A New Active Substance Derived from Lyzed Willaertia magna C2c Maky Cells to Fight Grapevine Downy Mildew
Abstract
:1. Introduction
2. Results
2.1. Elicitor Property
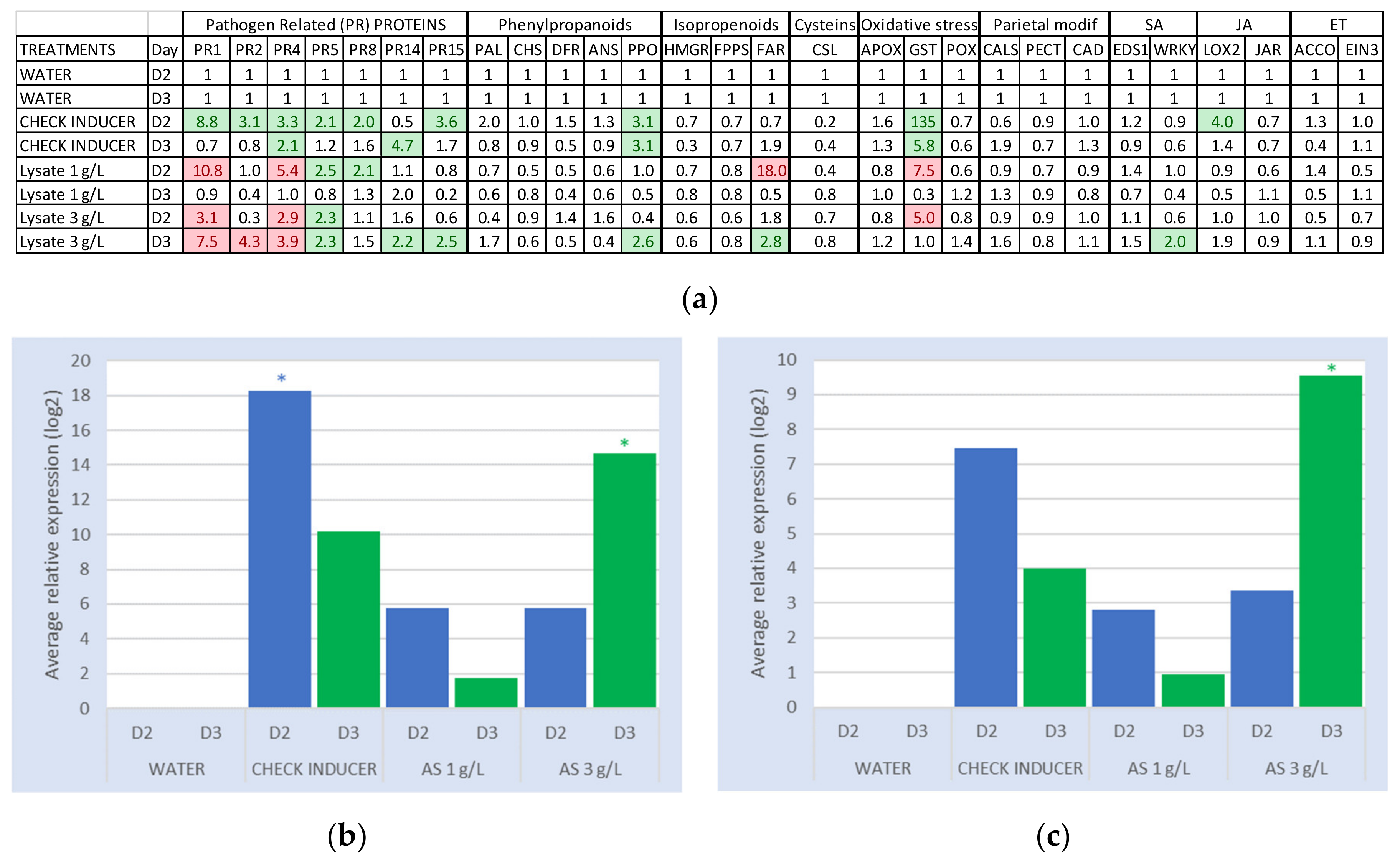
2.1.1. Comparative Analysis of Grapevine Gene Defense Induction
2.1.2. Fold Change of PR Protein Genes
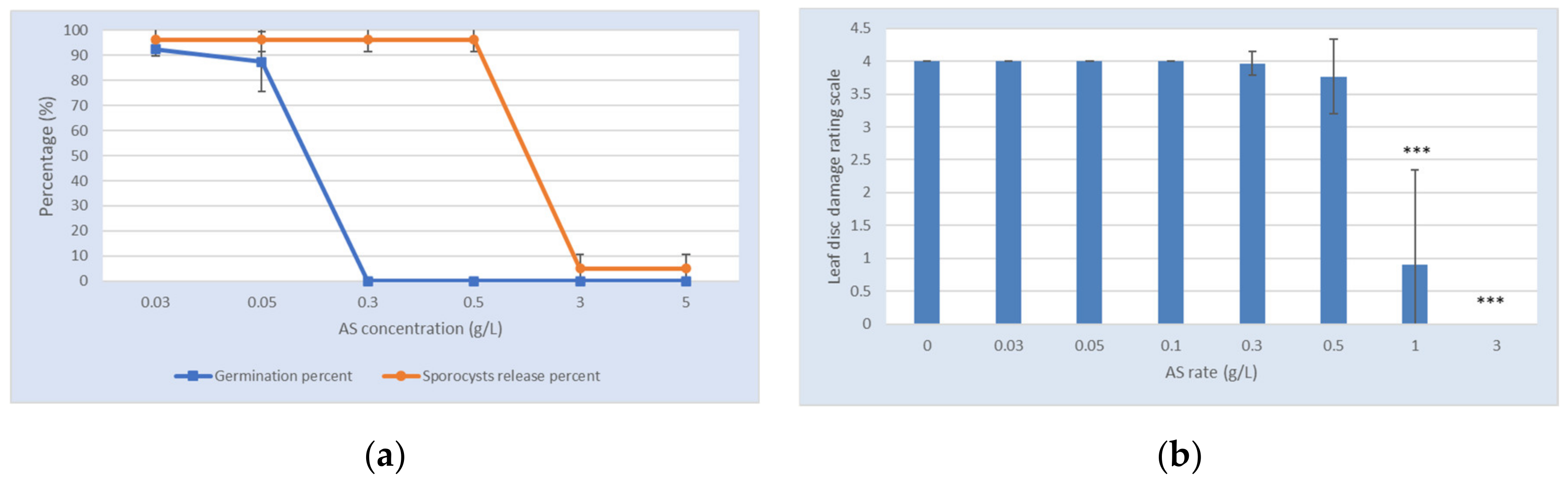
2.2. Anti-Oomycete Activity
2.3. Greenhouse Tests
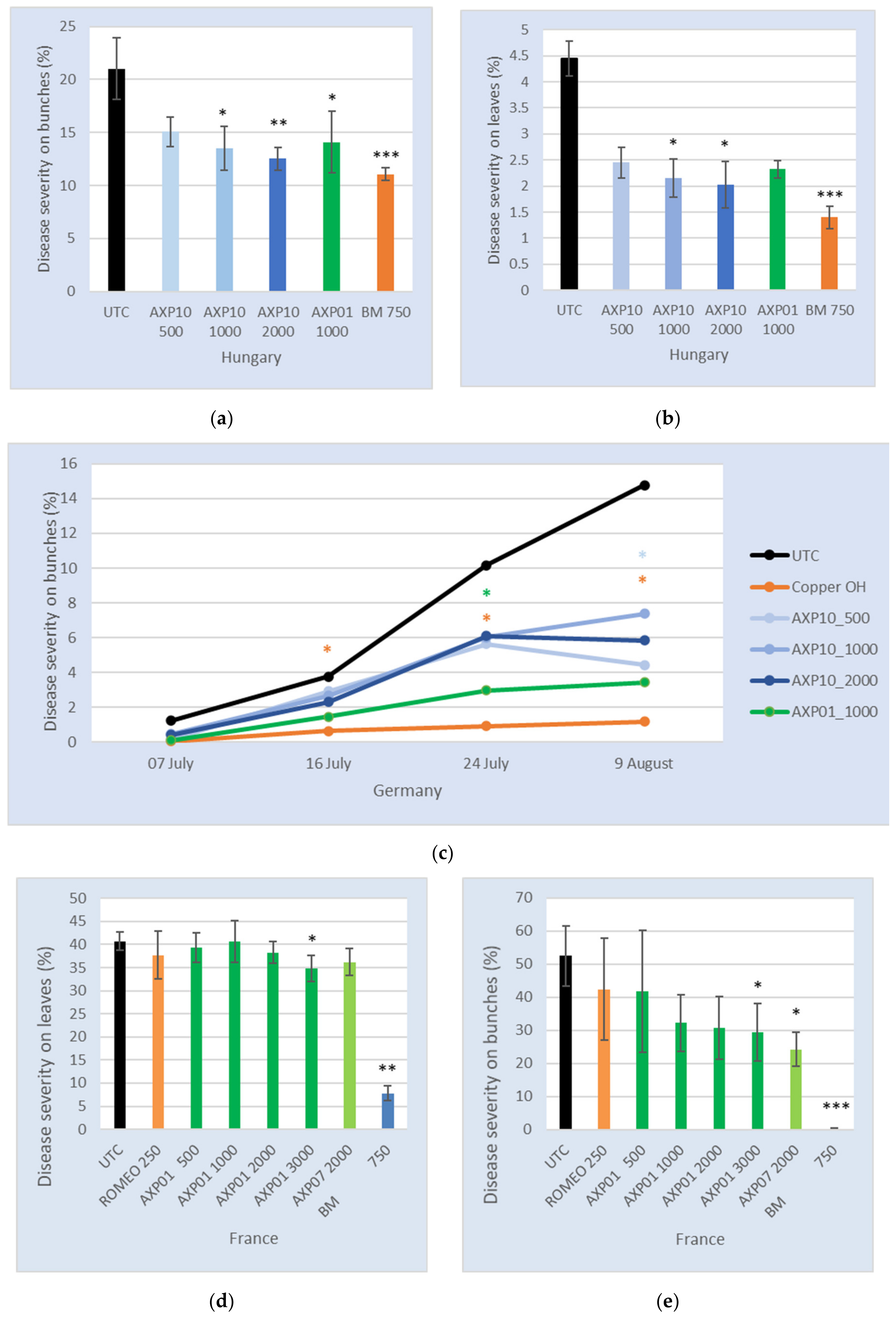
2.4. Efficacy in the Field
3. Discussion
4. Materials and Methods
4.1. Active Substance and Formulation Preparation
4.2. Study of the Stimulation of Plant Defense Genes by qPFD®
4.2.1. Biological Material
4.2.2. Plant Treatment and Samplings
- -
- D0: Initial sampling before any treatment;
- -
- D2 and D3: Samplings performed 2 and 3 days after treatment.
4.2.3. Marker Genes
4.3. Anti-Oomycetal Properties of the AS
4.3.1. Oomycete Strain
4.3.2. Active Substance Preparation
4.3.3. In Vitro Test
4.3.4. In Vivo Test
- Evaluation of the disease development on leaf discs
- Evaluation of the contamination properties of spores produced on leaf discs
4.4. Greenhouse Tests
4.4.1. Plant Material
4.4.2. AS Application
4.4.3. Plasmopara viticola Inoculation
4.4.4. Incubation and Notations
4.5. Field Tests
4.5.1. Field Test Conducted in Hungary
4.5.2. Field Test Conducted in Germany
4.5.3. Field Test Conducted in France
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The International Organisation of Vine and Wine Current Situation of the Vitivinicultural Sector at a Global Level. Available online: http://www.oiv.int/en/oiv-life/current-situation-of-the-vitivinicultural-sector-at-a-global-level (accessed on 30 April 2020).
- Agrios, G.N. Plant diseases caused by fungi. In Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Academic Press: New York, NY, USA, 2005; pp. 385–614. [Google Scholar]
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine’Merlot’(Vitis vinifera). Vitis 2010, 49, 77–85. [Google Scholar]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar] [CrossRef]
- Fröbel, S.; Zyprian, E. Colonization of Different Grapevine Tissues by Plasmopara viticola—A Histological Study. Front. Plant. Sci. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrel, L. Integrated Analysis of the Grapevine Response to Plasmopara viticola Infection: Through Study of Breakdown Resistance. Ph.D. Thesis, Université de Strasbourg, Grand Est, France, 2016. [Google Scholar]
- Dagostin, S.; Schärer, H.-J.; Pertot, I.; Tamm, L. Are there alternatives to copper for controlling grapevine downy mildew in organic viticulture? Crop Prot. 2011, 30, 776–788. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Rosa, D.J.; Corredor Prado, J.P.; Borghezan, M.; Bastos de Melo, G.W.; Fonsêca de Sousa Soares, C.R.; Comin, J.J.; Simão, D.G.; Brunetto, G. Reduction of copper phytotoxicity by liming: A study of the root anatomy of young vines (Vitis labrusca L.). Plant Physiol. Biochem. 2015, 96, 270–280. [Google Scholar] [CrossRef] [Green Version]
- European Commission A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 10 August 2020).
- De Jonckheere, J.F.; Dive, D.G.; Pussard, M.; Vickerman, K. Willaertia magna gen. nov., sp. nov.(Vahlkampfiidae), a thermophilic amoeba found in different habitats. Protistologica 1984, 20, 5–13. [Google Scholar]
- Hasni, I.; Chelkha, N.; Baptiste, E.; Mameri, M.R.; Lachuer, J.; Plasson, F.; Colson, P.; La Scola, B. Investigation of potential pathogenicity of Willaertia magna by investigating the transfer of bacteria pathogenicity genes into its genome. Sci. Rep. 2019, 9, 18318. [Google Scholar] [CrossRef]
- Hasni, I.; Decloquement, P.; Demanèche, S.; Mameri, R.M.; Abbe, O.; Colson, P.; La Scola, B. Insight into the Lifestyle of Amoeba Willaertia magna during Bioreactor Growth Using Transcriptomics and Proteomics. Microorganisms 2020, 8, 771. [Google Scholar] [CrossRef]
- Barbaree, J.M.; Fields, B.S.; Feeley, J.C.; Gorman, G.W.; Martin, W.T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl. Environ. Microbiol. 1986, 51, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Scheikl, U.; Sommer, R.; Kirschner, A.; Rameder, A.; Schrammel, B.; Zweimüller, I.; Wesner, W.; Hinker, M.; Walochnik, J. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur. J. Protistol. 2014, 50, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Clarholm, M. Protozoan grazing of bacteria in soil—Impact and importance. Microb. Ecol. 1981, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zaragoza, S. Ecology of Free-Living Amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Jarry, A.; Quelard, B.; Carlino, A.; Eberst, J.-B.; Abbe, O.; Demanèche, S. Intracellular Behaviour of Three Legionella pneumophila Strains within Three Amoeba Strains, Including Willaertia magna C2c Maky. Pathogens 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisset, M.-N.; Bernonville, T.D.D. Device for Determining or Studying the State of Stimulation of the Natural Defences of Plants or Portions of Plants. U.S. Patent No. 9,290,788, 22 March 2016. [Google Scholar]
- Van Aubel, G.; Buonatesta, R.; Van Cutsem, P. COS-OGA: A novel oligosaccharidic elicitor that protects grapes and cucumbers against powdery mildew. Crop Prot. 2014, 65, 129–137. [Google Scholar] [CrossRef]
- Plasson, F.; Mameri, M.O. Therapeutic or Non-Therapeutic Use of Protozoans of the Willaertia Genus as a Fungistatic and/or Fungicide. WO2019030459, 14 February 2019. [Google Scholar]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Paro, R.; Tiboni, G.M.; Buccione, R.; Rossi, G.; Cellini, V.; Canipari, R.; Cecconi, S. The fungicide mancozeb induces toxic effects on mammalian granulosa cells. Toxicol. Appl. Pharmacol. 2012, 260, 155–161. [Google Scholar] [CrossRef]
- Francesca, S.; Simona, G.; Nicola, T.F.; Andrea, R.; Vittorio, R.; Federico, S.; Cynthia, R.; Lodovica, G.M. Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Glob. Change Biol. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Falk, S.P.; Pearson, R.C.; Gadoury, D.M.; Seem, R.C.; Sztejnberg, A. Fusarium proliferatum as a biocontrol agent against grape downy mildew. Phytopathology 1996, 86, 1010–1017. [Google Scholar] [CrossRef]
- Bakshi, S.; Sztejnberg, A.; Yarden, O. Isolation and Characterization of a Cold-Tolerant Strain of Fusarium proliferatum, a Biocontrol Agent of Grape Downy Mildew. Phytopathology® 2001, 91, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Puopolo, G.; Giovannini, O.; Pertot, I. Lysobacter capsici AZ78 can be combined with copper to effectively control Plasmopara viticola on grapevine. Microbiol. Res. 2014, 169, 633–642. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, Y.; Fu, X.; Wang, Q. Screening and characterization of endophytic Bacillus for biocontrol of grapevine downy mildew. Crop Prot. 2017, 96, 173–179. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Li, Y.; Héloir, M.-C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, S.; Mochizuki, M.; Aoki, Y.; Kobayashi, H.; Takayanagi, T.; Shimizu, M.; Suzuki, S. Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci. Technol. 2011, 21, 705–720. [Google Scholar] [CrossRef]
- Dey, R. Étude Comparée de la Prolifération de Legionella pneumophila dans Différents Hôtes Amibiens et de Leurs Inter-Relations: Implication Potentielle de Phospholipides Aminés. Ph.D. Thesis, Université Claude Bernard-Lyon I, Villeurbanne, France, 2010. [Google Scholar]
- Pujos, P.; Martin, A.; Farabullini, F.; Pizzi, M. RomeoTM, cerevisane-based biofungicide against the main diseases of grape and of other crops: General description. In Proceedings of the Atti, Giornate Fitopatologiche, Chianciano Terme (Siena), Italy, 18–21 March 2014; Volume 2, pp. 51–56. [Google Scholar]
- De Miccolis Angelini, R.M.; Rotolo, C.; Gerin, D.; Abate, D.; Pollastro, S.; Faretra, F. Global transcriptome analysis and differentially expressed genes in grapevine after application of the yeast-derived defense inducer cerevisane. Pest Manag. Sci. 2019, 75, 2020–2033. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Faino, L.; Carli, P.; Testa, A.; Cristinzio, G.; Frusciante, L.; Ercolano, M.R. Potato R1 resistance gene confers resistance against Phytophthora infestans in transgenic tomato plants. Eur. J. Plant Pathol. 2010, 128, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Gamir, J.; Darwiche, R.; Van’t Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, J.E.; Sanchez, J.P.; Zumstein, K.; Gilchrist, D.G. Plant and animal PR1 family members inhibit programmed cell death and suppress bacterial pathogens in plant tissues. Mol. Plant Pathol. 2018, 19, 2111–2123. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-X.; Zhang, F.-C.; Zhang, W.-Z.; Song, L.-F.; Wu, W.-H.; Chen, Y.-F. Arabidopsis Di19 Functions as a Transcription Factor and Modulates PR1, PR2, and PR5 Expression in Response to Drought Stress. Mol. Plant 2013, 6, 1487–1502. [Google Scholar] [CrossRef] [Green Version]
- Charles, M.T.; Tano, K.; Asselin, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. V. Constitutive defence enzymes and inducible pathogenesis-related proteins. Postharvest Biol. Technol. 2009, 51, 414–424. [Google Scholar] [CrossRef]
- Thibaud, M.-C.; Gineste, S.; Nussaume, L.; Robaglia, C. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol. Biochem. 2004, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Chilosi, G.; Leonardi, L.; Bertini, L.; Magro, P.; Buonocore, V.; Caporale, C. A basic peroxidase from wheat kernel with antifungal activity. Phytochemistry 2001, 58, 743–750. [Google Scholar] [CrossRef]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.M.; Campo, S.; Murillo, I.; Coca, M.; San Segundo, B. Fungus- and wound-induced accumulation of mRNA containing a class II chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 2003, 52, 745–759. [Google Scholar] [CrossRef]
- Petre, B.; Major, I.; Rouhier, N.; Duplessis, S. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Kido, E.A.; Pandolfi, V.; Houllou-Kido, L.M.; Andrade, P.P.; Marcelino, F.C.; Nepomuceno, A.L.; Abdelnoor, R.V.; Burnquist, W.L.B.; Benko-Iseppon, A.M. Plant Antimicrobial Peptides: An Overview of SuperSAGE Transcriptional Profile and a Functional Review. Available online: http://www.eurekaselect.com/85038/article (accessed on 19 March 2020).
- Ferreira, R.B.; Monteiro, S.; Freitas, R.; Santos, C.N.; Chen, Z.; Batista, L.M.; Duarte, J.; Borges, A.; Teixeira, A.R. The role of plant defence proteins in fungal pathogenesis. Mol. Plant Pathol. 2007, 8, 677–700. [Google Scholar] [CrossRef]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biol. Control 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Krzyzaniak, Y.; Trouvelot, S.; Negrel, J.; Cluzet, S.; Valls, J.; Richard, T.; Bougaud, A.; Jacquens, L.; Klinguer, A.; Chiltz, A.; et al. A Plant Extract Acts Both as a Resistance Inducer and an Oomycide Against Grapevine Downy Mildew. Front. Plant. Sci. 2018, 9, 1085. [Google Scholar] [CrossRef]
- La Torre, A.; Talocci, S.; Spera, G.; Valori, R. Control of downy mildew on grapes in organic viticulture. Commun. Agric. Appl. Biol. Sci. 2008, 73, 169–178. [Google Scholar]
- Reuveni, M.; Zahavi, T.; Cohen, Y. Controlling downy mildew (Plasmopara viticola) in field-grown grapevine with β-aminobutyric acid (BABA). Phytoparasitica 2001, 29, 125–133. [Google Scholar] [CrossRef]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. N. Phytol. 2017, 213, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of microbial biocontrol agents—An industrial perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- De Jonckheere, J. Use of an axenic medium for differentiation between pathogenic and nonpathogenic Naegleria fowleri isolates. Appl. Environ. Microbiol. 1977, 33, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugé de Bernonville, T.; Marolleau, B.; Staub, J.; Gaucher, M.; Brisset, M.-N. Using Molecular Tools To Decipher the Complex World of Plant Resistance Inducers: An Apple Case Study. J. Agric. Food Chem. 2014, 62, 11403–11411. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; Van Den Boom, T. The BBCH system to coding the phenological growth stages of plants–history and publications. J. Kulturpflanzen 2009, 61, 41–52. [Google Scholar]

| Defence Classes and Subclasses | Defence Genes | ||
|---|---|---|---|
| Gene Codes | Complete Names | ||
| Chemical and/or physical barriers | PR proteins | PR-1 | Pathogenesis-related protein 1 |
| PR-2 | Pathogenesis-related protein 2 (glucanases) | ||
| PR-4 | Pathogenesis-related protein 4 (hevein-like) | ||
| PR-5 | Pathogenesis-related protein 5 (thaumatin-like, osmotin) | ||
| PR-8 | Pathogenesis-related protein 8 (class III chitinase) | ||
| PR-14 | Pathogenesis-related protein 14 (lipid transfer protein) | ||
| PR-15 | Pathogenesis-related protein 15 (oxalate oxidase) | ||
| Phenylpropanoids | PAL | Phenylalanine ammonia-lyase | |
| CHS | Chalcone synthase | ||
| DFR | Dihydroflavonol reductase | ||
| ANS | Anthocyanidin synthase | ||
| PPO | Polyphenol oxidase | ||
| Isoprenoids | HMGR | Hydroxymethyl glutarate-CoA reductase | |
| FPPS | Farnesyl pyrophosphate synthase | ||
| Far | (E,E)-alpha-farnesene synthase | ||
| Cysteines | CSL | Alliinase | |
| Oxidative stress | APOX | Ascorbate peroxidase | |
| GST | Glutathion S-transférase | ||
| POX | Peroxidase | ||
| Parietal modification | CalS | Callose synthase | |
| Pect | Pectin methyl esterase | ||
| CAD | Cinnamyl alcohol dehydrogenase | ||
| Hormonal signaling | Salicylic acid (SA) | EDS1 | Disease resistance protein EDS 1 |
| WRKY | WRKY transcription factor 30 | ||
| Jasmonic acid (JA) | LOX2 | Lipoxygenase AtLOX2 | |
| JAR | Jasmonate resistant 1 | ||
| Ethylene (ET) | ACCO | 1-aminocyclopropene-1-carboxylate oxidase | |
| EIN3 | EIN3-BINDING F BOX PROTEIN 1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demanèche, S.; Mirabel, L.; Abbe, O.; Eberst, J.-B.; Souche, J.-L. A New Active Substance Derived from Lyzed Willaertia magna C2c Maky Cells to Fight Grapevine Downy Mildew. Plants 2020, 9, 1013. https://doi.org/10.3390/plants9081013
Demanèche S, Mirabel L, Abbe O, Eberst J-B, Souche J-L. A New Active Substance Derived from Lyzed Willaertia magna C2c Maky Cells to Fight Grapevine Downy Mildew. Plants. 2020; 9(8):1013. https://doi.org/10.3390/plants9081013
Chicago/Turabian StyleDemanèche, Sandrine, Laurène Mirabel, Olivier Abbe, Jean-Baptiste Eberst, and Jean-Luc Souche. 2020. "A New Active Substance Derived from Lyzed Willaertia magna C2c Maky Cells to Fight Grapevine Downy Mildew" Plants 9, no. 8: 1013. https://doi.org/10.3390/plants9081013
APA StyleDemanèche, S., Mirabel, L., Abbe, O., Eberst, J.-B., & Souche, J.-L. (2020). A New Active Substance Derived from Lyzed Willaertia magna C2c Maky Cells to Fight Grapevine Downy Mildew. Plants, 9(8), 1013. https://doi.org/10.3390/plants9081013





