Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages
Abstract
:1. Introduction
2. Results
2.1. Content of Phenolic Compounds
2.1.1. Total Phenolic Compounds Content
2.1.2. Total Tannins Content
2.1.3. Total Flavonoids Content
2.1.4. Total Anthocyanins Content
2.1.5. Proanthocyanidins Content
2.1.6. Total Hydroxycinnamic Acids Content
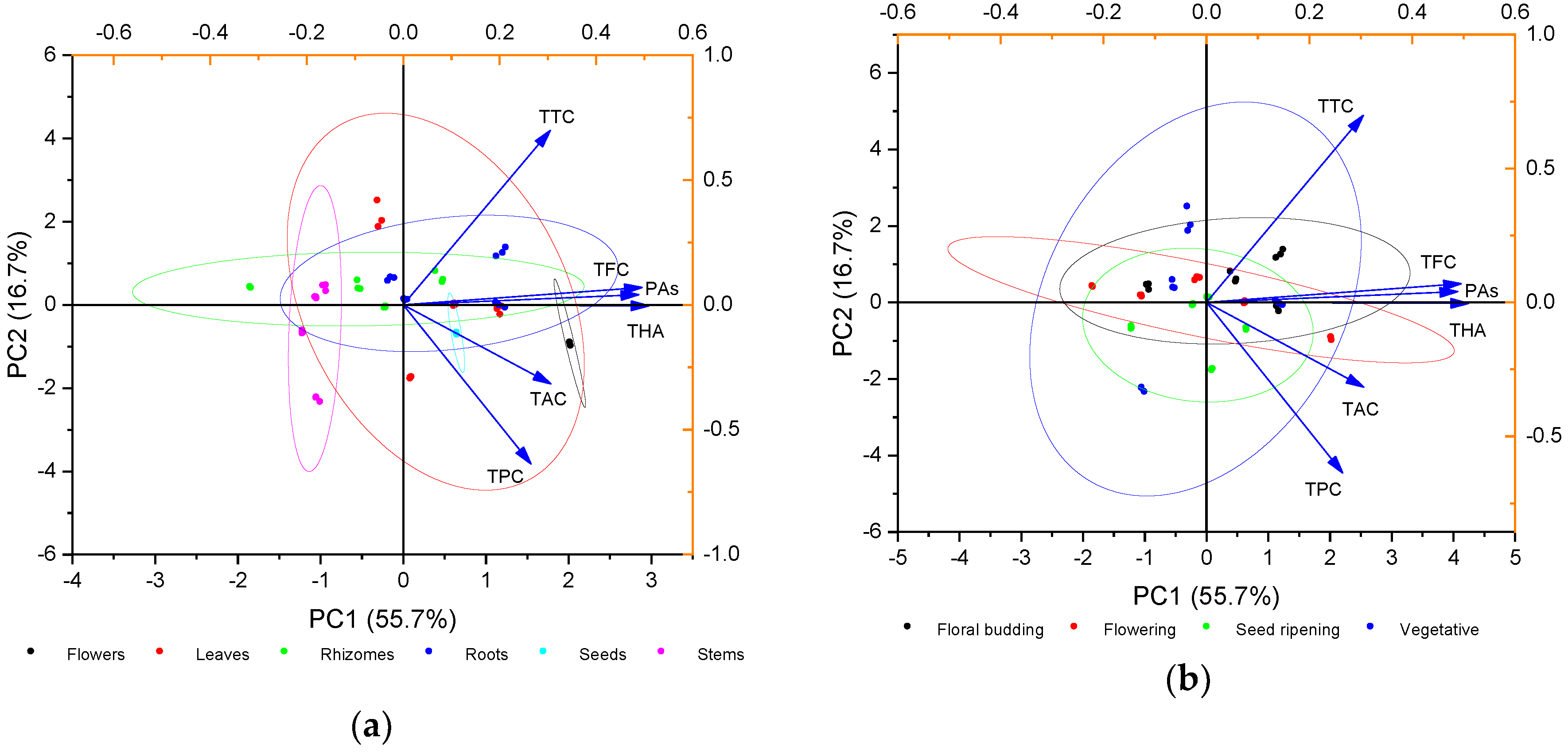
2.2. Principal Component Analysis (PCA) of Phenolic Compounds Content
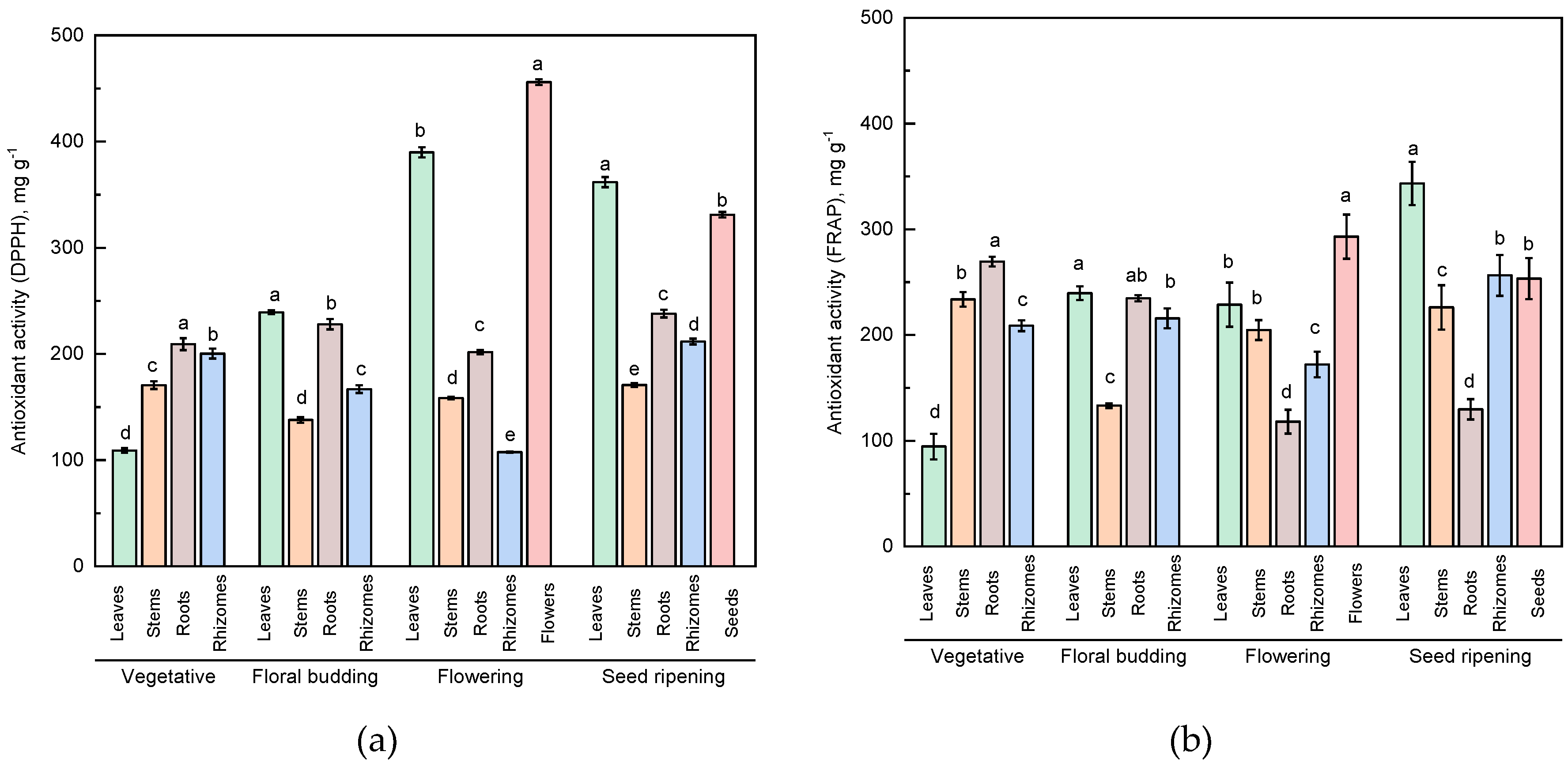
2.3. Antioxidant Activity
2.4. Antibacterial Activity
3. Discussion
4. Materials and Methods
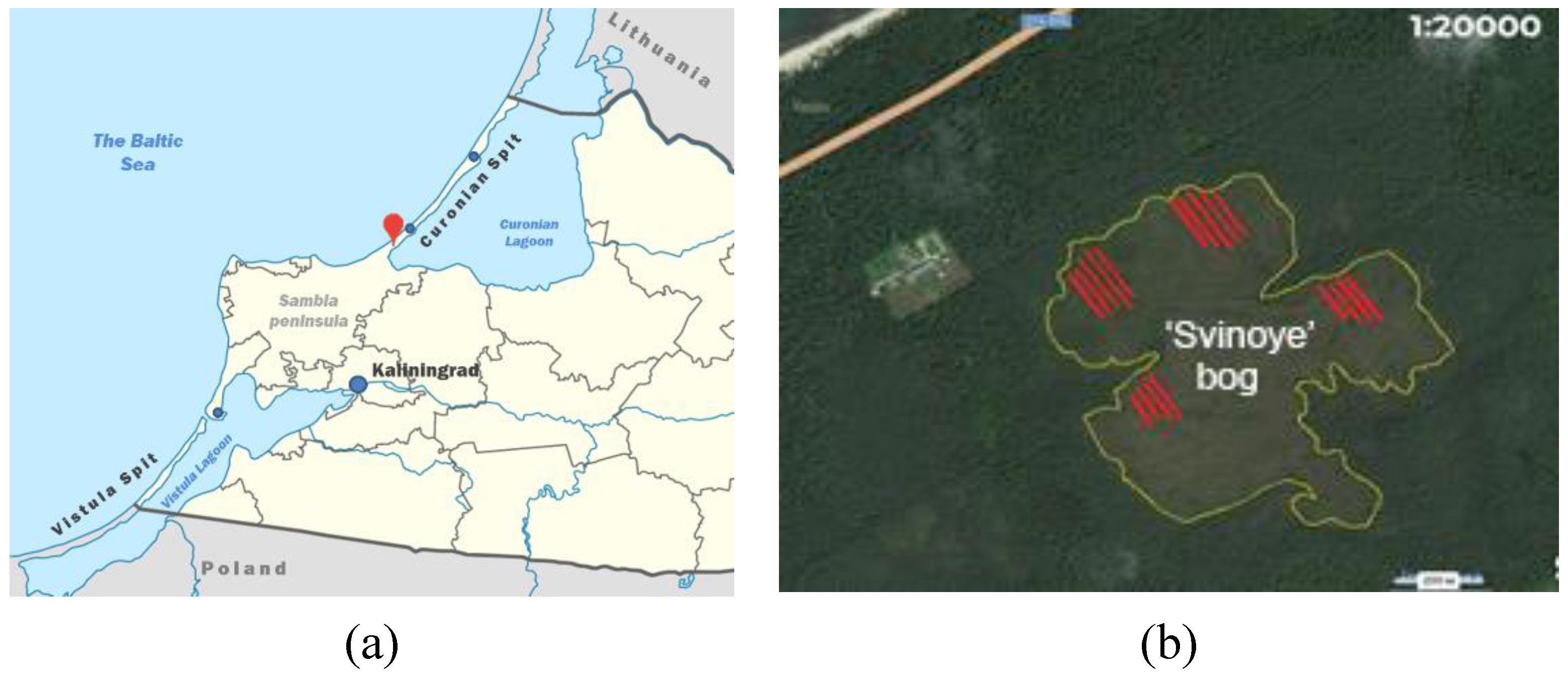
4.1. Plant Material
4.2. Determination of Phenolic Compounds
4.2.1. Extraction of Phenolic Compounds
4.2.2. Total Phenolics Content (TPC)
4.2.3. Total Tannins Content (TTC)
4.2.4. Total Flavonoids Content (TFC)
4.2.5. Total Anthocyanins Content (TAC)
4.2.6. Total Proanthocyanidins Content (PAs)
4.2.7. Total Hydroxycinnamic Acids Content (THA)
4.3. Assessment of Antioxidant Activity (AOA)
4.4. Assessment of Antibacterial Activity
4.4.1. Plant Extract Preparation
4.4.2. Bacteria Culture
4.4.3. Determination of the Antibacterial Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Main Effects | Factors | TPC 1 | TTC | TFC | TAC | PAs | THA | AOA (DPPH) | AOA (FRAP) |
|---|---|---|---|---|---|---|---|---|---|
| Plant part (P) | Leaves | 249 b | 15.0 c | 214 a | 1.16 a | 6.8 b | 4.8 a | 275 a | 227 a |
| Stems | 229 d | 10.6 d | 98 d | 0.46 b | 5.5 d | 3.2 d | 159 d | 199 b | |
| Roots | 265 a | 18.7 a | 186 b | 0.23 d | 7.9 a | 4.6 b | 219 b | 188 b | |
| Rhizomes | 240 c | 15.7 b | 122 c | 0.26 c | 6.3 c | 3.4 c | 171 c | 213 a | |
| Growth stage (G) | Vegetative | 228 c | 13.7 d | 167 b | 0.45 d | 6.4 b | 4.3 a | 172 d | 202 b |
| Floral budding | 250 b | 16.5 a | 210 a | 0.49 c | 8.2 a | 4.3 a | 193 c | 206 b | |
| Flowering | 231 c | 15.3 b | 126 c | 0.61 a | 5.6 c | 3.6 c | 214 b | 181 c | |
| Seed ripening | 274 a | 14.5 c | 116 d | 0.56 b | 6.4 b | 3.7 b | 246 a | 239 a | |
| Significance | P | * | * | * | * | * | * | * | * |
| G | * | * | * | * | * | * | * | * | |
| P*G | * | * | * | * | * | * | * | * |
References
- Beil, I.; Buras, A.; Hallinger, M.; Smiljanić, M.; Wilmking, M. Shrubs tracing sea surface temperature—Calluna vulgaris on the Faroe Islands. Int. J. Biometeorol. 2015, 59, 1567–1575. [Google Scholar] [CrossRef]
- Behrend, A.; Hohe, A. Inheritance of the ‘Bud Bloomer’ trait in Calluna vulgaris. Acta Hortic. 2016, 1127, 251–258. [Google Scholar] [CrossRef]
- Spindelböck, J.P.; Cook, Z.; Daws, M.I.; Heegaard, E.; Måren, I.E.; Vandvik, V. Conditional cold avoidance drives between-population variation in germination behaviour in Calluna vulgaris. Ann. Bot. 2013, 112, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delerue, F.; Gonzalez, M.; Achat, D.L.; Puzos, L.; Augusto, L. Competition along productivity gradients: News from heathlands. Oecologia 2018, 187, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Šoltés, R.; Gregušková, E.; Šoltésová, A. Bioindication of chemical elements deposition in the high Tatra mts (Slovakia) based on Calluna vulgaris (L.) Hull; comparative levels after the improvement of emissions. Carpathian J. Earth Environ. Sci. 2014, 9, 5–14. [Google Scholar]
- Wadekar, J.B.; Sawant, R.L.; Patal, U.B. Rheumatoid arthritis and herbal drugs: A review. J. Phytopharm. 2015, 4, 311–318. [Google Scholar]
- Moreira, T.; Santos, J.; Costa, A.S.G.; Pinto, D.; Pimentel, F.; Oliveira, A.I.; Rodrigues, F.; Beatriz, M.; Oliveira, P.P. Valorization of Calluna vulgaris (L.) Hull as a potential cosmetic ingredient: Characterization, antioxidante activity and cell viability study. In Proceedings of the Livro de Atas do XIII Encontro de Química dos Alimentos, Porto, Portugal, 14–16 September 2016; pp. 39–42. [Google Scholar]
- Orhan, I.; Küpeli, E.; Terzioğlu, S.; Yesilada, E. Bioassay-guided isolation of kaempferol-3-O-β-D-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L. J. Ethnopharmacol. 2007, 114, 32–37. [Google Scholar] [CrossRef]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. In vitro activity of heather (Calluna vulgaris (L.) Hull) extracts on selected urinary tract pathogens. Bosn. J. Basic Med. Sci. 2014, 14, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, A.K.; Gauguin, B.; Andersen, J.; Adsersen, A.; Gudiksen, L. Screening of plants used in Danish folk medicine to treat depression and anxiety for affinity to the serotonin transporter and inhibition of MAO-A. J. Ethnopharmacol. 2013, 145, 822–825. [Google Scholar] [CrossRef]
- Saaby, L.; Rasmussen, H.B.; Jäger, A.K. MAO-A Inhibitory Activity of Quercetin from Calluna vulgaris (L.) Hull. J. Ethnopharmacol. 2009, 121, 178–181. [Google Scholar] [CrossRef]
- Dróżdż, P.; Sentkowska, A.; Pyrzynska, K. Flavonoid content and antioxidant properties in different extracts of Calluna vulgaris (L.) flowers. J. Agric. Sci. Technol. A 2017, 7, 39–44. [Google Scholar] [CrossRef] [Green Version]
- García-Risco, M.R.; Vázquez, E.; Sheldon, J.; Steinmann, E.; Riebesehl, N.; Fornari, T.; Reglero, G. Supercritical fluid extraction of heather (Calluna vulgaris) and evaluation of anti-hepatitis C virus activity of the extracts. Virus Res. 2015, 198, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pironi, A.M.; de Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip, G.A.; Postescu, D.; Tatomir, C.; Muresan, A.; Clichici, S. Calluna vulgaris extract modulates NF-κB/ERK signaling pathway and matrix metalloproteinase expression in SKH-1 hairless mice skin exposed to ultraviolet B irradiation. J. Physiol. Pharmacol. 2012, 63, 423–432. [Google Scholar]
- Kalemba-Drożdż, M.; Cierniak, A. Antioxidant and genoprotective properties of extracts from edible flowers. J. Food Nutr. Res. 2019, 58, 42–50. [Google Scholar]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Monschein, M.; Neira, J.I.; Kunert, O.; Bucar, F. Phytochemistry of heather (Calluna vulgaris (L.) Hull) and its altitudinal alteration. Phytochem. Rev. 2010, 9, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Moreira, T.; Pinto, D.; Pimentel, F.B.; Costa, A.S.; Nunes, M.A.; Albuquerque, T.G.; Costa, H.S.; Palmeira-de-Oliveira, A.; Oliveira, A.I.; et al. The phytochemical and bioactivity profiles of wild Calluna vulgaris L. flowers. Food Res. Int. 2018, 111, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Szakiel, A.; Niżyński, B.; Pączkowski, C. Triterpenoid profile of flower and leaf cuticular waxes of heather Calluna vulgaris. Nat. Prod. Res. 2013, 27, 1404–1407. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, Z.B.; Song, P.; Hao, J.Y.; Zhang, D.Y.; Zhang, Y.F. Different harvest seasons modify bioactive compounds and antioxidant activities of Pyrola incarnata. Ind. Crop. Prod. 2016, 94, 405–412. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.A.; de Macêdo, D.G.; Boligon, A.A.; Menezes, I.R.A.; de Almeida Souza, M.M.; da Costa, J.G.M. Influence of seasonality on the phenolic composition of Secondatia floribunda A. DC (Apocynaceae) during its phenological cycle. Acta Physiol. Plant. 2019, 41, 185. [Google Scholar] [CrossRef]
- Rieger, G.; Müller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, W.; Li, Q.; Li, Y.; Luo, H.; Song, J.; Sun, C.; Qian, J.; Zhu, Y.; Hayward, A.; et al. Genome-wide identification of phenolic acid biosynthetic genes in Salvia miltiorrhiza. Planta 2015, 241, 711–725. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef]
- Jakovljevic, Z.D.; Stankovic, S.M.; Topuzovic, D.M. Seasonal variability of Chelidonium majus L. secondary metabolites content and antioxidant activity. EXCLI J. 2013, 12, 260–268. [Google Scholar]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; Da Cruz, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L.; et al. Influence of Harvest Season and Cultivar on the Variation of Phenolic Compounds Composition and Antioxidant Properties in Vaccinium ashei Leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef] [Green Version]
- Jalal, M.A.; Read, D.J.; Haslam, E. Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochemistry 1982, 21, 1397–1401. [Google Scholar] [CrossRef]
- Dróżdż, P.; Sentkowska, A.; Pyrzyńska, K. Biophenols and antioxidant activity in wild and cultivated heather. Nat. Prod. Res. 2017, 31, 1181–1184. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; Wiley-Blackwell: Oxford, UK, 2017; Volume 2, pp. 115–124. [Google Scholar]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Munakata, R.; Larbat, R.; Duriot, L.; Olry, A.; Gavira, C.; Mignard, B.; Bourgaud, F. Polyphenols from Plant Roots. Recent Adv. Polyphen. Res. 2019, 6, 207–236. [Google Scholar]
- Zaprometov, M.N. Biochemistry of phenolic compounds. Uspekhi Sovrem Biol. 1967, 63, 380–399. [Google Scholar]
- Pan, X.; Du, L.; Tao, J.; Jiang, S.; Qian, D.; Duan, J. Dynamic changes of flavonoids in Abelmoschus manihot different organs at different growth periods by UPLC–MS/MS. J. Chromatogr. B 2017, 1059, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugart, S.; Krumbein, A.; Zrenner, R. Influence of light and temperature on gene expression leading to accumulation of specific flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica). Front. Plant Sci. 2016, 7, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, B.S.; Bedair, M.F.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Yang, D.S.; Allen, S.N.; .Li, W.; Tang, Y.; Sumner, L.W. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015, 167, 1699–1716. [Google Scholar] [CrossRef] [Green Version]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Tech. 2011, 100, 12–35. [Google Scholar]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef] [Green Version]
- Govindasamy, C.; Srinivasan, R. In vitro antibacterial activity and phytochemical analysis of Catharanthus roseus (Linn.) G. Don. Asian Pac. J. Trop. Biomed. 2012, 2, 155–158. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure—Activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Padhi, E.M.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Gupta, C.; Verma, R. Visual estimation and spectrophotometric determination of tannin content and antioxidant activity of three common vegetable. Int. J. Pharm. Sci. Res. 2011, 2, 175–182. [Google Scholar]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.). Eur. J. Wood Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Sevket, A.L.P.; Ercisli, S.; Jurikova, T.; Cakir, O.; Gozlekci, S. Bioactive content of rose hips of different wildly grown Rosa dumalis genotypes. Not. Bot. Horti Agrobot. 2016, 44, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Chupakhina, G.N.; Shansky, M.; Parol, A.; Chupakhina, N.Y.; Feduraev, P.V.; Skrypnik, L.N.; Maslennikov, P.V. Comparative characteristics of antioxidant capacity of some forage plants of the Baltic Sea Region (a case study of the Kaliningrad Region and Estonia). Agron. Res. 2018, 16, 1976–1985. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crop. Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Štefan, M.B.; Rodríguez, J.V.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal. Methods 2014, 7, 326–336. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandim, F.; Barros, L.; Heleno, S.A.; Pires, T.C.; Dias, M.I.; Alves, M.J.; Santos, P.F.; Ferreira, I.C. Phenolic profile and effects of acetone fractions obtained from the inflorescences of Calluna vulgaris (L.) Hull on vaginal pathogenic and non-pathogenic bacteria. Food Funct. 2019, 10, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 2003, 84, 2347–2363. [Google Scholar] [CrossRef] [Green Version]

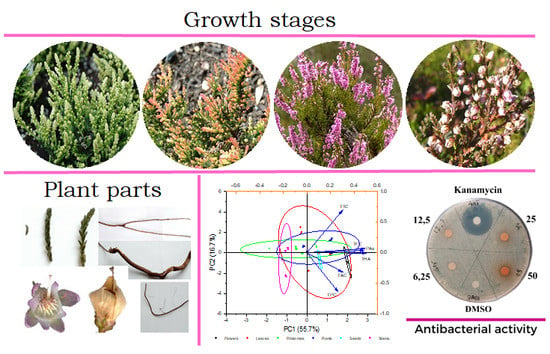
| Growth Stage | Plant Parts | Phenolic Compounds, mg g−1 | |||||
|---|---|---|---|---|---|---|---|
| TPC 1 | TTC | TFC | TAC | PAs | THA | ||
| Vegetative | Leaves | 14.52 ± 1.85d2 | 17.69 ± 0.39b | 172.7 ± 7.0b | 0.96 ± 0.043a | 5.80 ± 0.12c | 4.54 ± 0.08b |
| Stems | 27.46 ± 0.80b | 3.09 ± 0.30d | 120.5 ± 2.8d | 0.32 ± 0.007b | 6.27 ± 0.20b | 3.32 ± 0.09c | |
| Roots | 31.66 ± 0.53a | 18.16 ± 0.04a | 239.6 ± 8.3a | 0.26 ± 0.013bc | 7.75 ± 0.25a | 5.73 ± 0.10a | |
| Rhizomes | 24.53 ± 0.61c | 15.72 ± 0.33c | 136.9 ± 2.3c | 0.25 ± 0.008c | 5.87 ± 0.26c | 3.56 ± 0.09c | |
| Floral budding | Leaves | 28.15 ± 0.76a | 14.94 ± 0.45c | 305.6 ± 3.4a | 0.92 ± 0.043a | 8.82 ± 0.21bc | 4.73 ± 0.13a |
| Stems | 20.47 ± 0.34d | 12.39 ± 0.19d | 112.6 ± 5.8d | 0.30 ± 0.025c | 6.20 ± 0.11d | 3.17 ± 0.08c | |
| Roots | 27.58 ± 0.34b | 21.72 ± 0.86a | 245.7 ± 8.3b | 0.32 ± 0.015bc | 9.21 ± 0.15ab | 4.72 ± 0.04a | |
| Rhizomes | 25.35 ± 1.10c | 17.12 ± 0.30b | 176.2 ± 4.1c | 0.41 ± 0.006b | 8.45 ± 0.20c | 4.48 ± 0.13b | |
| Flowering | Leaves | 26.88 ± 0.11b | 15.79 ± 0.11c | 210.8 ± 2.2b | 1.17 ± 0.017b | 6.77 ± 0.21b | 5.34 ± 0.05b |
| Stems | 24.06 ± 0.15d | 14.95 ± 0.05d | 93.5 ± 0.6d | 0.81 ± 0.012c | 4.55 ± 0.20c | 3.11 ± 0.08d | |
| Roots | 26.18 ± 0.32c | 17.85 ± 0.41a | 139.4 ± 2.2c | 0.23 ± 0.002d | 6.95 ± 0.30b | 3.71 ± 0.10c | |
| Rhizomes | 20.23 ± 0.04e | 12.71 ± 0.09e | 58.7 ± 1.1e | 0.21 ± 0.005d | 3.98 ± 0.21d | 2.22 ± 0.08e | |
| Flowers | 27.88 ± 0.18a | 16.78 ± 0.12b | 263.2 ± 1.1a | 5.88 ± 0.026a | 9.18 ± 0.11a | 5.73 ± 0.12a | |
| Seed ripening | Leaves | 32.67 ± 0.12a | 11.66 ± 0.14e | 165.6 ± 3.5a | 1.56 ± 0.017b | 5.93 ± 0.26c | 4.44 ± 0.08b |
| Stems | 26.58 ± 0.19e | 11.95 ± 0.09d | 65.8 ± 3.3e | 0.42 ± 0.014c | 5.02 ± 0.31d | 2.97 ± 0.10e | |
| Roots | 28.77 ± 0.16d | 17.01 ± 0.09b | 119.3 ± 3.5c | 0.09 ± 0.003e | 7.67 ± 0.21a | 4.03 ± 0.07c | |
| Rhizomes | 30.13 ± 0.12b | 17.30 ± 0.05a | 115.0 ± 1.6d | 0.19 ± 0.007d | 7.06 ± 0.13b | 3.38 ± 0.09d | |
| Seeds | 29.77 ± 0.15c | 16.74 ± 0.11c | 145.9 ± 2.8b | 2.83 ± 0.021a | 7.24 ± 0.22b | 4.86 ± 0.07a | |
| Parameters | TPC 1 | TTC | TFC | TAC | PAs | THA | AOA (DPPH) | AOA (FRAP) |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.17 | 0.41 * | 0.22 | 0.55 ** | 0.45 * | 0.65 ** | 0.77 ** |
| TTC | 1 | 0.41 * | 0.11 | 0.48 * | 0.47 * | 0.15 | 0.09 | |
| TFC | 1 | 0.41 * | 0.81 ** | 0.86 ** | 0.52 ** | 0.37 * | ||
| TAC | 1 | 0.64 ** | 0.54 ** | 0.75 ** | 0.44 * | |||
| PAs | 1 | 0.74 ** | 0.47 * | 0.27 | ||||
| THA | 1 | 0.68 ** | 0.40 * | |||||
| AOA (DPPH) | 1 | 0.63 ** | ||||||
| AOA (FRAP) | 1 |
| Growth Stage | Plant Parts | Inhibition Zone, mm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | B. subtilis | ||||||||
| Extract Concentration, mg mL−1 | Extract Concentration, mg mL−1 | ||||||||
| 50 | 25 | 12.5 | 6.25 | 50 | 25 | 12.5 | 6.25 | ||
| Vegetative | Leaves | 9 (++) | 8 (+) | 7 (+) | – | – | – | – | – |
| Stems | 11 (+++) | 8 (+) | 8 (+) | 7 (+) | 7 (+) | 7 (+) | 6.5 (+) | – | |
| Roots | – | – | – | – | – | – | – | – | |
| Rhizomes | – | – | – | – | – | – | – | – | |
| Floral budding | Leaves | 9 (++) | 8 (+) | 8 (+) | 7 (+) | 10 (++) | 9 (++) | 8 (+) | – |
| Stems | 8 (+) | 7.5 (+) | 7 (+) | 6.5 (+) | 10 (++) | 9 (++) | 7 (+) | – | |
| Roots | – | – | – | – | 9.5 (++) | 8 (+) | 7 (+) | – | |
| Rhizomes | 9 (++) | 8 (+) | 7 (+) | 6.5 (+) | – | – | – | – | |
| Flowering | Leaves | – | – | – | – | – | – | – | – |
| Stems | 7 (+) | 7 (+) | 6.5 (+) | – | – | – | – | – | |
| Roots | – | – | – | – | 9 (++) | 8 (+) | 7 (+) | 6.5 (+) | |
| Rhizomes | – | – | – | – | – | – | – | – | |
| Flowers | 7.5 (+) | 7 (+) | 7 (+) | 6.5 (+) | – | – | – | – | |
| Seed ripening | Leaves | 9 (++) | 8 (+) | 7 (+) | 7 (+) | – | – | – | – |
| Stems | – | – | – | – | – | – | – | – | |
| Roots | 12 (+++) | 10 (+) | 7.5 (+) | 7 (+) | 10 (++) | 8 (+) | 7.5 (+) | 7 (+) | |
| Rhizomes | – | – | – | – | – | – | – | – | |
| Seeds | 9 (++) | 8 (+) | 8 (+) | 7 (+) | – | – | – | – | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. https://doi.org/10.3390/plants9080926
Chepel V, Lisun V, Skrypnik L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants. 2020; 9(8):926. https://doi.org/10.3390/plants9080926
Chicago/Turabian StyleChepel, Victoria, Valery Lisun, and Liubov Skrypnik. 2020. "Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages" Plants 9, no. 8: 926. https://doi.org/10.3390/plants9080926
APA StyleChepel, V., Lisun, V., & Skrypnik, L. (2020). Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants, 9(8), 926. https://doi.org/10.3390/plants9080926