Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max)
Abstract
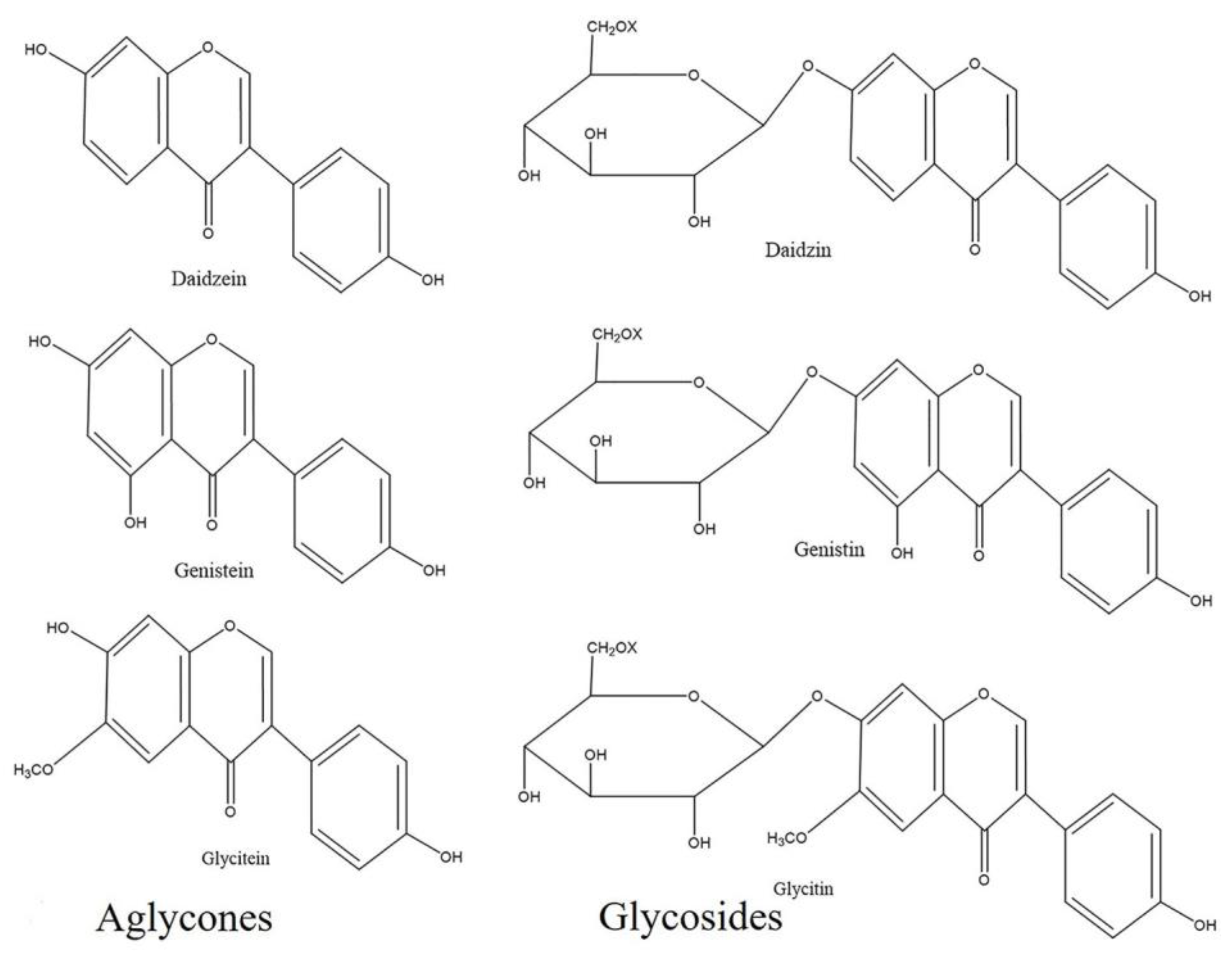
:1. Introduction
2. Results
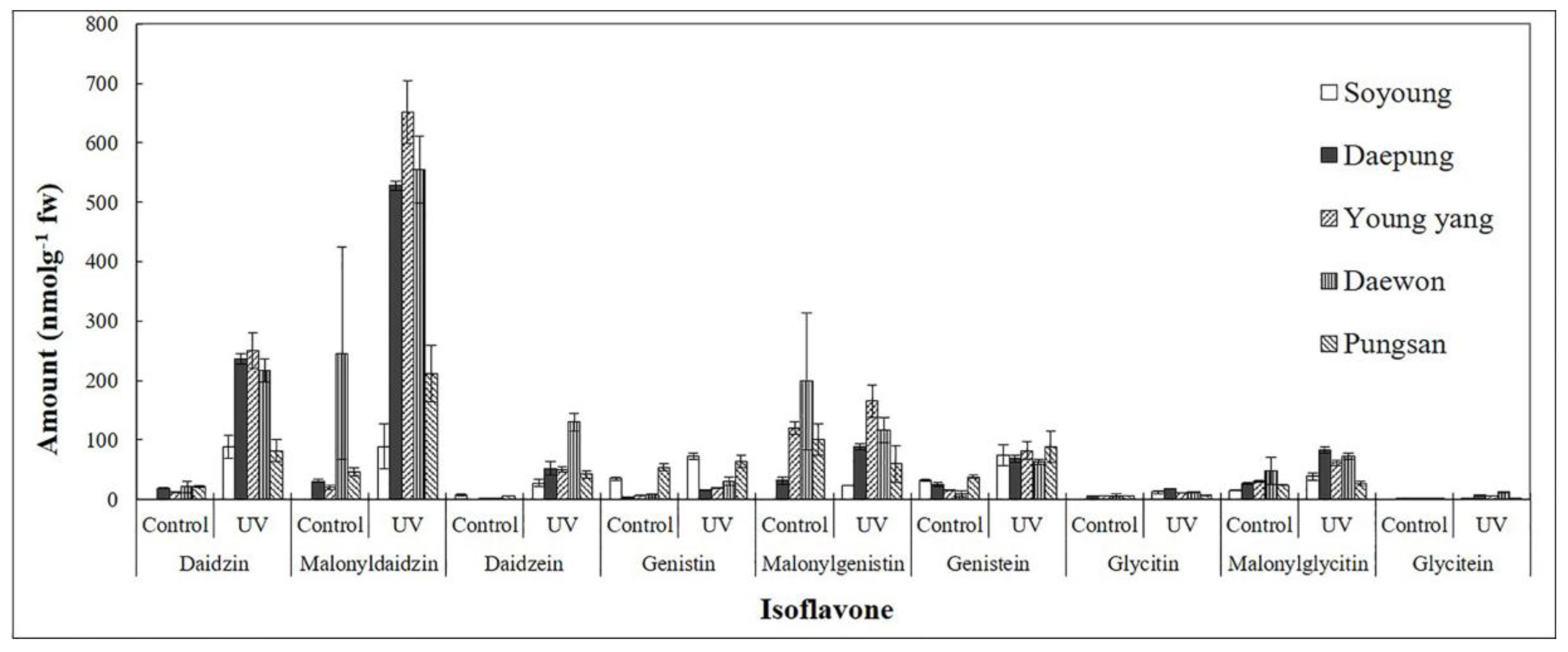
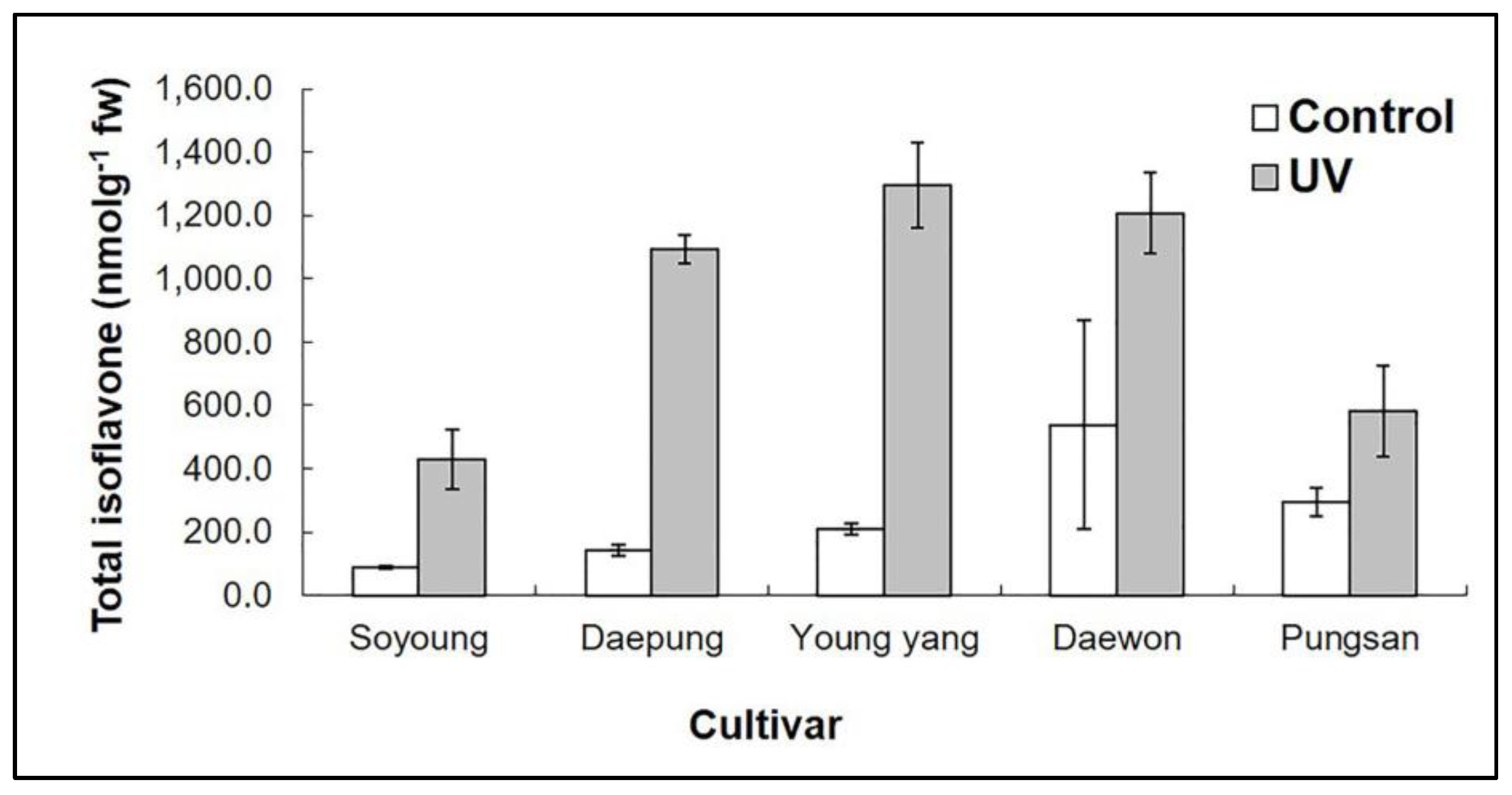
2.1. Effect of UV-C on Isoflavone Concentrations in Different Cultivars of Soybean
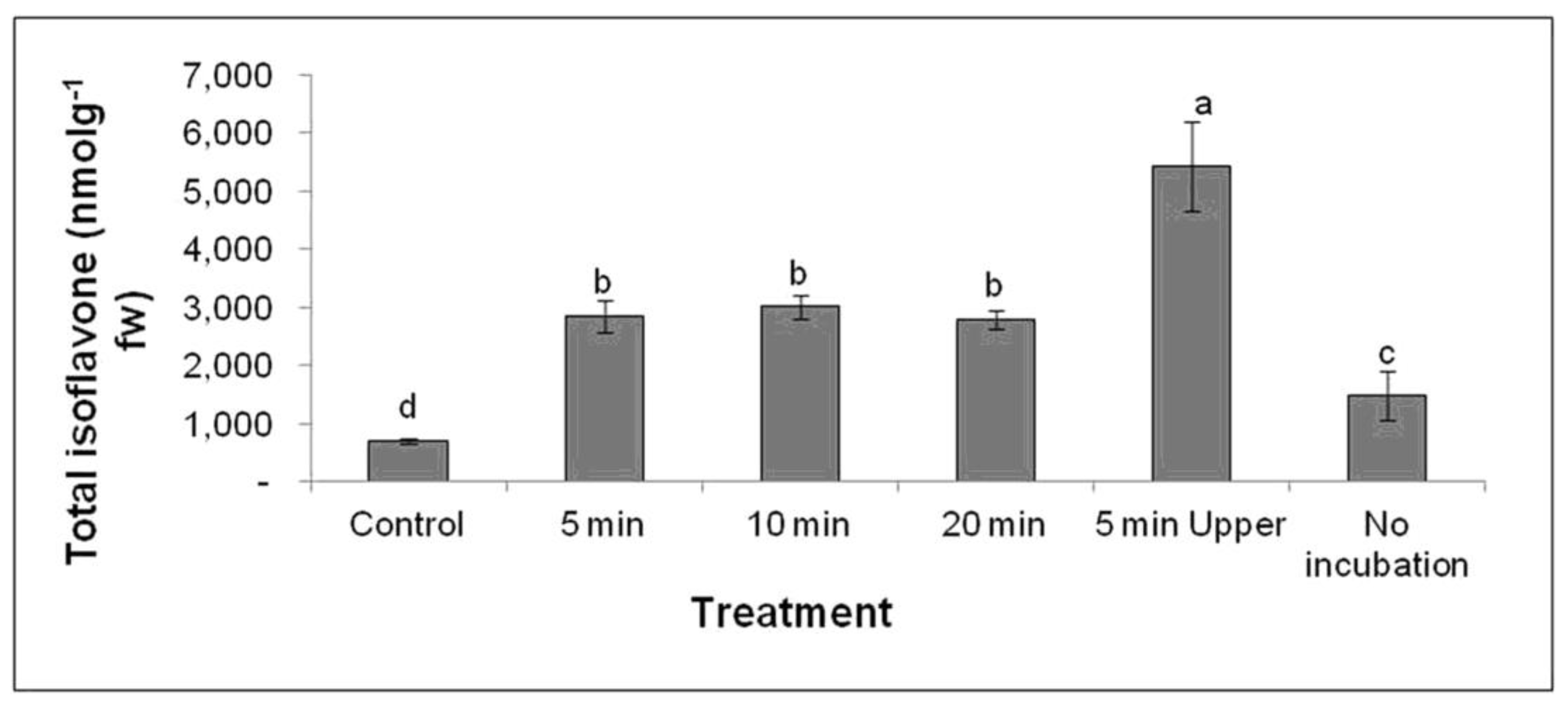
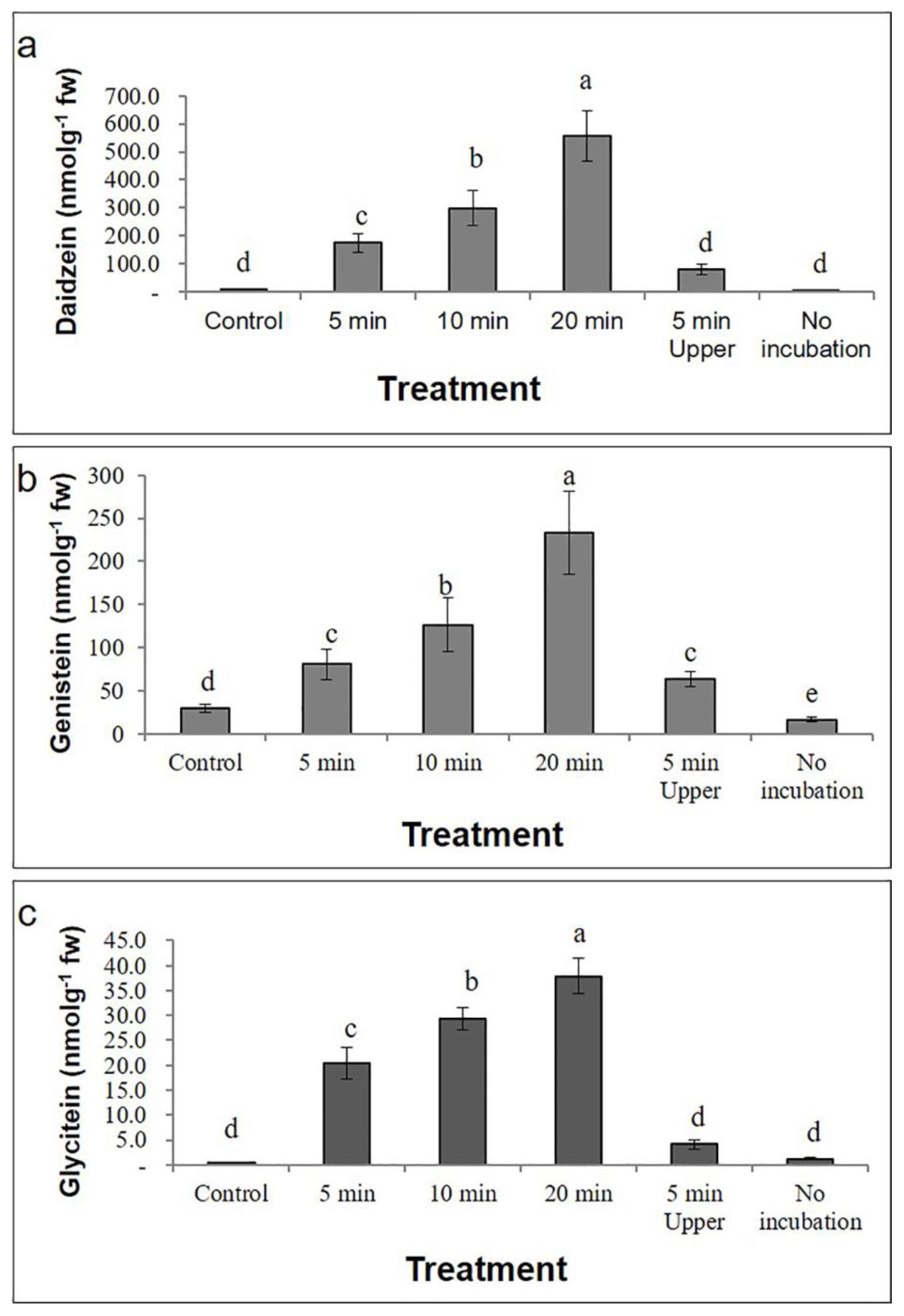
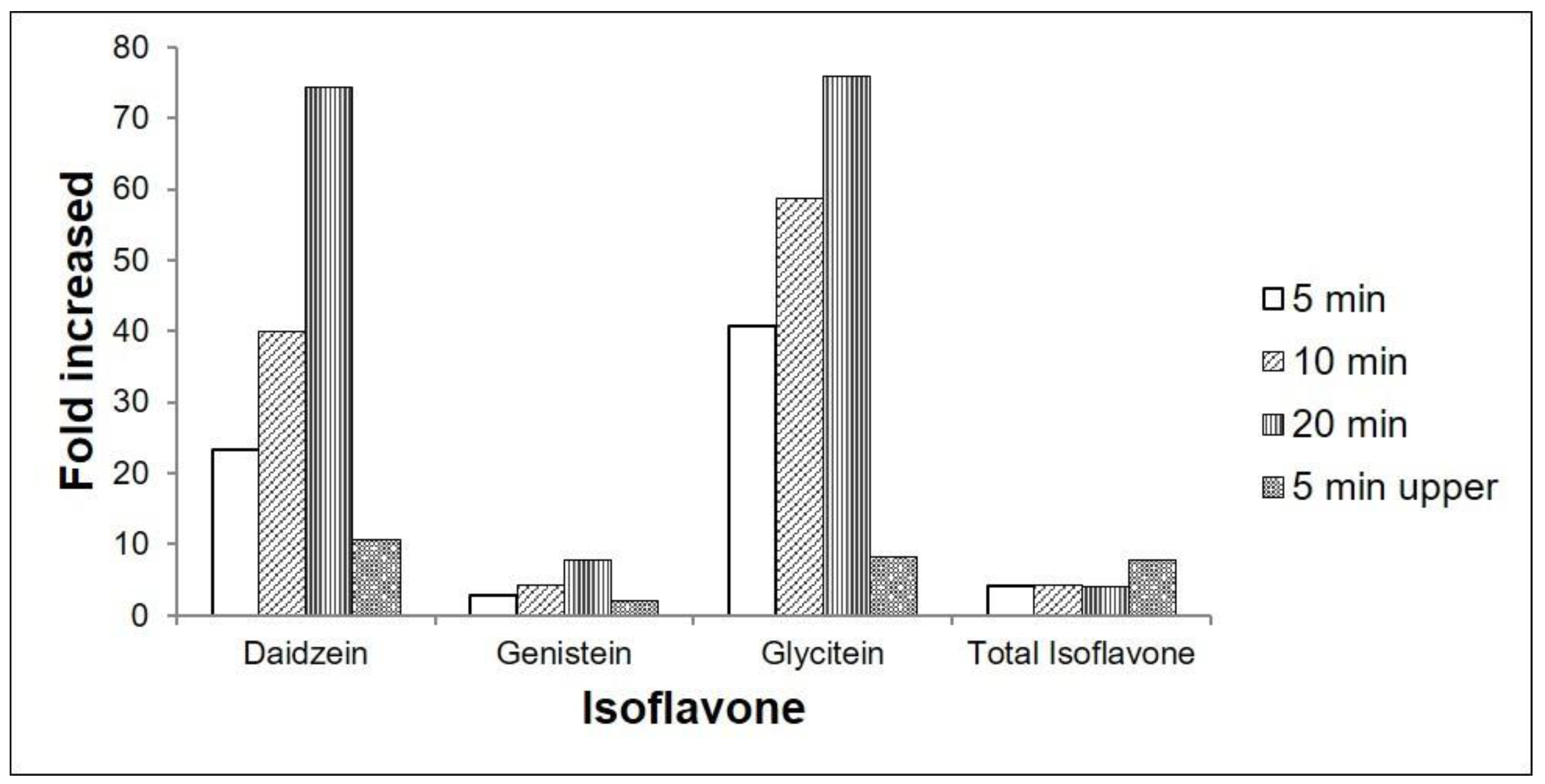
2.2. Effect of UV-C on Isoflavone Concentration in G. max cv. Daepung
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Ultraviolet-C Irradiation
4.3. Preparation of Plant Extracts
4.4. Quantification of Isoflavonoids by LC–MS Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, B.; Ribes, A.M.; Leandro, M.C.; Belchior, A.P.; Spranger, M.I. Stilbenes: Quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal. Chim. Acta 2006, 563, 382–390. [Google Scholar] [CrossRef]
- Mao, B.; Yin, H.; Wang, Y.; Zhao, T.-H.; Tian, R.-R.; Wang, W.; Ye, J.-S. Combined effects of O3 and UV radiation on secondary metabolites and endogenous hormones of soybean leaves. PLoS ONE 2017, 12, e0183147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Drouet, S.; Hano, C.; Abbasi, B.H. Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum L. Int. J. Mol. Sci. 2019, 20, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-Réguillon, A.; Hano, C. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. B Biol. 2017, 167, 216–227. [Google Scholar] [CrossRef]
- Loyall, L.; Uchida, K.; Braun, S.; Furuya, M.; Frohnmeyer, H. Glutathione and a UV light-induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 2000, 12, 1939–1950. [Google Scholar]
- Katerova, Z.; Ivanov, S.; Mapelli, S.; Alexieva, V. Phenols, proline and low-molecular thiol levels in pea (Pisum sativum) plants respond differently toward prolonged exposure to ultraviolet-B and ultraviolet-C radiations. Acta Physiol. Plant 2009, 31, 111–117. [Google Scholar] [CrossRef]
- Katerova, Z.; Todorova, D. Effect of enhanced UV-C irradiation on the growth, malondialdehyde, hydrogen peroxide, free proline, polyamines, IAA and IAA-oxidase activity in pea plants (Pisum sativum L.). Comptes Rendus L’Academie Bulg. Sci. 2011, 64, 1555–1562. [Google Scholar]
- Rai, R.; Meena, R.P.; Smita, S.S.; Shukla, A.; Rai, S.K.; Pandey-Rai, S. UV-B and UV-C pre-treatments induce physiological changes and artemisinin biosynthesis in Artemisia annua L.—An antimalarial plant. J. Photochem. Photobiol. B Biol. 2011, 105, 216–225. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Zacchini, M.; De Agazio, M. Spread of oxidative damage and antioxidative response through cell layers of tobacco callus after UV-C treatment. Plant Physiol. Biochem. 2004, 42, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Wilhelmová, N. The capacity of antioxidant protection during modulated ageing of bean (Phaseolus vulgaris L.) cotyledons. 1. The antioxidant enzyme activities. Cell Biochem. Funct. 2007, 25, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Inoue, T.; Takemura, K.; Hada, M.; Takahashi, S.; Ioki, M.; Nakajima, N.; Kondo, N. Induction and inhibition of cyclobutane pyrimidine dimer photolyase in etiolated cucumber (Cucumis sativus) cotyledons after ultraviolet irradiation depends on wavelength. J. Plant Res. 2007, 120, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Gallois, P. UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett. 1998, 437, 131–136. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Shono, T.; Takasugi, M.; Shirata, A.; Fujimura, T.; Machii, H. Mulberry moracins: Scavengers of UV stress-generated free radicals. Biosci. Biotechnol. Biochem. 2001, 65, 1402–1405. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Stapleton, A.E.; Yanovsky, M.J.; Scopel, A.L. Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiol. 1996, 112, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Choi, S.J.; Baek, K.H. Application of ultraviolet c irradiation for the increased production of secondary metabolites in plants. J. Anim. Plant Sci. 2020, 30, 1082–1091. [Google Scholar]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M. Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 2019, 256, 279–290. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef]
- Brössner, C.; Petritsch, K.; Fink, K.; Auprich, M.; Madersbacher, S.; Adlercreutz, H.; Rehak, P.; Petritsch, P. Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology 2004, 64, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.M.; Hoikkala, A.; Wähälä, K.; Adlercreutz, H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J. Steroid Biochem. Mol. Biol. 2003, 87, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.J.; Ka, M.N.; Luo, K.Q. Extraction and purification of isoflavones from soybeans and characterization of their estrogenic activities. J. Agric. Food Chem. 2007, 55, 6940–6950. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breinholt, V.; Larsen, J.C. Detection of weak estrogenic flavonoids using a recombinant yeast strain and a modified MCF7 cell proliferation assay. Chem. Res. Toxicol. 1998, 11, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.M.; McLachlan, J.A.; Arnold, S.F. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids 1997, 62, 365–372. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dietz, V.H. Occurrence and distribution of free flavonoid aglycones in plants. Phytochemistry 1981, 20, 869–932. [Google Scholar] [CrossRef]
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill). Agric. Biol. Chem. 1991, 55, 2227–2233. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, M.A.; Vanveldhuizen, P.J.; Thrasher, J.B. Effects of soy phytoestrogens on the prostate. Prostate Cancer Prostatic Dis. 2007, 10, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, A.A.; Custer, L.J.; Cerna, C.M.; Narala, K.K. Quantitation of phytoestrogens in legumes by HPLC. J. Agric. Food Chem. 1994, 42, 1905–1913. [Google Scholar] [CrossRef]
- Lee, K.H.; Xiao, Z. Lignans in treatment of cancer and other diseases. Phytochem. Rev. 2003, 2, 341–362. [Google Scholar] [CrossRef]
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative transcriptome analysis of waterlogging-sensitive and tolerant zombi pea (Vigna Vexillata) reveals energy conservation and root plasticity controlling waterlogging tolerance. Plants 2019, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Graham, T.L. Biosynthesis and distribution of phytoestrogens and their roles in plant defense, signal transduction, and cell-to-cell signaling. J. Med. Food 1999, 2, 93–97. [Google Scholar] [CrossRef]
- Reinli, K.; Block, G. Phytoestrogen content of foods—A compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 2011, 83, 2223–2235. [Google Scholar] [CrossRef]
- Barnes, S. The chemopreventive properties of soy isoflavonoids in animal models of breast cancer. Breast Cancer Res. Treat. 1997, 46, 169–179. [Google Scholar] [CrossRef]
- Messina, M.; Barnes, S. The role of soy products in reducing risk of cancer. J. Natl. Cancer Inst. 1991, 83, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Adlercreutz, H. Phytoestrogens: Epidemiology and a possible role in cancer protection. Environ. Health Perspect. 1995, 103, 103–112. [Google Scholar] [PubMed]
- Adlercreutz, H. Phyto-oestrogens and cancer. Lancet Oncol. 2002, 3, 364–373. [Google Scholar] [CrossRef]
- Strauss, L.; Santti, R.; Saarinen, N.; Streng, T.; Joshi, S.; Makela, S. Dietary phytoestrogens and their role in hormonally dependent disease. Toxicol. Lett. 1998, 102–103, 349–354. [Google Scholar] [CrossRef]
- Zhou, J.R.; Gugger, E.T.; Tanaka, T.; Guo, Y.; Blackburn, G.L.; Clinton, S.K. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999, 129, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.; Stoll, M.; Schapaugh, W.; Takemoto, L. Isoflavone content of Kansas soybeans. Am. J. Undergrad. Res. 2004, 2, 27–32. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Bou, S.M.; Shih, F.; Shih, B.Y.; Daigle, K.W.; Carter-Wientjes, C.H.; Cleveland, T.E. Effect of biotic elicitors on enrichment of antioxidant properties and induced isoflavones in soybean. J. Food Sci. 2008, 73, 43–49. [Google Scholar] [CrossRef]
- Cantos, E.; Garcia-Viguera, C.; De Pascual-Teresa, S.; Tomas-Barberan, F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef]
- Cetin, E.S. Induction of secondary metabolite production by UV-C radiation in Vitis vinifera L. Öküzgözü callus cultures. Biol. Res. 2014, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Palma, M.; Cantos-Villar, E. Induction of stilbenes in grapes by UV-C: Comparison of different subspecies of Vitis. Innov. Food Sci. Emerg. Technol. 2010, 11, 231–238. [Google Scholar] [CrossRef]
- Katerova, Z.; Todorova, D.; Tasheva, K.; Sergiev, I. Influence of ultraviolet radiation on plant secondary metabolite production. Genet. Plant Physiol. 2012, 2, 113–144. [Google Scholar]
- De Rijke, E.; Zafra-Gómez, A.; Ariese, F.; Brinkman, U.A.T.; Gooijer, C. Determination of isoflavone glucoside malonates in Trifolium pratense L. (red clover) extracts: Quantification and stability studies. J. Chromatogr. A 2001, 932, 55–64. [Google Scholar] [CrossRef]
- Tumova, L.; Tuma, J. The effect of UV light on isoflavonoid production in Genista tinctoria culture in vitro. Acta Physiol. Plant 2011, 33, 635–640. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Dayde, J.; Widholm, J.M. Effect of temperature and soil moisture status during seed development on soybean seed isoflavone concentration and composition. Crop Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Hettiarachchy, N.; Chen, P.; Horax, R.; Cornelious, B.; Zhu, D. Influence of the application of three different elicitors on soybean plants on the concentrations of several isoflavones in soybean seeds. J. Agric. Food Chem. 2006, 54, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Devi, M.K.A.; Giridhar, P.; Gokare, R.; Bot, A. Augmentation of major isoflavones in Glycine max L. through the elicitor-mediated approach. Acta Bot. Croat. 2013, 72, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Jeon, E.H.; Pak, J.H.; Ha, T.J.; Baek, I.Y.; Jung, W.S.; Lee, J.H.; Kim, D.H.; Choi, H.K.; Cui, Z.; et al. Increase of isoflavones in soybean callus by Agrobacterium-mediated transformation. Plant Biotechnol. Rep. 2010, 4, 253–260. [Google Scholar] [CrossRef]
- Jeng, T.L.; Shih, Y.J.; Wu, M.T.; Wang, C.S.; Sung, J.M. Evaluations and selections for high isoflavone black soybean mutants induced by NaN3 treatment. Am. J. Plant Sci. 2013, 4, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: A new “Functional” fruit? J. Agric. Food Chem. 2001, 49, 5052–5058. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.; He, Y.; Jiang, A.; Zhang, R. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biol. Technol. 2016, 111, 126–131. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wang, X.; Lin, Q.; Liu, W.; Xie, X.; Wang, Z.; Guan, W. Effects of UV-C irradiation on the physiological and antioxidant responses of button mushrooms (Agaricus bisporus) during storage. Int. J. Food Sci. Technol. 2016, 51, 1502–1508. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Ma, L.; Liu, G.; Wang, N.; Wang, J.; Wang, L.; Dai, Z.; Li, S.; Wang, L. Transcriptomic analysis of grape (Vitis vinifera L.) leaves after exposure to ultraviolet C irradiation. PLoS ONE 2014, 9, e113772. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Collison, M.W. Determination of total soy isoflavones in dietary supplements, supplement ingredients, and soy foods by high-performance liquid chromatography with ultraviolet detection: Collaborative study. J. AOAC Int. 2008, 91, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Mahdavian, K.; Kalantari, K.M.; Ghorbanli, M.; Torkzade, M. The effects of salicylic acid on pigment contents in ultraviolet radiation stressed pepper plants. Biol. Plant. 2008, 52, 170–172. [Google Scholar] [CrossRef]
- Balouchi, H.R.; Sanavy, S.A.M.M.; Emam, Y.; Dolatabadian, A. UV radiation, elevated CO2 and water stress effect on growth and photosynthetic characteristics in durum wheat. Plant Soil Environ. 2009, 55, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Ma, L.; Xi, H.F.; Wang, L.J.; Li, S.H. Resveratrol synthesis under natural conditions and after UV-C irradiation in berry skin is associated with berry development stages in “Beihong” (V. vinifera × V. amurensis). Food Chem. 2015, 168, 430–438. [Google Scholar] [CrossRef]
- Bravo, S.; García-Alonso, J.; Martín-Pozuelo, G.; Gómez, V.; Santaella, M.; Navarro-González, I.; Periago, M.J. The influence of post-harvest UV-C hormesis on lycopene, β-carotene, and phenolic content and antioxidant activity of breaker tomatoes. Food Res. Int. 2012, 49, 296–302. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, C.T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Yuan, L.; Guan, W.-Q.; Brennan, C.S.; Zhang, Y.-Y.; Zhang, J.; Wang, Z.-D. The influence of postharvest UV-C treatment on anthocyanin biosynthesis in fresh-cut red cabbage. Sci. Rep. 2017, 7, 5232. [Google Scholar] [CrossRef]
- Yin, X.; Singer, S.D.; Qiao, H.; Liu, Y.; Jiao, C.; Wang, H.; Li, Z.; Fei, Z.; Wang, Y.; Fan, C.; et al. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) cv. “Tonghua-3”. Front. Plant Sci. 2016, 7, 503. [Google Scholar]
- Sheng, K.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Effect of postharvest UV-B or UV-C irradiation on phenolic compounds and their transcription of phenolic biosynthetic genes of table grapes. J. Food Sci. Technol. 2018, 55, 3292–3302. [Google Scholar] [CrossRef]
- Takayanagi, T.; Okuda, T.; Mine, Y.; Yokotsuka, K. Induction of resveratrol biosynthesis in skins of three grape cultivars by ultraviolet irradiation. J. Jpn. Soc. Hort. Sci. 2004, 73, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Tang, K.; Yang, H.R.; Wen, P.F.; Zhang, P.; Wang, H.L.; Huang, W.D. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol. Biochem. 2010, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Yu, O.; Lau, S.M.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 2017, 58, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karki, K.B.; Mishra, A.K.; Choi, S.-J.; Baek, K.-H. Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max). Plants 2020, 9, 1043. https://doi.org/10.3390/plants9081043
Karki KB, Mishra AK, Choi S-J, Baek K-H. Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max). Plants. 2020; 9(8):1043. https://doi.org/10.3390/plants9081043
Chicago/Turabian StyleKarki, Krishna Bahadur, Awdhesh Kumar Mishra, Seong-Jin Choi, and Kwang-Hyun Baek. 2020. "Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max)" Plants 9, no. 8: 1043. https://doi.org/10.3390/plants9081043
APA StyleKarki, K. B., Mishra, A. K., Choi, S.-J., & Baek, K.-H. (2020). Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max). Plants, 9(8), 1043. https://doi.org/10.3390/plants9081043








