of Nesidiocoris tenuis (Hemiptera: Miridae) adults. J. Insect Sci. 2016, 103, 1–7. [Google Scholar]
- Wang, Z.; Dai, P.; Yang, X.; Ruan, C.C.; Biondi, A.; Desneus, N.; Zang, L.S. Selectivity of novel and traditinal insecticides used for management of whiteflies on the parasitoid Encarsia formosa. Pest Manag. Sci. 2019, 75, 2716–2724. [Google Scholar] [CrossRef]
- Jansen, J.P.; Defrance, T.; Warnier, A.M. Side effects of flonicamide and pymetrozine on five aphid natural enemy species. BioControl 2011, 56, 759–770. [Google Scholar] [CrossRef]
- Perring, T.M.; Gruenhagen, N.M.; Farrar, C.A. Management of plant viral disease through chemical control of insect vectors. Annu. Rev. Entomol. 1999, 44, 457–481. [Google Scholar] [CrossRef]
- D’Ambrosio, D.; George, A.; Kennedy, G.; Huseth, A.S. Feeding behavior of Frankliniella fusca on seedling cotton expressing Cry51Aa2. 834_16 Bt toxin. Pest Manag. Sci. 2020. [CrossRef]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.A.; Giurcanu, C. New insecticides for management of tomato yellow leaf curl, a virus vectored by the silverleaf whitefly, Bemisia tabaci. J. Insect Sci. 2014, 14, 183. [Google Scholar] [CrossRef]
- Kayser, H.; Kaufmann, L.; Schfirmann, E. Pymetrozine (CGA 215,944): A novel compound for aphid and whitefly control. An overview of its mode of action. In Proceedings of the Crop Protection Conference-Pests and Diseases, Brighton, UK, 21–24 November 1994; Volume 2, pp. 737–742. [Google Scholar]
- Gildow, F.E.; Reavy, B.; Mayo, M.A.; Duncan, G.H.; Woodford, J.A.; Lamb, J.W.; Hay, R.T. Aphid acquisition and cellular transport of potato leaf roll virus-like particles lacking P5 read through protein. Phytopathology 2000, 90, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morilla, G.; Janssen, D.; Garcia-Andres, S.; Moriones, E.; Cuadrado, I.M.; Bejarano, E.R. Pepper (Capsicum annuum) is a dead-end host for tomato yellow leaf curl virus. Phytopathology 2005, 95, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiser, R.M.; Ziegler-Graff, V.; Reutenauer, A.; Herrbach, E.; Lemaire, O.; Guilley, H.; Richards, K.; Jonard, G. Agroinfection as an alternative to insects for infecting plants with beet western yellows luteovirus. Proc. Natl. Acad. Sci. USA 1991, 89, 9136–9140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of Enzyme-Linked Immunosorbent Assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- IRAC (Insecticide Resistance Action Committee). Insecticide Resistance Action Committee. 2019. Available online: https://www.irac-online.org (accessed on 1 February 2019).
- Rodriguez-López, M.J.; Garzo, E.; Bonani, J.P.; Fernández-Muñoz, E.; Moriones, E.; Fereres, A. Acylsucrose-producing tomato plants forces Bemisia tabaci to shift its preferred settling and feeding site. PLoS ONE 2011, 7, e33064. [Google Scholar] [CrossRef] [Green Version]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Backus, E.A.; Cline, A.R.; Ellerseick, M.R.; Serrano, M.S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: New methods and parameters for analysis of nonsequential electrical penetration graph data. Ann. Entomol. Soc. Am. 2007, 100, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Abacus Concepts. Statview II; Abacus Concepts, Inc.: Berkeley, CA, USA, 1987. [Google Scholar]
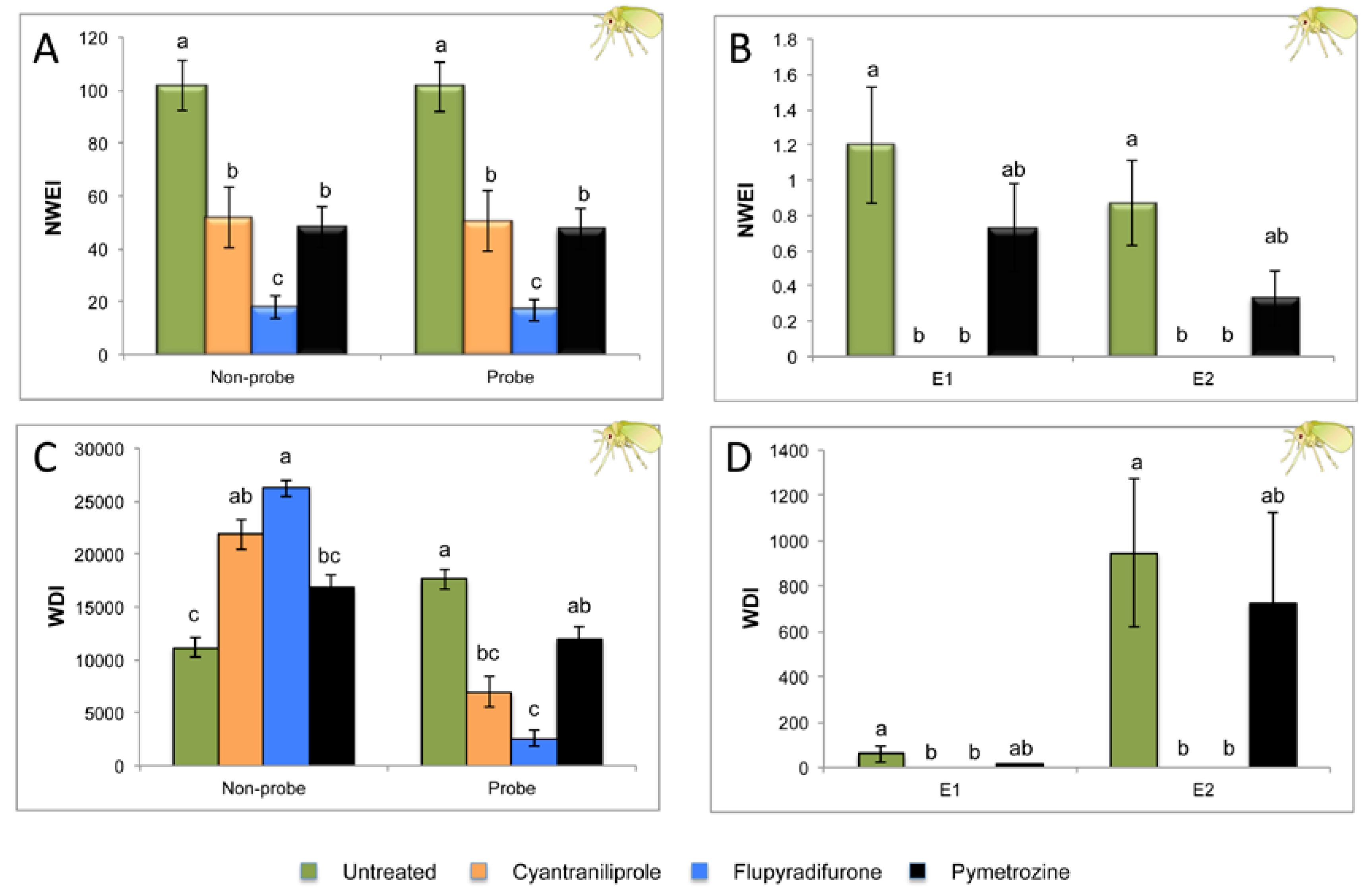
| Sequential Variables | Untreated Control n = 15 | Cyantraniliprole n = 15 | Flupyradifurone n = 15 | Pymetrozine n = 15 | P |
|---|---|---|---|---|---|
| Time to first probe from EPG start | 415.6 ± 78.7 a | 341.4 ± 86.3 a | 311.4 ± 83.6 a | 385.7 ± 105.1 a | 0.766 |
| Time from first probe to 1st E | 18,861.4 ± 2714.7 b | 28,458.5 ± 86.3 a | 28,488.6 ± 83.6 a | 22,438.1 ± 2016.8 ab | 0.003 |
| Time from the beginning of that probe to first E | 921.00 ± 202.8 a | - | - | 1390.5 ± 290.4 a | 0.101 |
| Time from the beginning of that probe to first E2 | 1091.1 ± 232.2 a | - | - | 1062.5 ± 187.3 a | 0.758 |
| Indices | |||||
| Probing % spent in C | 88.34 ± 3.1 b | 99.26 ± 0.7 a | 98.18 ± 1.8 a | 84.13 ± 4.37 b | 0.000 |
| Probing % spent in G | 5.82 ± 2.3 a | 0.74 ± 0.7 b | 1.82 ± 1.8 b | 11.02 ± 3.6 a | 0.005 |
| Probing % spent in E1 | 0.28 ± 0.2 a | 0 b | 0 b | 0.11 ± 0.0 a | 0.0001 |
| Probing % spent in E2 | 5.56 ± 1.9 a | 0 b | 0 b | 4.74 ± 2.7 ab | 0.0001 |
| Sequential Variables | Untreated Control n = 18 | Sulfoxaflor n = 19 | Flonicamid n = 17 | Spirotetramat n = 19 | P |
|---|---|---|---|---|---|
| Time to first probe from start of EPG | 267.1 ± 122.6 a | 196.4 ± 76.2 a | 1064.2 ± 558.6 a | 213.7 ± 70.5 a | 0.804 |
| Time from first probe to first E | 3897.7 ± 743.5 a | 7625.9 ± 2029.2 a | 8162.1 ± 2697.2 a | 6528.1 ± 1142.6 a | 0.303 |
| Time from beginning of that probe to first E1 | 679.2 ± 71.8 a | 1747.8 ± 610.7 a | 784.8 ± 65.4a | 1124.3 ± 353.1 a | 0.307 |
| Time from beginning of that probe to first E2 | 765.4 ± 04.9 b | 1800.5 ± 665.9 b | 824.3 ± 79.7 b | 1233.5 ± 351.5 a | 0.0001 |
| Indices | |||||
| Probing % spent in C | 26.36 ± 5.0 b | 47.85 ± 4.3 a | 48.45 ± 6.5 a | 23.59 ± 1.6 b | 0.0001 |
| Probing % spent in F | 10.07 ± 2.9 b | 35.38 ± 5.2 a | 20.26 ± 7.3 ab | 18.73 ± 2.2 ab | 0.015 |
| Probing % spent in E1 | 5.35 ± 1.2a | 5.53 ± 1.5 a | 6.57 ± 1.7 a | 5.26 ± 0.6 a | 0.999 |
| Probing % spent in E2 | 57.65 ± 6.7 a | 10.00 ± 2.9 b | 24.42 ± 8.1 b | 50.48 ± 3.7 a | 0.0001 |
| Bemisia tabaci | |||||
| Active Ingredient | Dose (ai) | Chemical Class | Commercial Product | Company | IRAC * |
| Cyantraniliprole | 150 ppm | Ryanoid | CyazypyrTM 10% | Dupont Corporation | 28 |
| Flupyradifurone | 150 ppm | Butenolides | SivantoTM 200SL | Bayer | 4 |
| Pymetrozine | 100 ppm | Pyridine azomethine | Plenum®50% (WG) P/P | Syngenta | 9 |
| Myzus persicae | |||||
| Active Ingredient | Dose (ai) | Chemical Class | Commercial Product | Company | IRAC * |
| Spirotretamat | 75 ppm | Ketoenols | Movento® 150 O-TEQ 15% [OD] P/V | Bayer | 23 |
| Flonicamid | 60 ppm | Pyridinocarboxamide | CarbineTM 50WG | FMC Corporation | 29 |
| Sulfoxaflor | 24 ppm | Sulfoximines | IsoclastTM active 30% | Dow Agrosciences | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. Feeding Behavior and Virus-transmission Ability of Insect Vectors Exposed to Systemic Insecticides. Plants 2020, 9, 895. https://doi.org/10.3390/plants9070895
Garzo E, Moreno A, Plaza M, Fereres A. Feeding Behavior and Virus-transmission Ability of Insect Vectors Exposed to Systemic Insecticides. Plants. 2020; 9(7):895. https://doi.org/10.3390/plants9070895
Chicago/Turabian StyleGarzo, Elisa, Aránzazu Moreno, María Plaza, and Alberto Fereres. 2020. "Feeding Behavior and Virus-transmission Ability of Insect Vectors Exposed to Systemic Insecticides" Plants 9, no. 7: 895. https://doi.org/10.3390/plants9070895







