A New Species of Mimosa L. ser. Bipinnatae DC. (Leguminosae) from the Cerrado: Taxonomic and Phylogenetic Insights
Abstract
:1. Introduction
2. Results
2.1. Taxonomic Treatment
2.1.1. Type and Diagnosis
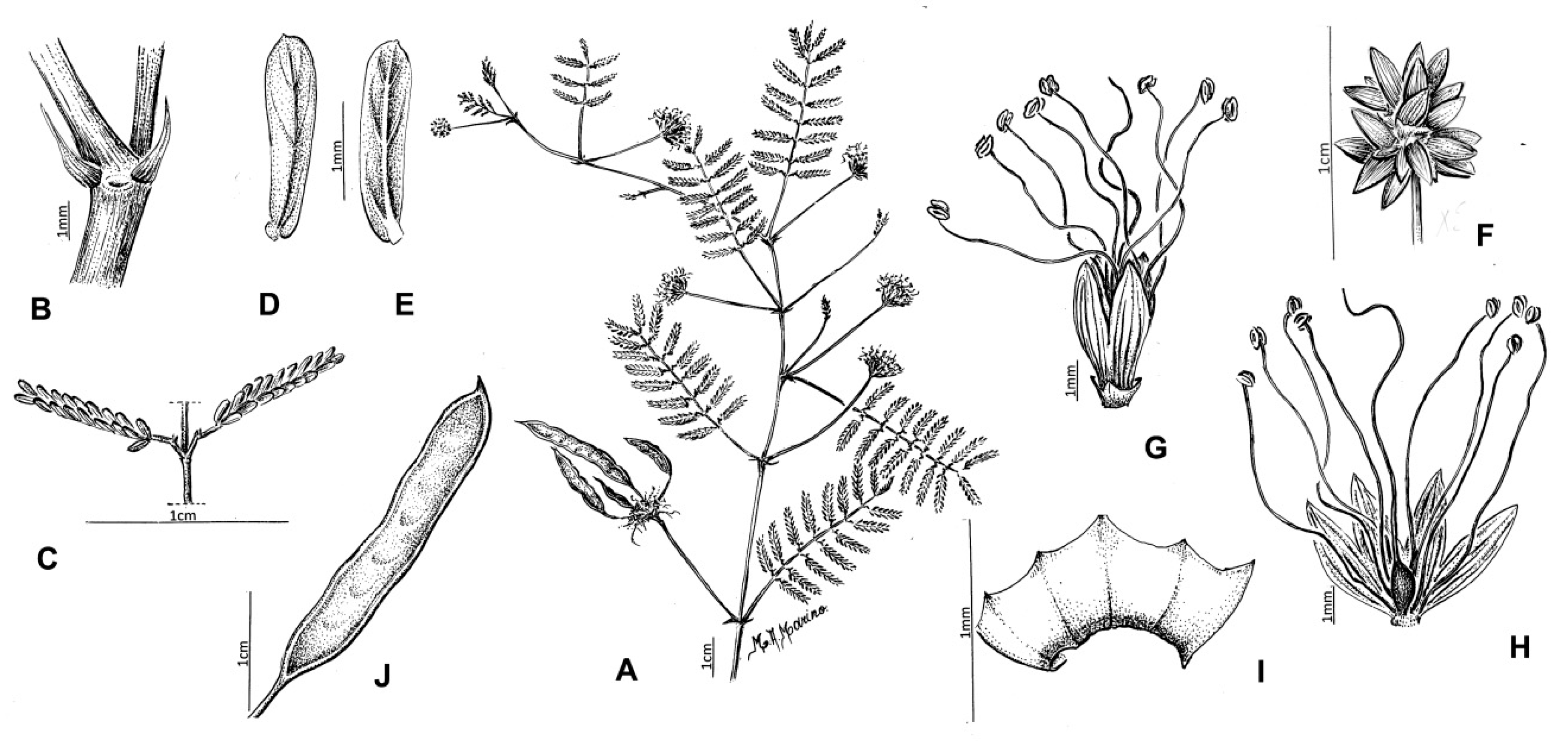
2.1.2. Description
2.1.3. Distribution and Ecology
2.1.4. Etymology
2.1.5. Conservation notes
2.2. Morphological and Phylogenetic Analyses
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based approach to protecting half the terrestrial realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Ratter, J.A.; Ribeiro, J.F.; Bridgewater, S. The Brazilian Cerrado vegetation and threats to its biodiversity. Ann. Bot. 1997, 80, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.F.; Pennington, T. Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Barneby, R.C. Sensitivae Censitae: A description of the genus Mimosa Linnaeus (Mimosaceae) in the New World. Mem. N. Y. Bot. Gard. 1991, 65, 1–835. [Google Scholar]
- Barneby, R.C. Toward a Census of Genus Mimosa (Mimosaceae) in the Americas: A New Species from Mexico (Baja California Sur) and Two from Planaltine Brazil (Goiás, Minas Gerais). Brittonia 1997, 49, 452–457. [Google Scholar] [CrossRef]
- Simon, M.F.; Hughes, C.E.; Harris, S.A. Four new species of Mimosa (Leguminosae) from the central highlands of Brazil. Syst. Bot. 2010, 35, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Dutra, V.F.; Garcia, F.C.P. Two new species and one new variety of Mimosa sect. Habbasia (Leguminosae: Mimosoideae) from Central Brazil. Kew Bull. 2013, 68, 163–171. [Google Scholar] [CrossRef]
- Dutra, V.F.; Garcia, F.C.P. Three new species of Mimosa sect. Mimosa (Leguminosae, Mimosoideae) from the campos rupestres of Minas Gerais, Brazil. Brittonia 2014, 66, 33–41. [Google Scholar] [CrossRef]
- Borges, L.M.; Simon, M.F.; Pirani, J.R. The census continues: Two new montane species of Mimosa (Leguminosae Mimosoideae) from Southeastern Brazil. Phytotaxa 2014, 177, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Atahuachi, M.; Van Der Bent, M.L.; Wood, J.R.I.; Lewis, G.P.; Hughes, C.E. Bolivian Mimosa (Leguminosae, Mimosoideae): Three new species and a species checklist. Phytotaxa 2016, 260, 201–222. [Google Scholar] [CrossRef]
- Jordão, L.S.B.; Morim, M.P.; Baumgratz, J.F.A.; Simon, M.F. A new species of Mimosa (Leguminosae) endemic to the Brazilian Cerrado. Phytotaxa 2017, 312, 237–246. [Google Scholar] [CrossRef]
- Morales, M.; Grohar, M.; Rosenfeldt, S.; Fortunato, R.H. A new species of Mimosa (Leguminosae) from the Paraguayan Cerrado. Phytotaxa 2019, 401, 24–32. [Google Scholar] [CrossRef]
- Simon, M.F.; Proença, C. Phytogeographic patterns of Mimosa (Mimosoideae, Leguminosae) in the Cerrado biome of Brazil: An indicator genus of high-altitude centers of endemism? Biol. Conserv. 2000, 96, 279–296. [Google Scholar] [CrossRef]
- Bessega, C.; Fortunato, R.H. Section Mimadenia: Its phylogenetic relationships within the genus Mimosa (Leguminosae, Mimosoideae) using plastid trnL-F sequence data. Aust. Syst. Bot. 2011, 24, 104–110. [Google Scholar] [CrossRef]
- Simon, M.F.; Grether, R.; Queiroz, L.P.; Särkinen, T.E.; Dutra, V.F.; Hughes, C.E. The evolutionary history of Mimosa (Leguminosae): Toward a phylogeny of the sensitive plants. Am. J. Bot. 2011, 98, 1201–1221. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Baena, M.S.; Garcia, L.C.; Peterson, A.T. Completeness of digital accessible knowledge of the plants of Brazil and priorities for survey and inventory. Divers. Distrib. 2014, 20, 369–381. [Google Scholar] [CrossRef]
- IUCN–Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 6 March 2020).
- Bentham, G. Notes on Mimoseae, with a short synopsis of species. J. Bot. 1842, 4, 393–418. [Google Scholar]
- Scatigna, A.V.; da Silva, N.G.; Válka, R.J.; Souza, V.C.; Simões, A.O. Two new species of the Carnivorous Genus Philcoxia (Plantaginaceae) from the Brazilian Cerrado. Syst. Bot. 2017, 42, 351–357. [Google Scholar] [CrossRef]
- Guarçoni, E.A.E.; Saraiva, R.V.C.; Ferraz, S.M. Dyckia maranhensis (Bromeliaceae, Pitcairnioideae), a new species from the Cerrado of Maranhão, northeastern Brazil. Syst. Bot. 2020, 45, 47–52. [Google Scholar] [CrossRef]
- JSTOR, Global Plant Database. Available online: https://plants.jstor.org/ (accessed on 6 February 2020).
- Kew Herbarium Catalogue. Available online: https://apps.kew.org/herbcat/navigator.do (accessed on 27 April 2020).
- The Barneby Legume Catalogue, New York Botanical Garden. Available online: http://sweetgum.nybg.org/science/projects/barneby/ (accessed on 27 April 2020).
- TROPICOS—Missouri Botanical Garden. Available online: https://www.tropicos.org/home (accessed on 27 April 2020).
- Species Link. Available online: http://splink.cria.org.br/tools?criaLANG=pt (accessed on 27 April 2020).
- Inglis, P.W.; Pappas, M.R.; Resende, V.L.; Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high throughput SNP genotyping and sequencing applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J.; Clustal, W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TreeBASE: A database of phylogenetic knowledge. Available online: http://treebase.org (accessed on 1 April 2020).



| Dichotomy | Step/Species |
|---|---|
| 1. Corolla 3–11–nerved | 2 |
| 1′. Corolla 1–3–nerved | 11 |
| 2. Setae of stems dilated and dorsiventrally compressed, scalelike | M. calliandroides Hoehne |
| 2′. Setae of stems subterete, sometimes transversally dilated scarce overall the stems | 3 |
| 3. Trichomes of indumentum branched, generally arborescent plumose–scabrous | M. surumuënsis Harms |
| 3′. Trichomes unbranched | 4 |
| 4. Leaflets up to 12–17 × 2–4.5 mm | M. somnambulans Barneby |
| 4′. Leaflets up to 10 × 2 mm | 5 |
| 5. Bracts of the lower flowers in each capitulum united into an involucre, generally heteromorphic | 6 |
| 5′. Floral bracts homomorphic, not forming an involucre | 7 |
| 6. Stems armed; pinnae constantly 2-pairs | M. monacensis Barneby |
| 6. Stems unarmed; pinnae 2–4-pairs | M. poculata Barneby |
| 7. Pinnae of leaves at mid-stem 2-pairs | M. glaucula Barneby |
| 7′. Pinnae of leaves at mid-stem more than 2-pairs | 8 |
| 8. Rachis of longer pinnae 4–8 mm long, and leaflets 6–10 pairs | 10 |
| 9. Subshrubs humifuse, procumbent, with stems slender. Insertion of pinnae on leaf rachis notably articulated forming a v-shaped, interpinnal spicule absent | M. carolina M.Morales & Marc.F.Simon |
| 9′. Subshrubs erect to procumbent with stems firm. Insertion of pinnae on leaf rachis notably not or barely articulated, interpinnal spicule 0.25–0.5 mm long | M. leptorhachis Benth. |
| 10. Craspedia breaking only in valves, mostly 1–4-seeded. Ovules and seeds 1–4. Leaves subsessile with petiole 0.1–4 mm long | M. brachycarpa Benth |
| 10′. Craspedia with typical dehiscence, breaking in articles. Ovules and seeds mainly more than 5. Leaves with petiole more than 5 mm long | M. somnians Humb. & Bonpl. ex Willd |
| 11. Pinnae 7–30-pairs; cauline setae smooth and subterete, sometimes basally spurred | M. microcephala Humb. & Bonpl. ex Willd |
| 11′. Pinnae 1–4-pairs; cauline setae scaberoulous | 12 |
| 12. Cauline setae dilated, scalelike, lanceolate–triangular, basifixed; calyx ± one tenth as long as corolla | M. scaberrima Hoehne |
| 12′. Cauline setae terete, spurred at base, thus laterally attached; calyx ± half as long as corolla | M. brachycarpoides Barneby |
| Taxon | Voucher (Herbarium) | Locality | GenBank Accession Number | Source |
|---|---|---|---|---|
| Mimosa adenocarpa Benth. | M.F. Simon 728 (CEN) | Brasília, Distrito Federal, Brazil | FJ981984 | [15] |
| Mimosa brachycarpa Benth. | L.P. Queiroz 10589 (HUEFS) | Porto Estrela, Mato Grosso, Brazil | FJ982011 | [15] |
| Mimosa camporum Benth. | S.M. Faria 729 (RB) | Oriximiná, Pará, Brazil | FJ982019 | [15] |
| Mimosa carolina sp. nov. | M.F. Simon 2828 (CEN) | Carolina, Maranhão, Brazil | MT459463 | This study |
| Mimosa monacensis Barneby | M.F. Simon 2780 (CEN) | Carolina, Maranhão, Brazil | MT459464 | This study |
| Mimosa orthocarpa Spruce ex Benth. | M.F. Simon 855 (FHO) | Acayucan, Veracruz, Mexico | FJ982141 | [15] |
| Mimosa poculata Barneby | L.P. Queiroz 10160 (HUEFS) | Oeiras, Piauí, Brazil | JF694269 | [15] |
| Mimosa somnambulans Barneby | M.F. Simon 2450 (CEN) | Nova Roma, Goiás, Brazil | MT459460 | This study |
| Mimosa somnians Humb. & Bonpl. ex Willd. var. lasiocarpa (Benth.) Barneby | M.F. Simon 736 (CEN) | São João D’aliança, Goiás, Brazil | FJ982194 | [15] |
| Mimosa somnians Humb. & Bonpl. ex Willd. var. lupulina (Benth.) Barneby | M.F. Simon 2436 (CEN) | Niquelândia, Goiás, Brazil | MT459461 | This study |
| Mimosa somnians Humb. & Bonpl. ex Willd. var. velascoënsis (Harms) Barneby | J.E.Q. Faria Jr. 3430 (CEN) | Cáceres, Mato Grosso, Brazil | MT459462 | This study |
| Mimosa somnians Humb. & Bonpl. ex Willd. var. viscida | J.R.I. Wood 27420 (K) | Velasco, Sta. Cruz, Bolivia | KJ802912 | [10] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, M.; Fortunato, R.H.; Simon, M.F. A New Species of Mimosa L. ser. Bipinnatae DC. (Leguminosae) from the Cerrado: Taxonomic and Phylogenetic Insights. Plants 2020, 9, 934. https://doi.org/10.3390/plants9080934
Morales M, Fortunato RH, Simon MF. A New Species of Mimosa L. ser. Bipinnatae DC. (Leguminosae) from the Cerrado: Taxonomic and Phylogenetic Insights. Plants. 2020; 9(8):934. https://doi.org/10.3390/plants9080934
Chicago/Turabian StyleMorales, Matías, Renée H. Fortunato, and Marcelo Fragomeni Simon. 2020. "A New Species of Mimosa L. ser. Bipinnatae DC. (Leguminosae) from the Cerrado: Taxonomic and Phylogenetic Insights" Plants 9, no. 8: 934. https://doi.org/10.3390/plants9080934





