Interactive Effects of N Form and P Concentration on Growth and Tissue Composition of Hybrid Napier Grass (Pennisetum purpureum × Pennisetum americanum)
Abstract
:1. Introduction
2. Results
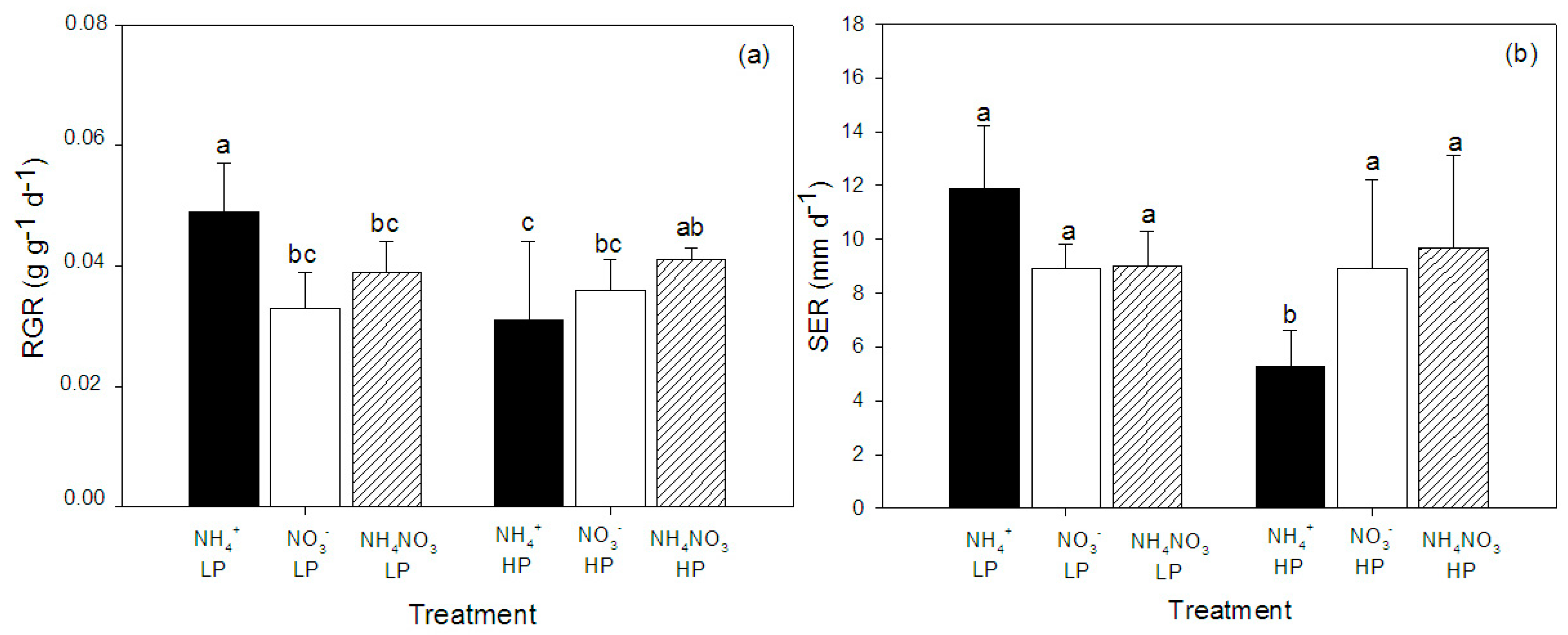
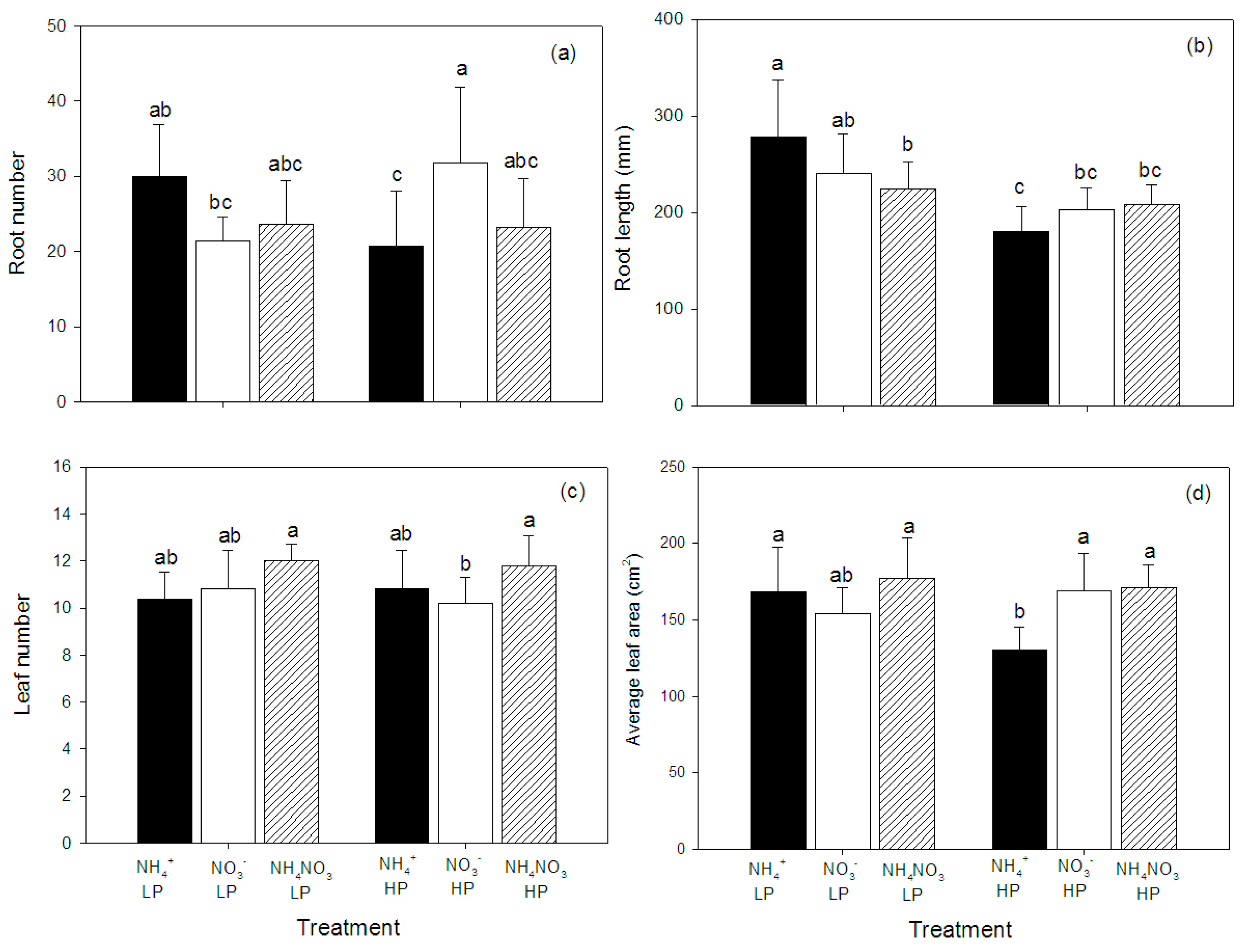
2.1. Growth and Morphology
2.2. Chlorophylls and Carotenoids
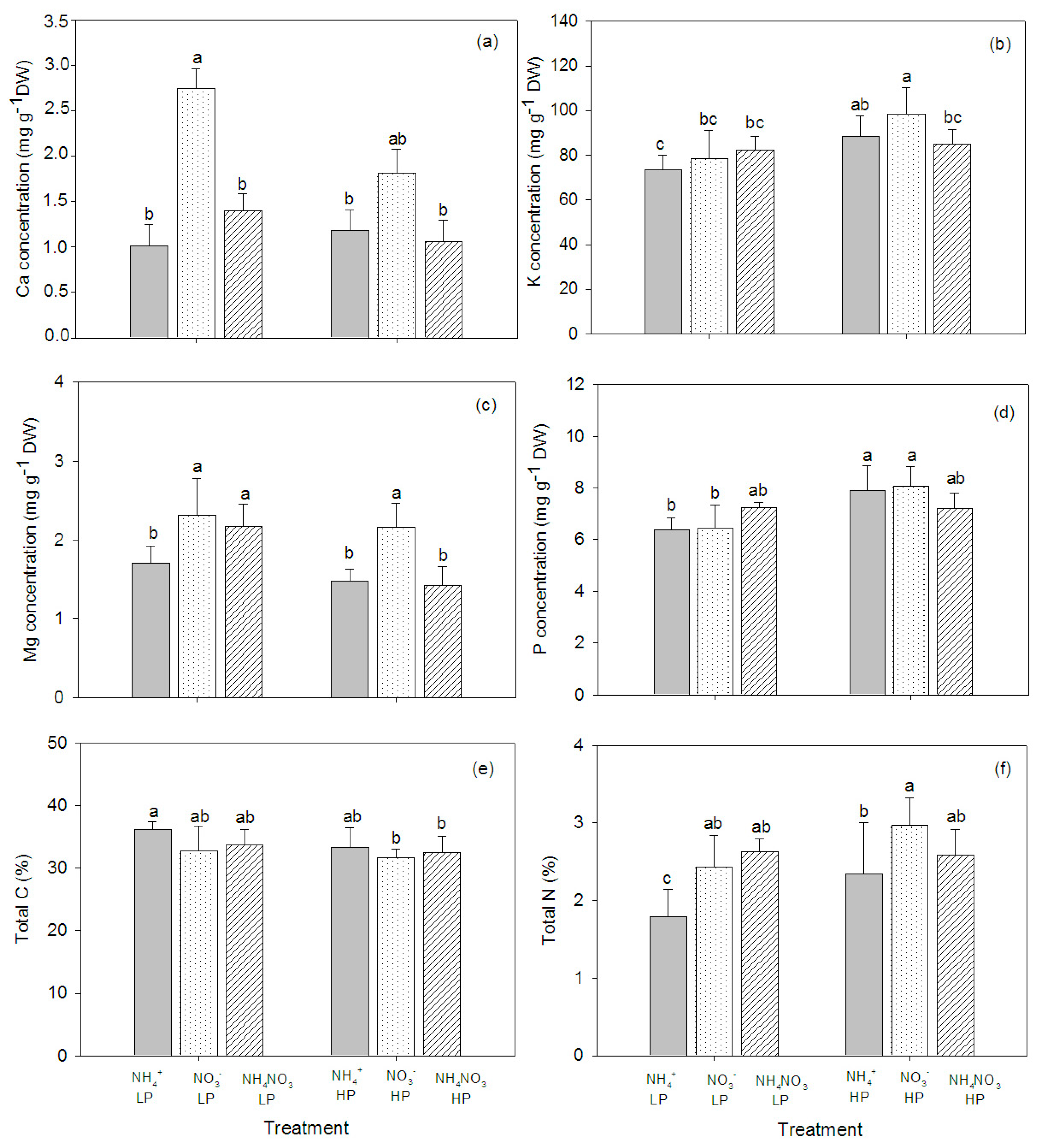
2.3. Mineral Element Concentrations in the Plant Tissue
2.4. CN in the Plant Tissue
3. Discussion
4. Materials and Methods
4.1. Plant Preparation and Experimental Design
4.2. Plant Harvest and Chemical Analyses
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruinsma, J. (Ed.) World Agriculture: Towards 2015/2030—An FAO Perspective; Earthscan: London, UK, 2003. [Google Scholar]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.H.; Wallace, S.D. (Eds.) Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Mintz, M.M.; Snyder, S.W. An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: Challenges and opportunities towards energy-neutral WWTPs. Renew. Sust. Energ. Rev. 2015, 50, 346–362. [Google Scholar] [CrossRef] [Green Version]
- Abdeshahian, P.; Lim, J.S.; Ho, W.S.; Hashim, H.; Lee, C.T. Potential of biogas production from farm animal waste in Malaysia. Renew. Sust. Energ. Rev. 2016, 60, 714–723. [Google Scholar] [CrossRef]
- Kirchmann, H.; Witter, E. Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresour. Technol. 1992, 40, 137–142. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Qadir, M.; Yamamoto, S.; Endo, T.; Zahoor, A. Global regional, and country level need for data on wastewater generator, treatment, and use. Agric. Water Manag. 2013, 130, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.Y.; Liu, X.L.; Li, X.H.; Jing, Q.C.; Wei, X.F.; Shi, T.H.; Dong, Y.L.; Yan, P.P. Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil. Open Chem. 2018, 16, 968–977. [Google Scholar] [CrossRef]
- Matheyarasu, R.; Bolan, N.; Naidu, R. Abattoir wastewater irrigation increases the availability of nutrients and influences on plant growth and development. Water Air Pollut. 2016, 227, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, R.M.P.; Firme, L.P.; Montes, C.R.; Melfi, A.J.; de Stefano Piedade, S.M. Soil exchangeable cations, sugarcane production and nutrient uptake after wastewater irrigation. Sci. Agric. (Piracicaba Braz.) 2009, 66, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Mo, W.; Zhang, Q. Energy-nutrients-water nexus: Integrated resource recovery in municipal wastewater treatment plants. J. Environ. Manage. 2013, 127, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Dzantor, E.K.; Chekol, T.; Vough, L.R. Feasibility of using forage grasses and legumes for phytoremediation of organic pollutants. J. Environ. Sci. Health 2000, 35, 1645–1661. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somsiri, S.; Vivanpatarakij, S. Potential of transforming Napier grass to energy. J. Energy Res. 2015, 12, 47–58. [Google Scholar]
- Waramit, N.; Chaugool, J. Napier grass: A novel energy crop development and the current status in Thailand. J. ISSAAS. 2014, 20, 139–150. [Google Scholar]
- Somjai, T.; Suwan, C. Carbon footprint analysis of Napier Pakchong 1 grass plantation in Prachinburi province. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 141, pp. 1–5. [Google Scholar]
- Jampeetong, A.; Brix, H.; Kantawanichkul, S. Effects of inorganic nitrogen form on growth, morphology, N uptake, and nutrient allocation in hybrid Napier grass (Pennisetum purpureum x P. americanum cv. Pakchong1). Ecol. Eng. 2014, 73, 653–658. [Google Scholar] [CrossRef]
- Tarvorasak, V.; Piwpuan, N.; Jampeetong, A. Responses and tolerance to high ammonium levels of hybrid Napier grass (Pennisetum purpureum × Pennisetum americanum cv. Pakchong 1): Assessing the potential for water treatment and agricultural management in southeast Asia. Chiang Mai J. Sci. 2016, 43, 1059–1069. [Google Scholar]
- Zeng, H.; Liu, G.; Kinoshita, T.; Zhang, R.; Zhu, Y.; Shen, Q.; Xu, G. Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil. 2012, 357, 205–214. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Heintz-Buschart, A.; Kirkman, K.; Spohn, M. Interactions of nitrogen and phosphorus cycling promote P acquisition and explain synergistic plant-growth responses. Ecology 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Dickson, R.W.; Fisher, P.R.; Argo, W.R.; Jacques, D.J.; Sartain, J.B.; Trenholm, L.E.; Yeager, T.H. Solution ammonium: Nitrate ratio and cation/anion uptake affect acidity or basicity with floriculture species in hydroponics. Sci. Hortic. 2016, 200, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, K.E.; Burton, T.M. An experimental comparison of the dry-matter and nutrient distribution patterns of Typha latifolia L., Typha angustifolia L., Sparganium eurycarpum Engelm. and Phragmites australis (Cav.) Trin. ex Steud. Aquat. Bot. 1988, 2, 129–139. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K. Interactive effects of N and P on growth but not on resource allocation of Canna indica in wetland microcosms. Aquat. Bot. 2008, 89, 317–323. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crop Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhu, X.F.; Hu, A.Y.; Wang, C.; Wang, B.; Dong, X.Y.; Shen, R.F. Differential effects of nitrogen forms on cell wall phosphorus remobilization are mediated by nitric oxide, pectin content, and phosphate transporter expression. Plant Physiol. 2016, 171, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, U.; Yuan, L.; Neumann, G. Improving the efficiency and effectiveness of global phosphorus use: Focus on root and rhizosphere levels in the agronomic system. Front. Agric. Sci. Eng. 2019, 6, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Bakht, J.; Shafi, M.; Khan, S.; Shah, W.A. Uptake nitrogen as affected by various contaminations of nitrogen and phosphorus. Asian J. Plant Sci. 2002, 1, 367–369. [Google Scholar]
- Romero, J.A.; Brix, H.; Comin, F.A. Interactive effects of N and P on growth, nutrient allocation and NH4+ uptake kinetics by Phragmites australis. Aquat. Bot. 1999, 64, 369–380. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Rychter, A.M. Nitrate uptake by bean (Phaseolus vulgaris L.) roots under phosphate deficiency. Plant Soil. 2000, 226, 79–85. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Q.; Gu, J.; Gong, J.; Guan, C.; Lepo, J.E.; Rong, X.; Song, H.; Zhang, Z. V-ATPase and V-PPase at the tonoplast affect NO3- content in Brassica napus by controlling distribution of NO3- between the cytoplasm and vacuole. J. Plant Growth Regul. 2015, 34, 22–34. [Google Scholar] [CrossRef]
- Rufty, T.W., Jr.; MacKown, C.T.; Israel, D.W. Phosphorus stress effects on assimilation on nitrate. Plant Physiol. 1990, 94, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.B.; Yuan, L.X.; Zhang, J.L.; Li, H.G.; Bai, Z.H.; Chen, X.P.; Zhang, W.F.; Zhang, F.S. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, D.; Li, T.; Zheng, Z.; Zhang, X.; Chen, G.; Yu, H. Root physiological adaptations involved in enhancing P assimilation in mining and non-mining ecotypes of Polygonum hydropiper grown under organic P media. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Cruz-Ramı´rez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Yano, K.; Kume, T. Root Morphological plasticity for heterogeneous phosphorus supply in Zea mays L. Plant Prod. Sci. 2005, 8, 427–432. [Google Scholar] [CrossRef]
- Bhatti, A.S.; Loneragan, J.F. The effect of early superphosphate toxicity on the subsequent growth of wheat. Aust. J. Agric. Res. 1970, 21, 881–892. [Google Scholar] [CrossRef]
- Chiou, T.J.; Aung, K.; Lin, S.I.; Wu, C.C.; Chiang, S.F.; Su, C.L. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 2006, 18, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves. Plant Cell Environ. 2020. [Google Scholar] [CrossRef]
- Shane, M.W.; McCully, M.E.; Lambers, H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J. Exp. Bot. 2004, 55, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Guo, Q.; Zhang, J.; Korpelainen, H.; Li, C. Effects of nitrogen and phosphorus supply on growth and physiological traits of two Larix species. Environ. Exp. Bot. 2016, 130, 206–215. [Google Scholar] [CrossRef]
- Smart, R.M.; Barko, J.W. Laboratory culture of submerged freshwater macrophytes on natural sediments. Aquat. Bot. 1985, 21, 251–263. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves: The Functional Approach to Plant Growth Analysis; Edward Arnold: London, UK, 1982. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]



| Main Effects | Interaction | ||
|---|---|---|---|
| N Form | P Concentration | N Form × P Concentration | |
| Relative growth rate (g·g−1·d−1) | 1.7 | 2.7 | 6.8 ** |
| Shoot elongation rate (mm·d−1) | 0.3 | 5.5 * | 7.8 ** |
| Root number | 0.5 | 0.01 | 5.0 * |
| Root length (cm) | 0.4 | 17.5 *** | 4.0 * |
| Leaf number | 3.6 * | 0.1 | 0.4 |
| Average leaf area (cm2) | 4.6 * | 0.7 | 2.91 |
| Chlorophyll a (mg·g−1·DW) | 6.4 ** | 19.4 *** | 5.9 ** |
| Chlorophyll b (mg·g−1·DW) | 6.2 ** | 12.7 *** | 13.8 *** |
| Total chlorophylls (mg·g−1·DW) | 7.3 ** | 23.9 *** | 9.1 ** |
| Total carotenoids (mg·g−1·DW) | 6.1 ** | 21.5 *** | 6.9 ** |
| Main Effects | Interaction | ||
|---|---|---|---|
| N Form | P Concentration | N Form × P Concentration | |
| Stems | |||
| Ca (mg·g−1 DW) | 4.6 * | 1.1 | 0.8 |
| K (mg·g−1 DW) | 1.5 | 12.6 ** | 2.1 |
| Mg (mg·g−1 DW) | 12.9 ** | 11.0 ** | 3.1 |
| P (mg·g−1 DW) | 0.1 | 16.0 *** | 4.0 * |
| Total C (%) | 2.3 | 3.0 | 0.4 |
| Total N (%) | 6.9 ** | 5.1 * | 1.6 |
| Leaves | |||
| Ca (mg·g−1 DW) | 2.1 | 3.8 | 2.4 |
| K (mg·g−1 DW) | 11.4 *** | 1.8 | 5.9 ** |
| Mg (mg g−1 DW) | 4.3 * | 2.4 | 6.5 ** |
| P (mg·g−1 DW) | 6.7 ** | 50.5 *** | 12.7 *** |
| Total C (%) | 1.8 | 0.5 | 0.7 |
| Total N (%) | 1.3 | 1.0 | 1.1 |
| Roots | |||
| Ca (mg·g−1 DW) | 13.8 *** | 0.6 | 0.4 |
| K (mg·g−1 DW) | 0.4 | 4.9 * | 1.1 |
| Mg (mg·g−1 DW) | 0.9 | 16.9 *** | 0.6 |
| P (mg·g−1 DW) | 5.2 * | 20.0 *** | 3.9 * |
| Total C (%) | 3.3 | 7.6 * | 1.1 |
| Total N (%) | 1.7 | 0.4 | 3.6 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakwan, C.; Jampeetong, A.; Brix, H. Interactive Effects of N Form and P Concentration on Growth and Tissue Composition of Hybrid Napier Grass (Pennisetum purpureum × Pennisetum americanum). Plants 2020, 9, 1003. https://doi.org/10.3390/plants9081003
Pakwan C, Jampeetong A, Brix H. Interactive Effects of N Form and P Concentration on Growth and Tissue Composition of Hybrid Napier Grass (Pennisetum purpureum × Pennisetum americanum). Plants. 2020; 9(8):1003. https://doi.org/10.3390/plants9081003
Chicago/Turabian StylePakwan, Chonthicha, Arunothai Jampeetong, and Hans Brix. 2020. "Interactive Effects of N Form and P Concentration on Growth and Tissue Composition of Hybrid Napier Grass (Pennisetum purpureum × Pennisetum americanum)" Plants 9, no. 8: 1003. https://doi.org/10.3390/plants9081003





